Abstract
We use regression models to investigate the effects of inbreeding in 119 zoo populations, encompassing 88 species of mammals, birds, reptiles and amphibians. Meta-analyses show that inbreeding depression for neonatal survival was significant across the 119 populations although the severity of inbreeding depression appears to vary among taxa. However, few predictors of a population's response to inbreeding are found reliable. The models are most likely to detect inbreeding depression in large populations, that is, in populations in which their statistical power is maximised. Purging was found to be significant in 14 populations and a significant trend of purging was found across populations. The change in inbreeding depression due to purging averaged across the 119 populations is <1%, however, suggesting that the fitness benefits of purging are rarely appreciable. The study re-emphasises the necessity to avoid inbreeding in captive breeding programmes and shows that purging cannot be relied upon to remove deleterious alleles from zoo populations.
Similar content being viewed by others
Introduction
Inbreeding depression, the reduction in fitness in the offspring of matings of close relatives, has been observed in many outbreeding species (e.g. Byers and Waller, 1999; Hedrick and Kalinowski, 2000; Keller and Waller, 2002) and threatens the survival of small populations, both wild and captive. However, since inbreeding increases the frequency of the deleterious homozygous genotype, selection against deleterious alleles is also increased during inbreeding (Crow and Kimura, 1970), thereby providing an opportunity for a population to be ‘purged’ of its mutational load (Lande and Schemske, 1985; Barrett and Charlesworth, 1991). Following purging, the fitness levels of the population may increase, possibly returning to or even exceeding those of a large, randomly mating population at mutation-selection balance (Lande and Schemske, 1985; Hedrick, 1994). Purging may therefore be highly relevant to conservation, where small changes in viability can make the difference between population survival and extinction. At present, little is known about the actual effects of purging in either wild or captive populations. Clearly, a greater understanding of the effects of purging would be highly beneficial to the breeding management of captive populations of threatened species.
Although significant purging has been detected in a variety of taxa in the laboratory (e.g. Saccheri et al., 1996; Lacy and Ballou, 1998; Byers and Waller, 1999; Swindell and Bouzat, 2006), selection in these studies was fairly intense and it would be inappropriate to extrapolate directly to populations of conservation concern. For example, zoo conditions are often benign and hence the selection they exert may not be sufficient to cause purging (but see King, 1939; Gilligan et al., 2003). Additionally, purging appears to be more effective when the rate of inbreeding is low (Bijlsma et al., 2000; Miller and Hedrick, 2001; Day et al., 2003) and is applied over a long period. As zoo populations often undergo unusually intense inbreeding, purging may be less efficient.
If purging improves fitness, it may be desirable to subject endangered populations to deliberate inbreeding. When this was tried in a zoo population of Speke's gazelle (Gazella spekei), the level of inbreeding depression was reported to be dramatically reduced after only two or three generations (Templeton and Read, 1984), leading to support for this approach (Ralls and Ballou, 1986; Templeton et al., 1986). However, reanalysis of the same data failed to find evidence of genetic improvement (Kalinowski et al., 2000). If purging is not occurring in captivity, deliberately inbreeding small, endangered populations could be intensely damaging. It is therefore necessary to establish whether or not purging is occurring in zoo populations. As many zoo populations are pedigreed and are known to have experienced inbreeding (either through necessity, or, predominantly in the past, through lack of concern), they are highly suited to such a study.
Computer simulations indicate that purging is more likely to occur when deleterious mutations are of a large effect and when inbreeding occurs slowly and over many generations (e.g. Hedrick, 1994; Boakes and Wang, 2005). Empirical data suggest that mutations of both large and small effect contribute to inbreeding depression (Simmons and Crow, 1977; Willis, 1999) and hence successive generations of inbreeding in a real population might therefore only purge the mutational load resulting from lethal and semilethal alleles, allowing mildly deleterious alleles to remain. It is difficult to investigate purging in real populations because of the need for a full pedigree spanning several generations. However, laboratory studies have shown that, although purging does occur, its effects can be highly variable (e.g. Byers and Waller, 1999; Fowler and Whitlock, 1999; Crnokrak and Barrett, 2002; Reed et al., 2003). Additionally, the extent of purging as measured by researchers is partly dependent on the methods used in their investigation (e.g. ancestral versus current inbreeding (e.g. Ballou, 1997), changes in fitness components of inbred lines relative to the original outbred line (e.g. Barrett and Charlesworth, 1991), founder-flush experiments (e.g. Miller and Hedrick, 2001) and comparison of populations with the same level of inbreeding but different histories of rates of inbreeding (e.g. Frankham et al., 2001)).
