Abstract
Hypertension is one of the most important cardiovascular risk factors and results in macrophage infiltration of blood vessels. However, how macrophages coordinate inflammatory responses with endothelial cells (ECs) remains unclear. In this study, we investigated whether exosomes upregulate the expression of inflammatory factors in ECs under hypertensive conditions. Hypertension was induced in rats by continuous infusion of angiotensin II (Ang II). Exosomes were purified from rat serum by density gradient and ultracentrifugation and used to stimulate human coronary artery ECs (HCAECs). Moreover, the interactions between HCAECs and exosomes from human THP-1-derived macrophages were analyzed. Administration of Ang II enhanced the expression of CD68, a macrophage marker, in rat hearts, suggesting enhanced infiltration of macrophages. In addition, the expression of intracellular adhesion molecule-1 (ICAM1) and plasminogen activator inhibitor-1 (PAI-1), a proinflammatory factor, was increased in hypertensive rat hearts compared with control rats. CD68 protein expression and an increase in the expression of some exosome markers were detected in exosomes from hypertensive rat serum. Moreover, the exosomes upregulated the expression levels of ICAM1 and PAI-1 in HCAECs. The level of miR-17, a negative regulator of ICAM1 expression, was markedly decreased in exosomes from hypertensive rat serum compared with exosomes from control rats. Interestingly, Ang II-stimulated THP-1-derived exosomes also enhanced the expression of ICAM1 and PAI-1 and contained reduced levels of miR-17 compared with exosomes from unstimulated cells. These results suggest that inflammation of ECs under hypertensive conditions is caused, at least in part, by macrophage-derived exosomes.
Similar content being viewed by others
Introduction
Hypertension is a major risk factor for cardiovascular disease, stroke and chronic renal failure. Furthermore, hypertension promotes left ventricular hypertrophy, enhancing the incidence of heart failure and ventricular arrhythmias.1, 2, 3 Target organ damage occurs from blood pressure (BP)-dependent and -independent effects of several hormones and neurotransmitters, particularly angiotensin II (Ang II). Ang II not only elevates BP but also causes target organ damage mediated largely by inflammation and fibrosis.4, 5, 6 Therefore, inhibition of Ang II may also have protective effects beyond BP reduction.7 Furthermore, various anti-inflammatory and immunosuppressive interventions reduce Ang II-induced hypertensive target organ damage.4, 6, 8, 9, 10 Inflammation is most likely associated with unfavorable outcomes as a result of increased infarct size or altered ventricular remodeling and, especially, macrophage and fibroblast infiltration.11 However, the crucial mechanisms underlying regulation of inflammation and cardiac remodeling by Ang II remain poorly understood.
Exosomes are released from intracellular compartments, specifically late endosomes and multivesicular bodies, after fusion with the plasma membrane.12 These particles (50–100 nm in size) are released from viable cells either constitutively or upon activation of cell secretion but not from lysed or apoptotic cells.13 Exosomes are enriched in various bioactive materials, including proteins, lipids, mRNA and microRNA (miRNA).14, 15 Exosomes are thus communicasomes (that is, extracellular organelles that play diverse roles in intracellular communication); they function as signaling complexes by directly stimulating target cells and by transferring membrane receptors, proteins and mRNA between cells.
In this study, we investigated whether exosomes from Ang II-stimulated macrophages communicate with endothelial cells (ECs). We demonstrated that macrophage-derived exosomes increase the protein levels of intracellular adhesion molecule-1 (ICAM1) and plasminogen activator inhibitor-1 (PAI-1) in ECs. This is the first study to investigate the effects of serum-derived exosomes under hypertensive conditions.
Methods
All procedures were performed in accordance with the Osaka City University animal care guidelines and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
The in vivo hypertensive models
The 8-week-old male Wistar rats were purchased from CLEA Japan (Osaka, Japan). To induce cardiac hypertrophy in rats, Ang II (200 ng kg−1 min−1) was dissolved in saline and continuously infused subcutaneously via an osmotic mini-pump (Alzet, Cupertino, CA, USA), as previously described.16, 17 At 3 and 7 days after Ang II infusion, BP was determined using the tail cuff method (BP98A; Softron, Tokyo, Japan). Under anesthesia, the rat abdomen was opened, and a blood sample was collected from the inferior vena cava.
The hearts were then immediately excised, and the ventricle was separated from the atrium and weighed. The lower portions of the ventricles and aorta were fixed in 10% formaldehyde overnight, embedded in paraffin and stored at −80 °C until analysis of the expression of protein by western blot. Exosomes were purified from the serum as described below.
Immunohistochemical analysis
The paraffin-embedded heart tissue was cut into 4 μm-thick slices. The sections were stained using the Envision System (Dako, Tokyo, Japan) according to the manufacturer’s instructions using polyclonal anti-ED1 antibody (BMA Biomedicals, Augst, Switzerland) (1:200). The chromogen, which was diluted with 3,3′-diaminobenzidine in substrate buffer, was added to each section. Finally, the sections were counterstained with hematoxylin. Brown-colored products indicated positive staining.
