Abstract
Objective:
Obesity is a serious public health problem associated with increased morbidity and mortality. Although the causes for obesity are unclear, it seems that environmental, genetic, neural and endocrine factors contribute to its development. However, the rapid global spread of obesity resembles epidemiologically the spread of an infectious disease. Thus far, little consideration has been given to the possibility that the epidemic of obesity could be due to an infectious agent. Seven viruses and a scrapie agent have been implicated in obesity.
Design:
This review evaluates the infectious pathogens and the evidence that these viruses are associated with obesity and concludes that a strong evidence base is emerging that associates certain viruses with obesity.
Conclusion:
More work is however required to elucidate the mechanisms of weight gain after viral infection. In the mean time, discounting viruses as a contributing factor to obesity would deprive us of a potential new avenue of investigating and treating the ever increasing epidemic of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pi-Sunyer FX . Health implications of obesity. Am J Clin Nutr 1991; 53 (Suppl 6): 1599s–1603s.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data. http://www.cdc.gov/nchs/nhanes.htm.
Sclafani A . Animal models of obesity: classification and characterization. Int J Obes 1984; 8: 491–508.
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB . A virally induced obesity syndrome in mice. Science 1982; 216: 82–85.
Carter JK, Ow CL, Smith RE . Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immun 1983; 39: 410–422.
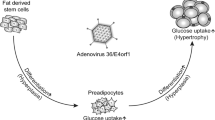
Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A . Effect of adenovirus infection on adiposity in chicken. Vet Microbiol 1992; 31: 101–107.
Gosztonyi G, Ludwig H . Borna disease – neuropathology and pathogenesis. Curr Top Microbiol Immunol 1995; 190: 39–73.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL . Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord 2000; 24: 989–996.
So PW, Herlihy AH, Bell JD . Adiposity induced by adenovirus 5 inoculation. Int J Obes (London) 2005; 29: 603–606.
Kim YS, Carp RI, Callahan SM, Wisniewski HM . Adrenal involvement in scrapie-induced obesity. Proc Soc Exp Biol Med 1988; 189: 21–27.
Whigham LD, Israel BA, Atkinson RL . Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am J Physiol Regul Integr Comp Physiol 2006; 290: R190–R194.
Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ et al. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes (London) 2007; 31: 87–96.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL . Association of adenovirus infection with human obesity. Obes Res 1997; 5: 464–469.
Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (London) 2005; 29: 281–286.
Harder TC, Osterhaus ADME . Canine distemper virus – a morbollivirus in search of new hosts? Trends Microbiol 1997; 5: 120–124.
Bernard A, Fevre-Montange M, Bencsik A, Giraudon P, Wild TF, Confavreux C et al. Brain structures selectively targeted by canine distemper virus in a mouse model infection. J Neuropathol Exp Neurol 1993; 52: 471–480.
Bernard A, Cohen R, Khuth ST, Vedrine B, Verlaeten O, Akaoka H et al. Alteration of the leptin network in late morbid obesity induced in mice by brain infection with canine distemper virus. J Virol 1999; 73: 7317–7327.
Verlaeten O, Griffond B, Khuth ST, Giraudon P, Akaoka H, Belin MF et al. Down regulation of melanin concentrating hormone in virally induced obesity. Mol Cell Endocrinol 2001; 181: 207–219.
Griffond B, Verlaeten O, Belin MF, Risold PY, Bernard A . Specific alteration of the expression of selected hypothalamic neuropeptides during acute and late mouse brain infection using a morbillivirus: relevance to the late-onset obesity? J Brain Res 2004; 1022: 173–181.
Carter JK, Smith RE . Specificity of avian leukosis virus-induced hyperlipidemia. J Virol 1984; 50: 301–308.
Carter JK, Smith RE . Rapid induction of hypothyroidism by an avian leukosis virus. Infect Immun 1983; 40: 795–805.
Johnson ES . Poultry oncogenic retroviruses and humans. Cancer Detect Prev 1994; 18: 9–30.
Johnson ES, Overby L, Philpot R . Detection of antibodies to avian leukosis/sarcoma viruses (ALSV) and reticuloendotheliosis viruses (REV) in humans by ELISA. Cancer Detect Prev 1995; 19: 394–404.
Johnson ES, Overby L, Philpot RJ . Detection of antibodies to avian leukosis/sarcoma viruses and reticuloendotheliosis viruses in humans by western blot assay. Cancer Detect Prev 1995; 19: 472–486.
Tsang SX, Switzer WM, Shanmugam V, Johnson JA, Goldsmith C, Wright A et al. Evidence of avian leukosis virus subgroup E and endogenous avian virus in measles and mumps vaccines derived from chicken cells: investigation of transmission to vaccine recipients. J Virol 1999; 73: 5843–5851.
Johnson JA, Heneine W . Characterization of endogenous avian leukosis viruses in chicken embryonic fibroblast substrates used in production of measles and mumps vaccines. J Virol 2001; 75: 3605–3612.
Zwick W . Bornasche Krankeit und Encephalomyelitis der Tiere. In: Gildenmeister E, Haagen E, Waldmann O (eds). Handbuch der Viruserkrankungen. Gustav Fischer Verlag: Jena, 1939, pp 252–354.
Briese T, de la Torre JC, Lewis A, Ludwig H, Lipkin WI . Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA 1992; 89: 11486–11489.
Briese T, Schneemann A, Lewis AJ, Park YS, Kim S, Ludwig H et al. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA 1994; 91: 4362–4366.
Hornig M, Briese T, Lipkin WI . Borna disease virus. J Neurovirol 2003; 9: 259–273.