Ballou (1997) tested for the presence of purging in 25 captive mammalian populations and found purging to be statistically significant in one species although there was a small but highly significant trend of purging on neonatal survival across species. However, a recent simulation study (Boakes and Wang, 2005) has shown that the ancestral inbreeding regression model that Ballou used to detect purging lacks statistical power when inbreeding depression is caused by mildly deleterious alleles. This finding suggests that significant purging may in fact have occurred in some populations additional to that in which Ballou (1997) detected it. Boakes and Wang (2005) developed an alternative model to Ballou's that performed up to 30% more powerfully. However, they concluded that, because of the nature of zoo pedigree data (i.e. small population sizes, small number of generations and small variations in inbreeding and ancestral inbreeding coefficients), any regression model used to investigate purging will be lacking in statistical power. Studies that aim to investigate the general effects of inbreeding depression and purging in zoo populations should therefore analyse as many populations as possible in order to obtain maximum statistical power.
In this study, we perform an analysis similar to Ballou's, using both his ancestral inbreeding model (Ballou, 1997) and the model developed by Boakes and Wang (2005), to look for evidence that inbreeding depression has partially been purged due to selection with inbreeding in 119 pedigreed zoo populations (105 mammals, 12 birds, 1 reptile and 1 amphibian). Following Ballou, the fitness trait used is survival to 7 days. By following Ballou's method exactly, we are able to make direct comparisons with his study. We then perform an additional analysis in which we find the minimum adequate model (MAM) that fits the data, hence allowing us to discard correlated or insignificant terms from the regression model. In this last analysis, we adopt the fitness measure of survival to 30 days in order to utilise more infant mortality data and so increase the power of the model. We also control for the effect of zoo as populations are made up of individuals kept at a variety of zoos and infant mortality may be partially dependent on housing conditions.
Methods
Regression models
Pedigree records were used to investigate the effects of inbreeding and purging on juvenile survival in zoo populations. We use two similar logistic regression models to analyse the pedigree data, one of which was devised by Ballou (1997) (hereafter known as ‘Ballou's model’) and the other by Boakes and Wang (2005) (hereafter known as the ‘alternative model’). The models are based on the assumption that inbred animals with inbred ancestry will be less susceptible to inbreeding depression than inbred animals with non-inbred ancestry as those inbred ancestors that are able to survive and to reproduce will be less likely to be carriers of deleterious alleles (Templeton and Read, 1984). Historical inbreeding can be measured by the ancestral inbreeding coefficient (fa), which is defined as the cumulative proportion of an individual's genome that has been previously exposed to inbreeding in its ancestors (Ballou, 1997). A method for calculating fa from pedigree data was described and exemplified in Ballou (1997). It should be noted that this method assumes the independence of f and fa, resulting in a slight overestimation of fa (Baumung, personal communication). However, the overestimation is small, particularly for low values of fa, and the approximation should not affect our results.
Ballou's logistic regression model was as follows

where u is the logit transformation of a measure of fitness such as juvenile survival, u0 is the mean fitness of non-inbred animals, fa is the ancestral inbreeding coefficient and βf,  ,
,  and βYOB are the regression coefficients associated with the inbreeding coefficient (f), the interaction term ffa, maternal inbreeding (fd) and year of birth (YOB), respectively. Inbreeding depression is characterised by a negative value of βf. If purging has occurred then the coefficient
and βYOB are the regression coefficients associated with the inbreeding coefficient (f), the interaction term ffa, maternal inbreeding (fd) and year of birth (YOB), respectively. Inbreeding depression is characterised by a negative value of βf. If purging has occurred then the coefficient  will be positive, thereby reducing the inbreeding effect. The larger the positive value of
will be positive, thereby reducing the inbreeding effect. The larger the positive value of  the greater is the effect of ancestral inbreeding on reducing inbreeding depression. Computer simulations have shown that the statistical power of Ballou's model increases with the selection coefficient of the deleterious allele, the population size and the number of generations of inbreeding, that is, with the proportion of lethal equivalents being purged (Boakes and Wang, 2005).
the greater is the effect of ancestral inbreeding on reducing inbreeding depression. Computer simulations have shown that the statistical power of Ballou's model increases with the selection coefficient of the deleterious allele, the population size and the number of generations of inbreeding, that is, with the proportion of lethal equivalents being purged (Boakes and Wang, 2005).
In Ballou's model,  ffa=0 when f=0, resulting in information about purging in individuals who have inbred ancestors but are not inbred themselves to be discarded from the analysis. Boakes and Wang (2005) therefore devised an alternative regression model which does not have an interaction term
ffa=0 when f=0, resulting in information about purging in individuals who have inbred ancestors but are not inbred themselves to be discarded from the analysis. Boakes and Wang (2005) therefore devised an alternative regression model which does not have an interaction term

where u, u0, f, fa, fd, fYOB, βf,  and βYOB are as described above and
and βYOB are as described above and  is the regression coefficient associated with ancestral inbreeding (fa). When alleles were mildly deleterious, this model showed up to a 30% relative improvement in detection of purging over Ballou's. However, when alleles were semilethal the alternative model detected up to 33% less of the purging events. The selection coefficients of the deleterious alleles that might cause inbreeding depression are not known although studies indicate that mildly deleterious alleles and lethals contribute simultaneously to inbreeding depression (Simmons and Crow, 1977; Willis, 1999). As both models are conservative and neither was found to make false detections of purging (Boakes and Wang, 2005), it was decided to use both models in the analysis and to take the less conservative answer as the more accurate estimate. Boakes and Wang (2005) tested various other regression models on their simulated data but were unable to find a model more powerful than the above mentioned two.