Culture of macrophages
Human monocytic leukemia cells (THP-1), which were provided by the Health Science Research Resource Bank (Osaka, Japan), were cultured in RPMI-1640 medium containing 10% fetal bovine serum, penicillin (100 units per ml) and streptomycin (100 μg ml−1) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. THP-1 cells were seeded onto a 100 mm dish at a density of 1 × 107 cells and differentiated into macrophages with 100 nmol l−1 phorbol 12-myristate-13-acetate in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum for 24 h. After replacing the old media with fresh media, THP-1-derived macrophages were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum for 24 h. Cells were stimulated with or without Ang II (100 ng ml−1) under normoxic or hypoxic conditions for 24 h. Thereafter, the culture medium was collected for purification of exosomes, and the cells were analyzed by western blot.
Exosome isolation
Serum (1 ml) or culture media (21 ml) was centrifuged (2000 g for 30 min and 10 000 g for 30 min, respectively) at 4 °C to remove cellular debris. The supernatant was filtered through a 0.22 μm membrane and ultracentrifuged (100 000 g, 3 h) at 4 °C. The exosomes were washed in phosphate-buffered saline, ultracentrifuged (100 000 g, 3 h) at 4 °C and then resuspended in phosphate-buffered saline on ice.18 Moreover, exosomes were purified using an OptiPrep (Axis-Shield PoC, Oslo, Norway) density gradient. Briefly, a discontinuous iodixanol gradient was prepared by diluting a stock solution of OptiPrep (60% w/v) with 0.25 M sucrose/10 mM Tris, pH 7.5, to generate 40, 20, 10 and 5% w/v iodixanol solutions. The discontinuous iodixanol gradient was generated by sequentially layering 3 ml of each of 40, 20 and 10% (w/v) iodixanol solutions followed by 2.5 ml of the 5% iodixanol solution in 16 × 102 mm Ultra-Clear Hitachi Coulter centrifuge tubes (Hitachi, Tokyo, Japan). A 500 μl volume of exosomes derived from each of Ang II-stimulated or untreated rat serum purified by ultracentrifuge was overlaid on the discontinuous iodixanol gradient and centrifuged using a P28S-1004 rotor (Hitachi) for 18 h at 100 000 g at 4 °C. A total of twelve 1 ml fractions were collected from the top of the iodixanol solution and diluted to 2.5 ml in phosphate-buffered saline and centrifuged at 100 000 g for 3 h at 4 °C with a P70-AT-687 rotor (Hitachi).
Purified exosomes were characterized by electron microscopy. Moreover, we also assessed the size homogeneity of the obtained vesicles using a Zetasizer Nano ZS90 (Malvern Instruments, Kobe, Japan) instrument. Protein concentrations were determined using a commercial bicinchoninic acid kit.
Treatment of ECs with exosomes
Human coronary artery ECs (HCAECs; 4.5 × 105 cells) were cultured in 60 mm dishes and starved with endothelial growth medium-2 containing 0.2% fetal bovine serum for 18 h. Cells were stimulated with serum-derived exosomes in 10% serum or 0.3 or 2.5 μg of THP-1-derived exosomes for 24 h. After washing twice with phosphate-buffered saline, adherent cells were collected in 20 mM Tris-HCl (pH 7.4) containing 1% Triton X-100, 150 mM NaCl, 5 mM ethylene glycol tetra acetic acid, 50 mM NaF, 5 mM Na3VO4 and 100 μM phenylmethylsulfonyl fluoride (radioimmunoprecipitation assay (RIPA) buffer).
Western blot analysis
Left ventricle and aorta from Ang II-treated and control rats were homogenized with RIPA buffer by a Physcotron homogenizer (Niti-on, Chiba, Japan). Whole-cell lysates were also prepared in RIPA buffer, and then proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 10% gel. Immunoblot analysis was performed using antibodies against heat shock protein 90 (HSP90) (BD, New York, NY, USA) (1:200), 70 kDa heat shock cognate protein (HSC70) (Enzo, Farmingdale, NY, USA) (1:1000), CD63 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:500), CD9 (Abcam, Cambridge, UK) (1:1000), CD68 (Santa Cruz Biotechnology) (1:500), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abnove, Taipei City, Taiwan) (1:1000), phospho-endothelial nitric oxide synthase (p-eNOS) (Cell Signaling, Tokyo, Japan) (1:1000), eNOS (Cell Signaling) (1:1000), human ICAM1 (Bioss, Atlanta, GA, USA) (1:1000), rat ICAM1 (R&D Systems, Minneapolis, MN, USA) (1:5000), PAI-1 (Santa Cruz Biotechnology) (1:1000) and β-actin (Sigma, St Louis, MO, USA) (1:10 000). Blotted proteins were visualized using horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Dako) and Immobilon Western Chemiluminescent HRP Substrate (Millipore, Darmsadt, Germany). The amounts of protein detected by some antibodies were measured using a computed image analysis system (ImageQuant LAS-4000 mini, GE Healthcare, Buckinghamshire, UK).