Richt JA, Herzog S, Haberzettl K, Rott R . Demonstration of Borna disease virus-specific RNA in secretions of naturally infected horses by the polymerase chain reaction. Med Microbiol Immunol (Berl) 1993; 182: 293–304.
Richt JA, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S . Borna disease virus infection in animals and humans. Emerg Infect Dis 1997; 3: 343–352.
Sierra-Honigmann AM, Rubin SA, Estafanous MG, Yolken RH, Carbone KM . Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol 1993; 45: 31–36.
Berg M, Johansson M, Montell H, Berg AL . Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol Infect 2001; 127: 173–178.
Bode L, Ludwig H . Borna disease virus infection, a human mental-health risk. Clin Microbiol Rev 2003; 16: 534–545.
Dauphin G, Legay V, Pitel PH, Zientara S . Borna disease: current knowledge and virus detection in France. Vet Res 2002; 33: 127–138.
Herden C, Herzog S, Richt JA, Nesseler A, Christ M, Failing K et al. Distribution of Borna disease virus in the brain of rats infected with an obesity-inducing virus strain. Brain Pathol 2000; 10: 39–48.
Lyons MJ, Nagashima K, Zabriskie JB . Animal models of postinfectious obesity: hypothesis and review. J Neurovirol 2002; 8: 1–5.
Herden C, Herzog S, de Lecea L, Frese K, Richt JA . An experimentally induced obesity syndrome in rats infected with Borna disease virus: expression of various neuropeptides. J NeuroVirol 2000; 6: 451 (abstract).
Johnson RT . Prion diseases. Lancet Neurol 2005; 4: 635–642.
Kim YS, Carp RI, Callahan SM, Wisniewski HM . Scrapie induced obesity in mice. J Infect Dis 1987; 156: 402–405.
Outram GW . Changes in drinking and feeding habits of mice with experimental scrapie. J Comp Pathol 1972; 82: 415–427.
Markovitz P, Dormont D, Delpech B, Court L, Latarjet R . Essais de propagation in vitro de l'agent scrapie dans des cellules nerveuses de souris. Comptes Rend Seances l'Acad Sci (Paris) 1981; 293: 413–417.
Carp RI, Callahan SM, Sersen EA, Moretz RC . Preclinical changes in weight of scrapie-infected mice as a function of scrapie agent-mouse strain combination. Intervirology 1984; 21: 61–69.
Carp RI, Kim YS, Callahan SM . Scrapie-induced alterations in glucose tolerance in mice. J Gen Virol 1989; 70: 827–835.
Ye X, Scallet AC, Carp RI . The 139H scrapie agent produces hypothalamic neurotoxicity and pancreatic islet histopathology: electron microscopic studies. Neurotoxicology 1997; 2: 533–545.
De Jong JC, Wermenbol AG, Verweij-Uijterwaal MW, Slaterus KW, Wertheim-Van Dillen P, Van Doornum GJ et al. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbiol 1999; 37: 3940–3945.
Hierholzer JC . Adenoviruses. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds). Manual of Clinical Microbiology, 6th edn. ASM Press: Washington, DC, 1995, pp 947–955.
Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr 2002; 132: 3155–3160.
Vangipuram SD, Sheele J, Atkinson RL, Holland TC, Dhurandhar NV . A human adenovirus enhances preadipocyte differentiation. Obes Res 2004; 12: 770–777.
Rogers P, Fusinski K, Loiler S, Rathod M, Holland TC, Dhurandhar NV . E4 orf-1 gene of adipogenic human adenovirus Ad-36 enhances cAMP and insulin signaling pathways and induces differentiation in preadipocytes. In: Experimental Biology 2006. San Francisco, CA, 1–5 April 2006, abstract.
Pasarica M, Mahida M, Ou Yang H, Yu M, Mohankumar S, Jen K-L C et al. Human adenovirus-36 (Ad-36) induces adiposity in rats. Obes Res 2004; 12 (Suppl): A122.
Kapila M, Khosla P, Dhurandhar NV . Novel short-term effects of adenovirus Ad-36, on hamster lipoproteins. Int J Obes Relat Metab Disord 2004; 28: 1521–1527.
Rathod M, Vangipuram SD, Krishnan B, Heydari AR, Holland TC, Dhurandhar NV . Viral mRNA expression but not DNA replication is required for lipogenic effect of human adenovirus Ad-36 in preadipocytes. Int J Obes (London) 2007; 31: 78–86.
Marshall BJ, Warren JR . Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 1: 1311–1315.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasilakopoulou, A., le Roux, C. Could a virus contribute to weight gain?. Int J Obes 31, 1350–1356 (2007). https://doi.org/10.1038/sj.ijo.0803623
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803623
Keywords
This article is cited by
-
Obesity induced by Borna disease virus in rats: key roles of hypothalamic fast-acting neurotransmitters and inflammatory infiltrates
Brain Structure and Function (2020)
-
Polyinosinic‐polycytidylic acid inhibits the differentiation of mouse preadipocytes through pattern recognition receptor‐mediated secretion of tumor necrosis factor‐α
Immunology & Cell Biology (2016)
-
Pattern recognition receptor‐initiated innate antiviral response in mouse adipose cells
Immunology & Cell Biology (2014)
-
Progressive dementia associated with ataxia or obesity in patients with Tropheryma whipplei encephalitis
BMC Infectious Diseases (2011)
-
Response to “Prevalence of Infection With Adenovirus‐36 in Belgium and Holland and Association With Obesity”
Obesity (2011)