is the regression coefficient associated with ancestral inbreeding (fa). When alleles were mildly deleterious, this model showed up to a 30% relative improvement in detection of purging over Ballou's. However, when alleles were semilethal the alternative model detected up to 33% less of the purging events. The selection coefficients of the deleterious alleles that might cause inbreeding depression are not known although studies indicate that mildly deleterious alleles and lethals contribute simultaneously to inbreeding depression (Simmons and Crow, 1977; Willis, 1999). As both models are conservative and neither was found to make false detections of purging (Boakes and Wang, 2005), it was decided to use both models in the analysis and to take the less conservative answer as the more accurate estimate. Boakes and Wang (2005) tested various other regression models on their simulated data but were unable to find a model more powerful than the above mentioned two.
The simulations showed that, when alleles were of a mildly deleterious effect, the models detected ancestral inbreeding effects more often than they detected inbreeding depression (Boakes and Wang, 2005). Therefore, although inbreeding depression must be present for purging to occur, a detection of a significant increase in fitness with ancestral inbreeding was recorded as a detection of purging, regardless of whether significant inbreeding depression had been detected. Additionally, if levels of inbreeding and ancestral inbreeding are positively correlated, the occurrence of purging will actually reduce the power of the model to detect inbreeding depression. This is because individuals with very high inbreeding coefficients may actually be fitter than individuals with lower inbreeding coefficients. It would therefore be unjustified to dismiss the possibility of purging in populations in which significant inbreeding depression has not been detected.
Data and analyses
Owing to an increased participation in conservation activities over the last 20–30 years, many zoos are involved in coordinated captive breeding programmes (Frankham et al., 2002). Wherever possible, zoos record individuals' basic life histories in studbooks. However, medical records, morphological measurements or incidents of stillbirths are rarely logged. The pedigrees in this study were taken from the 2003 ISIS database that contains 877 studbooks. Pedigrees with sufficient inbreeding, ancestral inbreeding, neonatal mortality and known ancestry were selected for analysis. The 119 of the 877 pedigrees that fulfilled these criteria are listed in the Supplementary information. Some of the pedigrees contain overlapping data as, depending on the taxon, there may be several regional studbooks and/or an international studbook, with individuals sometimes appearing in two or more of these. In such instances, each studbook was analysed as a separate population.
The fitness trait examined was survival to 7 days. Individuals with unknown ancestry or unknown birth or death dates were excluded from the analysis. Wild-caught individuals were also discarded as they might bias the data set. Clearly, only surviving animals would be selected to be brought into zoo populations.
For species that did not produce litters, each individual was coded as either surviving beyond 7 days (1) or dying (0). The survival of offspring within a litter is not independent (Lacy and Ballou, 1998). We controlled for the non-independence of within-litter mortality by coding each litter as an independent data point. A litter was coded as surviving if half or more of the individuals within it survived. Inbreeding coefficients (f) were calculated for each individual using SPARKS v1.42 (Scobie, 1997). It was assumed that founders were unrelated with inbreeding coefficients of f=0. Ancestral inbreeding coefficients were calculated according to Ballou (1997).
Multiple logistic regression was used to estimate the regression coefficients in Equations (1) and (2) using the statistical software R (Ihaka and Gentleman, 1996). Comparison of model fits was based on comparison of the Akaike Information Criterion (AIC) values for each model. A lower AIC value indicated a better fit. A negative value of βf indicated inbreeding depression and a positive value of  indicated purging. Tests for the significance of trends in the signs of the regression coefficients across populations were conducted using a weighted Z-method combined probability test.
indicated purging. Tests for the significance of trends in the signs of the regression coefficients across populations were conducted using a weighted Z-method combined probability test.
The change in inbreeding depression due to ancestral inbreeding was calculated in a similar way to Ballou (1997). Inbreeding depression, δ, was calculated as

where ωf is the fitness of animals with inbreeding coefficient f and ω0 is the fitness of non-inbred animals (Lande and Schemske, 1985). Fitness values were obtained from Equations (1) and (2) using the regression coefficients estimated from the data. The change in inbreeding depression due to purging was estimated by calculating the inbreeding depression for individuals with no ancestral inbreeding (δ) and for individuals who had experienced ancestral inbreeding (δ′). The difference between the two inbreeding depression measures (δ′–δ) is the change in inbreeding depression due to purging. To obtain a standard measure that was comparable across populations, the change in inbreeding depression was calculated for individuals born in 2003 (the year the pedigrees were published) with f=0.25, fa=0.25 and fd=0.