mRNA and miRNA analysis
Total RNA was collected by using Isogen (Nippon Gene, Toyama, Japan) and extracted according to the manufacturer’s instructions. Total RNA at 500 ng was transcribed into cDNA with the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) in a total volume of 10 μl as follows: incubation for 15 min at 37 °C, followed by heating for 5 min at 98 °C to stop the reaction. cDNAs were then used as templates for PCR amplification using the SYBR Green PCR Master Mix (Roche, Tokyo, Japan) and the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The following primers were used: inducible nitric oxide synthase (iNOS) (NM_000625), forward 5′-TTCAGTATCACAACCTCAGCAAG-3′ and reverse 5′-TGGACCTGCAAGTTAAAATCCC-3′ primers; mannose receptor (MR) (NM_002438), forward 5′-TCCGGGTGCTGTTCTCCTA-3′ and reverse 5′-CCAGTCTGTTTTTGATGGCC T-3′ primers; interleukin-12 (IL-12) (p40) (NM_002187), forward 5′-CCCTGACATTCTGCGTTCA-3′ and reverse 5′-AGGTCTTGTCCGTGAAGACTCTA-3′ primers; IL-10 (NM_000572), forward 5′-GATGCCTTCAGCAGAGTGAA-3′ and reverse 5′-GCAACCCAGGTAACCCTTAAA-3′ primers; 18S rRNA, forward 5′-GCAATTATTCCCCATGAACG-3′ and reverse 5′-AGGGCCTCACTAAACCATCC-3′. Here, 18S rRNA was used as the housekeeping gene. The interpolated values for each sample were divided by the corresponding values for 18S rRNA, and the results are expressed as the ratio of relative intensity for specific gene/18S rRNA. Real-time PCR reactions were performed for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C.
MicroRNA was collected by using Isogen II (Nippon Gene) and extracted according to the manufacturer’s instructions. MicroRNA at 100 ng was transcribed into cDNA with the RT Kit (Qiagen, Hilden, Germany) in a total volume of 10 μl as follows: incubation for 60 min at 37 °C, followed by heating for 5 min at 95 °C to stop the reaction. cDNAs were then used as templates for PCR amplification using the miScript PCR system (Qiagen) and the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). The following primers were used: miR-17, forward 5′-ACTGCAGTGAAGGCACTTGTGG-3′; RNU6, forward 5′-AGACCCATTATGCTCCCCTGA-3′; miR-145-5p, forward 5′-GTCCAGTTTTCCCAGGAATCCCT-3′; miR-221-3p, forward 5′-AGCTACATTGTCTGCTGGGTTTC-3′; miR-222-5p, forward; 5′-GGCTCAGTAGCCAGTGTAGAT-3′. The interpolated values for intracellular samples were divided by the corresponding values for RNU6, which served as the housekeeping gene, and the results are expressed as the relative intensity ratio of miR-17/RNU6. Real-time PCR reactions were performed for 15 min at 95 °C, followed by 40 cycles of 15 s at 94 °C, 30 s at 55 °C and 30 s at 70 °C.
Statistical analysis
All data are presented as mean±s.d. Comparisons among groups were made using one-way analysis of variance. If the one-way analysis of variance result was significant, differences between individual groups were estimated using the Mann–Whitney U-test. The differences were considered statistically significant at a P-value of <0.05.
Results
Macrophages infiltrate the hearts of Ang II-treated rats
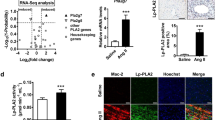
Ang II elevated BP at days 3 and 7 and increased left ventricular weights in treated rats (Figures 1a and b). Hematoxylin and eosin staining revealed that a large number of leukocytes infiltrated the inflamed region of the hearts of Ang II-treated rats after 7 days of Ang II infusion. In contrast, this phenomenon was not observed in the hearts of the control rats (Figure 1c). To determine the type of cells that infiltrated the rat heart, an anti-ED1 (CD68) antibody, which indicates the presence of macrophages, was used. Striking macrophage accumulation was observed in the hearts of hypertensive rats. In addition, CD68 levels were moderately increased in the left ventricle of Ang II-infused rat hearts (Figures 1d and e). Moreover, the expression levels of ICAM1 and PAI-1 were increased in the left ventricle of Ang II-infused rats compared with control rats (Figures 1f and g). Similarly, the increase in the expression of their inflammatory factors was detected in aorta after the administration of Ang II (Figures 1h and i). These results suggest that the administration of Ang II causes the accumulation of macrophages in rat heart and enhances the expression of ICAM1 and PAI-1 in rat heart and aorta. Hence, the amount of protein in rat serum-derived exosomes was investigated.