It was important to conduct an analysis that was directly comparable to Ballou's (1997) analysis, hence our use of the methods described above. However, we believed that there were several ways in which the analysis could be improved. Variation may have been introduced into the data due to animals being housed in different zoos which may have differing standards of husbandry or differing protocols regarding the minimisation of inbreeding. In order to control for this potential variation, a mixed effects model was used to reanalyse the pedigree data. Zoo was entered as a random effect as opposed to as a fixed effect as the aim of the analysis was not to make inferences about the zoos themselves but about the populations from which they were drawn. The fitness measure was changed to survival to 30 days in order that additional infant mortality data could be utilised. This new fitness measure was still comparable across species as all of the animals in the analysis were below reproductive age at 30 days.
The mixed-model analysis was performed using the glmmPQL function from the MASS package (v. 7.2–20) in R (Venables and Ripley, 1999). We applied the model

and used stepwise logistic regression to obtain the MAM for the data. We thought that this method of analysis might prove more powerful since correlated or insignificant terms would not be included in the model. The variance in survival to 30 days between zoos was compared to the variance in survival within zoos using the F-test.
Results
There was wide variation in inbreeding (f) and ancestral inbreeding (fa) coefficients among the 119 zoo populations (Table 1). As would be expected, small populations had higher levels of inbreeding and ancestral inbreeding than large populations. The genetic and demographic parameters of the populations are given in the Supplementary information.
Full results of the analyses using Ballou's ancestral inbreeding regression model (Equation (1)) and the alternative regression model (Equation (2)) are given in the Supplementary information. Using a mixed effects model, the MAM was found for each population (Supplementary information) although the model did not converge, that is was unable to fit the data, for one population. In 55 populations, the null model provided the optimum fit, that is the best-fit was achieved by dropping all of the fixed effect terms from the model.
Inbreeding effects
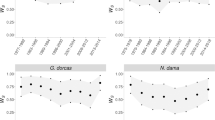
The inbreeding depression experienced by each population when f=0.25 is shown in Figure 1. Weighted Z-method combined probability tests (Mosteller and Bush, 1954; Liptak, 1958) showed that both Ballou's and the alternative models detected a highly significant trend in inbreeding depression across populations (Table 2). However, within populations, significant inbreeding depression was detected by at least one of the models in only 36 of the 119 populations and by all three models in just nine populations (Table 3). The relatively low power of these regression models (Boakes and Wang, 2005) and large number of tests suggests that although some significant results could be due to type 1 errors, in other populations significant inbreeding depression may have gone undetected. Our results are similar to those of Ballou (1997), who found significance in slightly more populations (37% as opposed to 30%) and the same direction of trend for the nine of the 10 populations considered by both studies, the exception being the Sumatran tiger.
The extent of inbreeding depression in the zoo populations. Values are from (a) Ballou's model (119 populations), (b) the alternative model (119 populations) and (c) the MAMs (31 populations). Values were calculated for individuals born in 2003 with f=0.25 and no ancestral or maternal inbreeding. A negative value of inbreeding depression indicates that fitness increased with inbreeding.
Despite the relative lack of significance in individual populations, overall inbreeding was over twice as likely to correlate negatively as opposed to positively with fitness, the ratio tending to be significant in groups with larger sample sizes such as primates and ungulates (Table 4). Only Caniformes show a neutral or even converse pattern. However, the number of species in this group is quite low and in many cases the offspring are hidden in dens and are prone to being eaten by their parents if they die, potentially biasing our measure of fitness. Consequently, it would be premature to conclude that Caniformes are not susceptible to the deleterious effects of inbreeding. Trends in rodents, marsupials, amphibians and reptiles could not be assessed because of small sample sizes.
Ancestral inbreeding effects
Ancestral inbreeding could not be investigated by Ballou's model in two populations as ffa=0 for all individuals. Significant purging, as measured by an increase in fitness with ancestral inbreeding, was detected in a total of only 14 populations (Table 3). However, using the alternative model, a cross-population trend of purging was detected and both models detected a trend of purging across populations, which experienced negative inbreeding effects (Table 2). It therefore seems that although purging is occurring in some zoo populations, its effects are highly variable and cannot be relied upon to reduce inbreeding depression.
It is of interest to note that we did not detect significant purging in the Sumatran tiger, yet this was the only population in which Ballou did detect significant purging. As mentioned above, we also found inbreeding to have a positive effect on neonatal survival in the tiger, as opposed to the negative effect that Ballou detected. Our analysis of the Speke's gazelle population found that neonatal survival decreased with ancestral inbreeding. This result is in contrast to that of Templeton and Read (1983, 1984), who reported purging in the Speke's gazelle population although their result was later disputed (Willis and Wiese, 1997; Kalinowski et al., 2000).
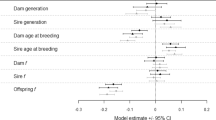
The predicted change in inbreeding depression due to ancestral inbreeding (δ′−δ) was calculated using the regression models for animals born in 2003 with f=0.25, fa=0.25 and fd=0 (Figure 2) and ranged from a 790% decrease in inbreeding depression to a 499% increase (see Table 5). However, it can be seen from Figure 2 that only a few populations showed large changes in inbreeding depression as a consequence of ancestral inbreeding. The large ranges given by Ballou's and the alternative models result from a few populations in which regression coefficients were estimated with very large standard errors and hence it is unlikely that these outlying values are accurate. The median change in inbreeding depression due to ancestral inbreeding in the 119 populations was less than 1%. Ballou obtained a median value of a 2% decrease in inbreeding depression. It can therefore be concluded that, in the majority of populations, the change in inbreeding depression due to purging is negligible. However, the data shown in Figure 2c suggest that in a few populations, ancestral inbreeding is able to significantly mitigate inbreeding depression. In this graph, changes in inbreeding depression are shown only for populations in which significant inbreeding and ancestral inbreeding effects were detected, meaning that the values should be fairly accurate. The fact that values were only calculated for populations in which significant inbreeding and ancestral inbreeding effects were detected also explains why the average changes in inbreeding depression are larger than those calculated for all 119 populations. Figure 3 shows the significant relationship between the change in inbreeding depression due to purging and the inbreeding depression suffered by a population. As would be expected, purging is more effective in populations that suffer from severe inbreeding depression.
Estimated change in inbreeding depression due to ancestral inbreeding (δ′–δ) against inbreeding depression (δ). (a) Estimates from Ballou's model. (δ′–δ)=0.077–0.933δ, R2=0.234, P<0.001. (b) Estimates from the alternative model. (δ′–δ)=−0.026–0.383δ, R2=0.184, P<0.001. Inbreeding depression has been reduced by purging in the populations plotted in the lower right quadrant of the graphs.
Maternal inbreeding and year of birth effects
The maternal inbreeding term was dropped from the models in the analysis of populations in which ancestral inbreeding was so highly correlated with maternal inbreeding that the inclusion of both terms in the model was impossible. In these cases, it must be recognised that ancestral and maternal inbreeding effects are confounded. Table 2 shows that, across populations, maternal inbreeding tended to have a negative effect on fitness. Many other studies of both mammals and birds have shown that maternal inbreeding can lead to reduced fitness (e.g. Ballou, 1997; Reid et al., 2003; Adamec et al., 2006), studies from wild bird populations suggesting that the effects of maternal inbreeding will be greatest on early-life fitness traits (Reid et al., 2003; Richardson et al., 2004). Our analysis therefore further emphasises that, when circumstances permit, inbred females should not be used for breeding, even if the potential offspring will not themselves be inbred.
As hypothesised, fitness showed a significant increase with year of birth across populations (Table 2), perhaps because of the improving standards of husbandry within zoos and/or adaptation to captivity. However, fitness decreased significantly with year of birth in 23 populations (Table 3), a finding that may be a cause of concern for these particular captive breeding programmes.
Comparison of models
Firstly, we shall compare the performance of Ballou's and the alternative models. The results of the analysis made using the two models were in general agreement, although the alternative model detected fewer cross-population effects and detected significant regression terms in 25 instances additional to Ballou's model. The AIC values show that the alternative model provided the better fit for 59 of the 119 populations meaning that there was no significant difference between the models' fits (P=0.93, sign test). If the genetic architecture of inbreeding depression in zoo populations differs then the two models would be expected to perform differently on different pedigrees.
The MAMs detected significant inbreeding and ancestral inbreeding effects in more populations than the other two models (Table 3). However, 14 of the populations in which Ballou's or the alternative model had detected significant effects of inbreeding, ancestral inbreeding, maternal inbreeding or year of birth were found to be fitted best by a null model. It is likely that the discarding of correlated and/or insignificant factors explains the model's improved fit to the data. Some of the differences in results may also stem from controlling for the effect of zoo. The variance in survival to 30 days between zoos was found to be significantly greater than the variance within zoos (P<0.05) in 15 of the 119 populations (Supplementary information). Additionally, changing the fitness measure from survival to 7 days to survival to 30 days may have resulted in the detection of terms that had previously not been found to be significant.
Discussion
Inbreeding depression
Our study shows that inbreeding depression is a common phenomenon in zoo populations. A significant trend of inbreeding depression across populations was detected, fitness decreasing with inbreeding in approximately 65% of the pedigrees. Other cross-species analyses of zoo populations have also detected significant trends of inbreeding depression (Ralls et al., 1979; Ralls and Ballou, 1983; Ralls et al., 1988) although in these studies a much higher proportion of populations (∼90%) were found to be affected by inbreeding. The lower incidence of inbreeding depression in the populations in our analysis might be because of the fact that they had experienced, on average, lower levels of inbreeding than the populations from the three aforementioned studies.