Macrophage infiltration of the hearts of angiotensin II (Ang II)-treated rats. Systolic blood pressure (BP) (a) and left ventricular (LV) weight (b) after 3 and 7 days of Ang II infusion are presented. Values are presented as the mean±s.d. (n=5 to 12). (c) Heart tissue sections stained with hematoxylin–eosin (HE) from control rats (i) or rats treated with Ang II for 7 days (ii); macrophages infiltration was visualized using anti-ED1 (anti-CD68) antibodies in immunohistochemically stained sections from control rats (iii) or rats treated with Ang II for 7 days (iv). (d) CD68 expression was analyzed using western blotting in the hearts of rats treated with Ang II for 7 days. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is presented as a loading control. (e) The ratios of CD68/GAPDH expression are presented as mean±s.d. (n=4 to 5). (f) Intracellular adhesion molecule-1 (ICAM1) and plasminogen activator inhibitor-1 (PAI-1) expression levels were analyzed by western blotting in the hearts of rats treated with Ang II for 7 days. The expression of GAPDH is presented as a loading control. (g) The ratios of the expression levels of ICAM1/GAPDH and PAI-1/GAPDH are presented as mean±s.d. (n=4 to 5). (h) The expression of ICAM1 and PAI-1 was analyzed using western blotting in the aorta of rats treated with Ang II for 7 days. The expression of β-actin is presented as a loading control. (i) The ratios of ICAM1/β-actin and PAI-1/β-actin expression are presented as mean±s.d. (n=4 to 5). Significant differences were determined by analysis of variance (ANOVA) and the Mann–Whitney U-test.
Macrophage marker proteins are present in rat serum-derived exosomes
To compare serum-derived exosomes from Ang II-treated rats at day 7 (AII-serum-exo) and control rats (Cont-serum-exo), we determined the protein levels of the exosome markers HSP90, HSC70, CD63, CD9 (a tetraspanin) and GAPDH. The relative intensity of HSP90 and CD63 proteins was increased in AII-serum-exo compared with Cont-serum-exo (Figures 2a and b). In this study, the protein expression of CD68, a macrophage marker, was first confirmed in rat serum-derived exosomes. These results indicate that the quality and quantity of exosomes may be altered in rat serum during hypertension.
Circulating exosomes in hypertensive rat serum. (a) Exosomes from the serum of rats infused with angiotensin II (Ang II) for 7 days (AII-serum-exo) (n=6) and serum of control rats (Cont-serum-exo) (n=7) were purified by ultracentrifugation. Heat shock protein 90 (HSP90), heat shock cognate protein 70 (HSC70), CD63, CD9 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as exosome protein markers and CD68 as a macrophage marker were analyzed by western blotting. Exosomes from 60 μl of serum were applied on a lane. (b) The amount of protein in each exosome is shown as the ratio of the expression of HSP90 in cont-serum-exo (AII-serum-exo; n=6, Cont-serum-exo; n=7). A difference with *P<0.05 was considered significant. (c) The exosomes were purified by ultracentrifugation from rat serum followed by an OptiPrep density gradient. HSP90, HSC70, CD63, CD9, GAPDH and CD68 were analyzed by western blotting in fraction numbers 5 to 10.
To remove contaminant proteins from exosome preparations, rat serum-derived exosomes were purified by an iodixanol gradient and collected in fractions 6 to 9 (Supplementary Figures 1 and 2C). The presence of the CD68 protein was successfully confirmed in positive exosomal fractions.
Serum-derived exosomes from hypertensive rats induce gene expression in ECs
Exosomes contain not only some proteins but also genomic DNA, mRNA and miRNA, and deregulation of miRNA levels in body fluids is associated with hypertension. Thus, we investigated miRNAs that play a role in the function of ECs. The level of miR-17, which targets ICAM1, was decreased in exosomes from rats with hypertension (Figure 3a). The levels of miR-145-5P as well as miR-221 and miR-222-5P, which target PAI-1 and eNOS, respectively, did not differ between AII-serum-exo and Cont-serum-exo.
The function of circulating exosomes in angiotensin II (Ang II)-treated rats. (a) The levels of microRNAs were analyzed by real-time PCR in serum-derived exosomes from rats that were treated with Ang II for 3 days or 7 days (AII-D3 or AII-D7, n=3) or from control rats (Cont, n=4). Significant differences were determined by analysis of variance (ANOVA) and the Mann–Whitney U-test. *P<0.05, NS, not significant. (b) Serum-derived exosomes from rats treated with Ang II for 7 days affect endothelial cell signals. The exosomes (serum-exo; exosomes including in 5% serum/dish) from the sera of rats treated with Ang II for 7 days (AII-serum-exo for D7) were co-cultured with human coronary artery endothelial cells. After stimulation for 24 h, intracellular adhesion molecule-1 (ICAM1) (b), plasminogen activator inhibitor-1 (PAI-1) (c), endothelial nitric oxide synthase (eNOS) and phosphorylated eNOS (d) were analyzed by western blotting. The expression of β-actin is presented as a loading control.