The cost of inbreeding at f=0.25 was found to vary considerably across populations and species (Figure 1). Inbreeding depression is often more severe under stressful conditions (e.g. Joron and Brakefield, 2003; Armbruster and Reed, 2005). It might therefore be more pronounced in populations of zoo animals in which neonates were particularly susceptible to environmental effects. Additionally, the effects of inbreeding depression will increase with the number of lethal equivalents in a population. Purging, either pre- or post-captivity, may reduce the number of lethal equivalents in a population and random founder effects may also dictate the numbers and the severity of the mutant alleles carried in zoo populations. Wild populations that have been isolated for many generations or have experienced severe population declines might be expected to have undergone purging and hence be less likely to experience inbreeding depression when brought into captivity, a possibility supported by the observation that threatened taxa have low heterozygosity compared with equivalent non-threatened taxa (Spielman et al., 2004). We tested whether island endemicity or IUCN threat rating were correlated with the detection of inbreeding depression across populations but found no relationships (data not shown). Nevertheless, the severity of inbreeding depression, δ, as calculated for the 27 populations in which significant inbreeding depression was detected, decreased significantly as threat rating increased (δ=0.533–0.062 (threat rating), R2=0.20, P=0.02), suggesting that threatened populations may have undergone purging before captivity. However, caution should be taken when interpreting this result. Threat rating was strongly correlated with a population's maximum inbreeding coefficient (R=0.61, P<0.001) and the two parameters explained equal amounts of the variance of the data (R2=0.20 and 0.20, respectively). It should also be remembered that populations of highly threatened taxa still expressed inbreeding depression, albeit less severely than in threatened taxa. In these populations, therefore, purging has not removed the entire genetic load and breeding programmes should still minimise inbreeding.
The detection of inbreeding depression will also depend on the parameters of the population that is analysed. The levels of inbreeding in a population that has only been in captivity for a couple of generations may not be high enough for the effects of inbreeding depression to be detected. A population that has been in captivity for many generations may have purged its genetic load resulting in fitness initially decreasing but then increasing with inbreeding coefficient. Such a relationship might mask the effects of inbreeding depression if they were exhibited only in the earlier part of the pedigree, although simulations did not suggest this to be a problem in typical zoo pedigrees (Boakes and Wang, 2005; but see Kalinowski and Hedrick, 2001). We investigated whether the detection of inbreeding depression was affected by the number of founders, the size of the population used in the analysis (N), the current and mean effective population sizes, the mean number of generations, the mean, variance and maximum values of the inbreeding and ancestral inbreeding coefficients and mean survival to 7 and 30 days. The detection of inbreeding depression increased with N and decreased with a population's maximum ancestral inbreeding coefficient (Table 6). The finding that the detection of inbreeding depression increases with population size is not unexpected as it shows the model was more likely to detect inbreeding depression in populations in which it had more power (Boakes and Wang, 2005).
Ancestral inbreeding effects
Following the results of Ballou (1997) and Boakes and Wang (2005), we expected the detectable effects of purging within populations to be very weak. Indeed, little evidence of purging is apparent within individual populations although a trend of purging was observed across populations. The mitigating effects of purging were found, on average, to be small (median<1% decrease in inbreeding depression) and hence can be considered, overall, as having a negligible benefit. However, inbreeding depression was appreciably reduced by purging (>20% reduction) in 23 populations. The variation in purging between populations was not unexpected given previous studies (e.g. Byers and Waller, 1999; Fowler and Whitlock, 1999) and might be explained by species' different life table traits and by differences in the demographic pre-capture history and genetic structure of populations.
Expectations of the occurrence of purging in zoo populations
There are several reasons that may explain the variation in purging effects detected in zoo populations. First, purging may have already occurred in founder populations, prior to their being taken into captivity, particularly in populations that were historically small or had experienced a sudden and dramatic decline in size. However, wild populations that have been through a genetic bottleneck can still experience inbreeding depression (e.g. Packer et al., 1991; Laikre et al., 1997) and hence, presumably, purging. How common this is is unclear and some of the effect may be due to stress caused by a shift to the zoo environment. Moreover, in our study, the detection of ancestral inbreeding effects as measured by z-values was not found to correlate with island endemicity or IUCN threat rating although the statistical weakness of the ancestral inbreeding regression models might preclude the detection of such relationships were they to exist.
Secondly, the strength of selection under zoo conditions may vary, hence leading to variation in the extent of purging. For example, conditions that would be lethal in the wild, for example albinism, cataracts and epilepsy, may be far less detrimental to captive individuals. Veterinary care will reduce selection on individuals' immune responses and there will be little opportunity for mate-competition. In this light, it is interesting that wild data sets reveal much stronger evidence of inbreeding depression (Crnokrak and Roff, 1999).
Thirdly, ancestral inbreeding will only purge inbreeding depression due to partially recessive alleles, being ineffective for loci exhibiting overdominance (Ohta, 1971; Rumball et al., 1994). Thus, the level of purging possible will depend on the relative contribution of these two mechanisms to inbreeding depression. Deleterious recessive alleles have been claimed to be the major cause of inbreeding depression (e.g. Simmons and Crow, 1977; Lande and Schemske, 1985), and simulations suggest that associative overdominance plays only a minor role in maintaining inbreeding load in small randomly mating populations of mammals (Wang et al., 1999). Against this, there are a number of recent studies that suggest overdominance may be more widespread than previously suspected (e.g. Latter, 1998; Li et al., 2001; Swanson-Wagner et al., 2006). Clearly, further work is needed to resolve this debate.
Finally, purging will be less likely to occur if the rate of inbreeding is rapid and occurs over a small number of generations, as is the case in many of the zoo populations analysed. Additionally, purging will be less efficient if inbreeding depression is due to many mildly deleterious alleles, and if there are few lethal equivalents in the genome (Hedrick, 1994; Fu et al., 1998; Wang, 2000). However, as little is known about the genetic architecture of the inbreeding depression of zoo populations, we are not yet in a position to assess what impact this will have on the rate of purging in real populations.
Statistical performance of the models
Simulations have shown that, under conditions likely to be found in zoo populations, regression models lack the power to detect purging (Boakes and Wang, 2005). For a fixed level of inbreeding, the power of the models to detect purging increases with population size and number of generations. The small mean effective sizes of the zoo populations (median=22.6) and low mean number of generations (median=3.0) indicate that even if zoo populations were undergoing purging, its effects might often go undetected, a possibility supported by the increased rate of detection of purging in empirical data with increased population size and maximum f (Table 6). The idea that failure to detect purging is more to do with lack of power and less because it is not occurring is also given credence by the trend of purging detected across populations.
The potential presence of errors within some pedigrees may also prevent regression models from detecting purging effectively. Simulations showed that the power of the models to detect purging decreased with increasing pedigree error rate (unpublished data). The earlier in the pedigree that errors appear, the greater the likelihood of them having an effect on the outcome of an analysis. Without genetic marker analyses, it is impossible to measure the accuracy of the pedigrees used in this analysis. However, previous molecular studies have found errors in studbooks (e.g. Waldrapp ibis (Signer et al., 1994), Bali starling (Ashworth and Parkin, 1992) and Arabian oryx (Marshall et al., 1999)) meaning that it is probable that some of the pedigrees analysed here contained errors.
Ballou's and the alternative models both detected significant inbreeding depression and/or purging in 17 populations but differences in the results obtained from the models sometimes arose (Table 3). It is possible that the differences in the models' performances on the empirical data stem from differences in the genetic architecture of the zoo populations as simulations showed that Ballou's model performed more powerfully if mutant alleles were semilethal and the alternative model if alleles were mildly deleterious (Boakes and Wang, 2005). Population size will also impact on the probability of purging, particularly in founder-flush situations (Miller and Hedrick, 2001; Thevenon and Couvet, 2002). Another explanation for the differences between the models is that they provided a poor fit to the data. Significant effects within a data set may not be detected if terms within the model are correlated or over-parameterise the data. One solution to this problem is to find the MAM that describes the data by removing correlated and/or insignificant terms in a stepwise procedure.
Our analysis investigated the effects of inbreeding and purging on juvenile survival. Inbreeding studies often concentrate on neonatal survival (e.g. Sausman, 1984; Lacy et al., 1993; Zschokke and Baur, 2002), presumably because it is an easily obtained measure of an individual's fitness. However, it may not necessarily be the fitness measure at which inbreeding depression is most strongly expressed. Indeed, inbreeding depression may affect different fitness traits in different species. For example, studies on Peromyscus species have reported greater inbreeding depression in litter size than in juvenile survival or growth (Brewer et al., 1990; Keane, 1990; Ribble and Millar, 1992). In some populations, the models might therefore have had more power to detect inbreeding depression and purging had alternative fitness traits such as adult mortality, disease resistance or fecundity been investigated. The statistical power of the models will also be reduced by random variation in the survival data brought about by non-genetic mortality.