To elucidate whether circulating exosomes from rats with hypertension affect ECs, serum-derived exosomes in fractions 6 to 9 (Figure 2c) were co-cultured with HCAECs. As expected, ICAM1 and PAI-1 levels were significantly increased by AII-serum-exo 24 h after the addition of exosomes to HCAECs (Figures 3b and c) compared with Cont-serum-exo or media alone. Interestingly, Cont-serum-exo drastically decreased ICAM1 protein levels. AII-serum-exo did not increase the protein levels of total or phosphorylated eNOS (Figure 3d). These results suggest that AII-serum-exo could regulate ICAM1 protein expression via the miRNAs contained within the exosomes.
Macrophage-derived exosomes induce inflammation in ECs
As shown in Figure 2, CD68 was detected in rat serum-derived exosomes, suggesting the presence of macrophage-derived exosomes in the serum. Therefore, we examined whether macrophage-derived exosomes (Mϕ-exo) could activate signaling pathways in ECs. Mϕ-exo were purified from the culture media of human THP-1-derived macrophages and cultured with or without Ang II (AII-Mϕ-exo or Cont-Mϕ-exo, respectively) for 24 h. Electron microscopy confirmed that the exosomes released from THP-1-derived macrophages were nanometer-sized particles with bilayer membranes (Supplementary Figure 2A). We also analyzed the particle size distribution using a Zetasizer nano ZS (Supplementary Figures 2B–G). Moreover, exosomes were also prepared from THP-1-derived macrophages cultured under hypoxic conditions (Hypo-Mϕ-exo) for 24 h because hypoxia regulates macrophage functions in inflammation.
In macrophages treated with Ang II for 24 h, iNOS, MR, IL-12 and IL-10 mRNA levels were enhanced compared with that in control macrophages (Figure 4a). In macrophages under hypoxic conditions, iNOS mRNA levels were upregulated, whereas IL-12 mRNA levels were drastically decreased compared with control macrophages. These results indicate that Ang II and hypoxia induce slightly different responses in macrophages. Next, the levels of HSP90 and HSC70, which are exosome marker proteins, were increased in Hypo-Mϕ-exo and AII-Mϕ-exo compared with Cont-Mϕ-exo (Figure 4b).
The function of macrophage-derived exosomes. THP-1-derived macrophages were cultured with or without angiotensin II (Ang II; 100 ng ml−1) under normoxic (Cont or AII) or without Ang II under hypoxic conditions (Hypo) for 24 h. (a) Inducible nitric oxide synthase (iNOS), mannose receptor (MR), interleukin-12 (IL-12) and interleukin-10 (IL-10) in the whole-cell lysates from THP-1-derived macrophages were analyzed by western blotting (n=3 in each set of experiments). A difference with *P<0.05 was considered significant. (b) After ultracentrifugation of the macrophage-cultured media, heat shock protein 90 (HSP90), heat shock cognate protein (HSC70), CD63, cofilin-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as exosome protein markers and CD45 as a leukocyte marker in macrophage-derived exosomes (Mϕ-exo) were analyzed by western blotting. (c) The levels of miR-17-3p and miR-145-5p were analyzed by real-time PCR in Mϕ-exo (Cont, Hypo and AII) and intracellular Mϕ (n=3 in each set of experiments). A difference with *P<0.05 was considered significant. (d) Exosomes (0.3 or 2.5 μg per dish) from macrophages (Cont, Hypo and Ang II) were co-cultured with human coronary artery endothelial cells (HCAECs). After stimulation for 24 h, intracellular adhesion molecule-1 (ICAM1) and plasminogen activator inhibitor-1 (PAI-1) were analyzed by western blotting. The expression of β-actin is presented as a loading control. Significant differences were determined by analysis of variance (ANOVA) and the Mann–Whitney U-test.
The level of miR-17 was decreased in AII-Mϕ-exo but not in Hypo-Mϕ-exo (Figure 4c). In contrast, the intracellular level of miR-17 was not different in macrophages among various conditions. Moreover, miR-145-5P levels in AII-Mϕ-exo were reduced compared with that in Hypo-Mϕ-exo or Cont-Mϕ-exo.
To determine whether macrophage-derived exosomes affect ECs, the exosomes were co-cultured with HCAECs. A marked dose-dependent increase in the level of ICAM1 was noted for AII- and Hypo-Mϕ-exo 24 h after addition of exosomes to the ECs (Figure 4d). PAI-1 expression was also upregulated by AII- and Hypo-Mϕ-exo compared with Cont-Mϕ-exo. These results indicate that macrophage-derived exosomes may play a role in vascular inflammation through upregulation of the expression of the adhesion molecules ICAM1 and PAI-1.
Discussion
In the present study, we elucidated the intracellular communication role of serum-derived exosomes under hypertensive conditions. It was demonstrated that hypertension increased the amount of accumulated macrophages in cardiac tissue and that macrophage-derived exosomes could partially lead to the activation of proinflammatory signaling pathways in ECs.
Early infiltration of macrophages into the heart is a key event in Ang II-induced hypertensive cardiac remodeling.19, 20, 21 Hypoxia has also been proposed as an important player in the pathogenesis of myocardial fibrosis. In addition, hypoxia increases the expression of proinflammatory cytokines, chemokines and adhesion molecules and enhances the accumulation of leukocytes in blood vessels.22 Thus, Ang II and hypoxia are characteristics of a proinflammatory microenvironment.22, 23 However, the mechanism and players involved in crosstalk between macrophages and endothelial cells lining blood vessels in hypertension are currently unknown. As an acellular tool for intercellular communication, mammalian cells secrete nano-sized extracellular vesicles either constitutively or in a regulated manner.24 Various types of mammalian cells, such as platelets, leukocytes, epithelial cells, ECs and tumor cells, secrete extracellular vesicles.25, 26, 27, 28 Hence, we postulated that extracellular vesicles, such as exosomes, play a crucial role in cell-to-cell communication under hypertensive conditions.
In the present study, we compared the protein content of serum-derived exosomes from hypertensive rats and control rats. Hypertension increased the amount of HSP90 and CD63 proteins, which are known exosome markers, in the circulating exosomes, suggesting that there is a qualitative difference between the two exosomes. However, the expression levels of these proteins in the heart were not different between hypertensive rats and control rats (data not shown). This finding indicates that these exosome markers might be occluded into exosomes selectively or they might be increased in the vesicle. Moreover, the expression levels of the proinflammatory factors ICAM1 and PAI-1 were increased in the left ventricle and aorta of Ang II-infused rats compared with control rats. Therefore, we examined whether circulating exosomes upregulate the expression levels of proinflammatory factors in ECs. Interestingly, ICAM1 and PAI-1 expression levels were increased by exosomes isolated from hypertensive rat serum but not from control rat serum. We observed that exosomes did not contain ICAM1 and PAI-1 proteins in these exosomes (data not shown). In contrast, the level of vascular cell adhesion molecule-1 was not affected by Ang II-serum-exo in EC; however, Ang II activates vascular cell adhesion molecule-1 and ICAM1.29 Moreover, ICAM1 expression levels were also increased by circulating exosomes from other hypertensive rats that were treated with Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), an inhibitor of NOS (Supplementary Figure 3). It is suggested that the circulating exosomes in hypertensive rats but not the direct effects of Ang II play an important role in cell-to-cell communication as proinflammatory factors. In a future study, it will be important to analyze serum exosomes from spontaneously hypertensive rats. ICAM1 is an adhesion protein expressed by ECs that is a key factor in vascular inflammation. Notably, ICAM1 on ECs participates in the migration of leukocytes out of the blood in response to pulmonary inflammation30 and acts as a molecular target for leukocyte adherence. Thus, ICAM1 has been implicated in the persistent migration and accumulation of neutrophils and macrophages. As the macrophage marker CD68 was contained within the circulating exosomes, we next prepared macrophage-derived exosomes from PMA-differentiated THP-1 cells that were cultured with Ang II or under hypoxic conditions. The human monocytic leukemia cell line THP-1 is a multifaceted model for the study of differentiation from monocytes to macrophages. Macrophages face pathophysiological hypoxia in inflamed areas because monocytes migrate toward hypoxic sites of inflammation where they differentiate into inflammatory macrophages. In this study, the EC expression level of ICAM1 is enhanced by exosomes from Ang II-stimulated or hypoxic THP-1 macrophages. Thus, these data indicate that circulating extracellular multivesicular bodies, especially macrophage-derived exosomes, could enhance the increase in expression of ICAM1 in ECs, thus upregulating inflammation. Macrophages differentiated from THP-1 monocyte cells expressed the Ang II type 1 (AT1) and AT2 receptors, resulting in binding of exogenous Ang II to macrophages but not monocytes.31 Furthermore, peroxide, which is a relatively stable metabolite of reactive oxygen species, was induced by Ang II through AT1 receptor signaling in THP-1-derived macrophages, suggesting that Ang II contributes to oxidative stress through autocrine or paracrine pathways.32 These investigations suggest that Ang II plays a role in macrophages through the paracrine system. In addition, a variety of signaling pathways are activated by hypoxia.33 In this study, Ang II induced iNOS, IL-12, MR and IL-10 mRNA expression, whereas hypoxia induced iNOS mRNA expression and decreased IL-12 mRNA expression. Several studies have suggested that macrophages can be classified into two major groups: M1 and M2.34 M1 macrophages act as antigen-presenting cells and release iNOS-dependent NO and proinflammatory cytokines such as tumor necrosis factor-α, IL-6, IL-1 and IL-12. In contrast, M2 macrophages exhibit increased expression of MR, a scavenger receptor, and can release anti-inflammatory cytokines, such as IL-4, IL-13, IL-10 or transforming growth factor-β. Our results demonstrate the different phenotypes between Ang II-stimulated and hypoxic THP-1 macrophages, but they were not identified as either of the two phenotypes.
The miRNAs regulate fundamental cellular processes, including extracellular signaling, through the secretion of exosomes.35 The in vitro studies demonstrated that miRNAs are critical for gene expression and function of ECs. Suárez et al.36 transfected human umbilical vein ECs with sense and antisense miR-17-3p and observed that sense miRNA significantly reduced the binding between neutrophils and ECs, whereas inhibition of miR-17-3p increased neutrophil adherence to tumor necrosis factor-α-simulated ECs. The results of the study indicated that miR-17-3p is essential for neutrophil adhesion via regulation of ICAM1 expression. Poliseno et al.37 noted 27 highly expressed miRNAs in human umbilical vein ECs and reported that miRNAs, including miR-31, miR-17-3p, miR-155, miR-221, miR-222 and miR-126, are important factors in the regulation of vascular inflammation. Thus, we examined the amount of miR-17-3p in the exosomes from rat serum and THP-1-derived macrophages. ICAM1 expression was increased in AII-serum-exo treated ECs but decreased in Cont-exo. Similarly, the amount of miR-17-3p was diminished in THP-1-macrophage-derived exosomes that upregulated the expression of ICAM in ECs. These results suggest that Ang II-stimulated macrophages induce the proinflammatory factor ICAM1 in ECs by decreasing the level of miR-17-3p in exosomes under hypertensive conditions. In contrast, the level of miR-17-3p was not downregulated intracellularly in THP-1-macrophages. Thus, the amount of miR-17-3p inside exosomes may be limited. However, the mechanisms for sorting microRNA into the internal vesicles of exosomes remain poorly understood.
Yan et al.38 demonstrated that the Toll-like receptor 4 (TLR4) ligand lipopolysaccharide induces the expression of ICAM1 in ECs. Previously, exosomes from cells infected with a human gamma-herpes virus, the Epstein–Barr virus, also upregulate the expression of ICAM1 in recipient cells.39 Thus, macrophage-derived exosomes might increase the level of ICAM1 through TLR4 signaling. Hypertension may induce the infiltration of inflammatory cells, including macrophages, into tissues, followed by the release of exosomes from these infiltrating cells into the blood, resulting in endothelial damage. Eissler et al.40 determined that hypertension is accompanied by enhanced TLR4 expression together with elevated BP and heart weight in spontaneously hypertensive rats. In addition, TLR4 was also upregulated in L-NAME-induced hypertension. This finding suggests that TLR4 signaling in hypertension can promote an inflammatory response based on cell damage, such as that induced by prolonged periods of elevated BP. However, the role of the innate immune system (that is, TLR4) in hypertension remains uncertain. These effects must be clarified in future studies.
In this study, increased PAI-1 expression was not regulated by miRNA in exosomes. A previous study demonstrated that Ang II activates nuclear factor-κB, resulting in an increase in PAI-1 expression.41 In the present study, nuclear factor-κB was phosphorylated by serum exosomes from hypertensive rats (data not shown). Exosomes derived from glioma cells induce angiogenesis through phenotypic modulation of ECs.42 The tumor-derived exosomes activated several cell surface receptors such as epidermal growth factor receptor and vascular endothelial growth factor receptor 2, and then the receptor kinase activation converges on the phosphoinositide 3-kinase/Akt pathway. Thus, PAI-1 levels might be regulated by exosomes via surface receptor activation rather than miRNA.
In summary, cardiac hypertrophy was associated with inflammation in developing hypertension. Our results indicate that the administration of exogenous hypertensive agents, such as Ang II and L-NAME, increases the amount of proteins in serum exosomes. Similar to exosomes derived from the serum of Ang II-infused rats, Ang II-stimulated macrophage-derived exosomes activated proinflammatory signaling pathways. Thus, macrophage-derived exosomes might be associated with cardiovascular remodeling in hypertension. Elucidating the precise role of exosomes in hypertension might provide new therapeutic targets for the treatment of hypertension-induced cardiovascular diseases.
References
Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C . Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005; 165: 923–928.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913.
Vakili BA, Okin PM, Devereux RB . Prognostic implications of left ventricular hypertrophy. Am Heart J 2001; 141: 334–341.
Bouzegrhane F, Thibault G . Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovasc Res 2002; 53: 304–312.
Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H . Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res 2003; 93: 874–883.
Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC . NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 2000; 35: 193–201.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869.
Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffiman TM . Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 2008; 295: F515–F524.
Mervaala E, Müller DN, Park JK, Dechend R, Schmidt F, Fiebeler A, Bieringer M, Breu V, Ganten D, Haller H, Luft FC . Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension 2000; 35: 360–366.
Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heideche H, Haller H, Zenke M, Luft FC . Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 2002; 161: 1679–1693.
Seropian IM, Toldo S, Van Tassell BW, Abbate A . Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014; 63: 1593–1603.
Lakkaraju A, Rodriguez-Boulan E . Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol 2008; 18: 199–209.
Thery C, Amigorena S, Raposo G, Clayton A . Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3: Unit 3.22.
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, Mochizuki T . Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 2010; 5: e13247.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Kim-Mitsuyama S, Izumi Y, Izumiya Y, Namba M, Yoshida K, Wake R, Yoshiyama M, Iwao H . Dominant-negative c-Jun inhibits rat cardiac hypertrophy induced by angiotensin II and hypertension. Gene Therapy 2006; 13: 348–355.
Yamazaki T, Yamashita N, Izumi Y, Nakamura Y, Shiota M, Hanatani A, Shimada K, Miura T, Iwao H, Yoshiyama M . The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res 2012; 35: 34–40.
Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, Iwao H, Yoshiyama M . Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun 2014; 445: 327–333.
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML . Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 2010; 49: 499–507.
Kagitani S, Ueno H, Hirade S, Takahashi T, Takata M, Inoue H . Tranilast attenuates myocardial fibrosis in association with suppression of monocyte/macrophage infiltration in DOCA/salt hypertensive rats. J Hypertens 2004; 22: 1007–1015.
Ren J, Yang M, Qi G, Zheng J, Jia L, Cheng J, Tian C, Li H, Lin X, Du J . Proinflammatory protein CARD9 is essential for infiltration of monocytic fibroblast precursors and cardiac fibrosis caused by Angiotensin II infusion. Am J Hypertens 2011; 24: 701–707.
Stenmark KR, Fagan KA, Frid MG . Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006; 99: 675–691.
Usui M, Egashira K, Tomita H, Koyanagi M, Katoh M, Shimokawa H, Takeya M, Yoshimura T, Matsushima K, Takeshita A . Important role of local angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation 2000; 101: 305–310.
Choi DS, Kim DK, Kim YK, Gho YS . Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev 2015; 34: 474–490.
Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Pirillo A, Mussoni L, Tremoli E . Proteome of endothelial cell-derived procoagulant microparticles. Proteomics 2005; 5: 4443–4455.
Choi DS, Lee JM, Park GW, Lim HW, Bang JY, Kim YK, Kwon HJ, Kim KP, Gho YS . Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res 2007; 6: 4646–4655.
Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF . The platelet microparticle proteome. J Proteome Res 2005; 4: 1516–1521.
Miguet L, Pacaud K, Felden C, Hugel B, Martinez MC, Freyssinet JM, Herbrecht R, Potier N, van Dorsselaer A, Mauvieux L . Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics 2006; 6: 153–171.
Han Y, Runge MS, Brasier AR . Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κB transcription factors. Circ Res 1999; 84: 695–703.
Nario RC, Hubbard AK . Localization of intercellular adhesion molecule-1 (ICAM-1) in the lungs of silica-exposed mice. Environ Health Perspect 1997; 105: 1183–1190.
Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T . Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens 1999; 17: 537–545.
Yanagitani Y, Rakugi H, Okamura A, Moriguchi K, Takiuchi S, Ohishi M, Suzuki K, Higaki J, Ogihara T . Angiotensin II type 1 receptor-mediated peroxide production in human macrophages. Hypertension 1999; 33: 335–339.
Benizri E, Ginouvès A, Berra E . The magic of the hypoxia-signaling cascade. Cell Mol Life Sci 2008; 65: 1133–1149.
Gordon S . Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35.
Das S, Halushka MK . Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 2015; 24: 199–206.
Suárez Y, Wang C, Manes TD, Pober JS . Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol 2010; 184: 21–25.
Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G . MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006; 108: 3068–3071.
Yan W, Zhao K, Jiang Y, Huang Q, Wang J, Kan W, Wang S . Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock 2002; 17: 433–438.
Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H . Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol 2014; 87: 10334–10347.
Eissler R, Schmaderer C, Rusai K, Kuhne L, Sollinger D, Lahmer T, Witzke O, Lutz J, Heemann U, Baumann M . Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res 2011; 34: 551–558.
Theuer J, Dechend R, Muller DN, Park JK, Fiebeler A, Barta P, Ganten D, Haller H, Dietz R, Luft FC . Angiotensin II induced inflammation in the kidney and in the heart of double transgenic rats. BMC Cardio Dis 2002; 2: 3.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M . Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA 2013; 110: 7312–7317.
Acknowledgements
We thank Dr Oogita, Dr S Hama and Dr K Kogure in the Department of Biophysical Chemistry, Kyoto Pharmaceutical University, for analyzing exosome size using the Zetasizer nano ZS. This study was partially supported by a Grant-in-Aid for Scientific Research (24390064, 15K15132, 24591101 and 26460344) from the Ministry of Education, Science, Culture, Sports and Technology of Japan and the Hoansha Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Rights and permissions
About this article
Cite this article
Osada-Oka, M., Shiota, M., Izumi, Y. et al. Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res 40, 353–360 (2017). https://doi.org/10.1038/hr.2016.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.163
Keywords
This article is cited by
-
Macrophage-based therapeutic approaches for cardiovascular diseases
Basic Research in Cardiology (2024)
-
Engineering biomaterials to tailor the microenvironment for macrophage–endothelium interactions
Nature Reviews Materials (2023)
-
Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications
Biomarker Research (2022)
-
Human pluripotent stem cell-derived macrophages and macrophage-derived exosomes: therapeutic potential in pulmonary fibrosis
Stem Cell Research & Therapy (2022)
-
Differential and targeted vesiculation: pathologic cellular responses to elevated arterial pressure
Molecular and Cellular Biochemistry (2022)