The concept of purging is at present topical among zoo managers (Christie and Walter, personal communication). Our analysis has shown that although, in general, purging does slightly reduce the genetic load of a zoo population, its effects are unpredictable and are rarely strong enough to eliminate inbreeding depression. Our results therefore indicate that a strategy of deliberate inbreeding would be inadvisable as, in the vast majority of cases, it would not remove inbreeding depression from zoo populations. We might additionally question whether purging in zoo populations is in fact desirable. Lacy (2000) warns that all individuals carry the equivalent of at least one lethal allele (Morton et al., 1956; Ralls et al., 1988) and that a rigorous programme of selection would require culling the entire population! He also cautions that it should not be presumed that an allele which is deleterious in captivity will necessarily be deleterious in the wild and vice versa, an observation that is particularly relevant for those captive populations that ultimately are to be reintroduced to the wild. We therefore believe that the results of our study further emphasise that breeding programmes must continue in their efforts to maximise genetic diversity and minimise inbreeding.
References
Adamec V, Cassell BG, Smith EP, Pearson RE (2006). Effects of inbreeding in the dam on dystocia and stillbirths in US Holsteins. J Dairy Sci 89: 307–314.
Armbruster P, Reed DH (2005). Inbreeding depression in benign and stressful environments. Heredity 95: 235–242.
Ashworth D, Parkin DT (1992). Captive breeding: can genetic fingerprinting help? In: Moore HDM, Holt WV, Mace GM (eds). Biotechnology and the Conservation of Genetic Diversity. Columbia University Press: New York. pp 135–149.
Ballou JD (1997). Ancestral inbreeding only minimally affects inbreeding depression in mammalian populations. J Heredity 88: 169–178.
Barrett SCH, Charlesworth D (1991). Effects of a change in the level of inbreeding on the genetic load. Nature 352: 522–524.
Bijlsma R, Bundgaard J, Boerema AC (2000). Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. J Evol Biol 13: 502–513.
Boakes EH, Wang J (2005). A simulation study on detecting purging of inbreeding depression in captive populations. Genet Res (Cambr) 86: 139–148.
Brewer BA, Lacy RC, Foster ML, Alaks G (1990). Inbreeding depression in insular and central populations of Peromyscus mice. J Hered 81: 257–266.
Byers DL, Waller DM (1999). Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annu Rev Ecol System 30: 479–513.
Crnokrak P, Barrett SCH (2002). Purging the genetic load: a review of the experimental evidence. Evolution 56: 2347–2358.
Crnokrak P, Roff DA (1999). Inbreeding depression in the wild. Heredity 83: 260–270.
Crow JF, Kimura M (1970). An Introduction to Population Genetics Theory. Harper and Row: New York.
Day SB, Bryant EH, Meffert LM (2003). The influence of variable rates of inbreeding on fitness, environmental responsiveness and evolutionary potential. Evolution 57: 1314–1324.
Fowler K, Whitlock MC (1999). The variance in inbreeding depression and the recovery of fitness in bottlenecked populations. Proc Roy Soc London B 266: 2061–2066.
Frankham R, Ballou JD, Briscoe DA (2002). Introduction to Conservation Genetics. University Press: Cambridge.
Frankham R, Gilligan DM, Morris D, Briscoe DA (2001). Inbreeding and extinction: effects of purging. Conserv Genet 2: 279–285.
Fu YB, Namkoong G, Carlson JE (1998). Comparison of breeding strategies for purging inbreeding depression via simulation. Conserv Biol 12: 856–864.
Gilligan DM, Briscoe DA, Frankham R (2003). Dynamics of genetic adaption to captivity. Conserv Genet 4: 189–197.
Hedrick P, Kalinowski ST (2000). Inbreeding depression in conservation biology. Annu Rev Ecol System 31: 139–162.
Hedrick PW (1994). Purging inbreeding depression and the probability of extinction: full-sib mating. Heredity 73: 363–372.
Ihaka R, Gentleman R (1996). R: A language for data analysis and graphics. J Comput Graph Stat 5: 299–314.
Joron M, Brakefield PM (2003). Captivity masks inbreeding effects on male mating success in butterflies. Nature 424: 191–194.
Kalinowski ST, Hedrick PW (2001). Inbreeding depression in captive bighorn sheep. Anim Conserv 4: 319–324.
Kalinowski ST, Hedrick PW, Miller PS (2000). Inbreeding depression in the Speke's gazelle captive breeding program. Conserv Biol 14: 1375–1384.
Keane B (1990). The effect of relatedness on reproductive success and mate choice in the white-footed mouse, Peromyscus leucopus. Anim Behav 39: 264–273.
Keller LF, Waller DM (2002). Inbreeding effects in wild populations. Trends Ecol Evol 17: 230–241.
King HD (1939). Life processes in gray Norway rats during fourteen years in captivity. Am Anat Mem 17: 1–77.
Lacy RC (2000). Should we select genetic alleles in our conservation breeding programs? Zoo Biol 19: 279–282.
Lacy RC, Ballou JD (1998). Effectiveness of selection in reducing the genetic load in populations of Peromyscus polionotus during generations of inbreeding. Evolution 42: 900–909.
Lacy RC, Petric A, Warneke M (1993). Inbreeding and outbreeding in captive populations of wild animal species. In: Thornhill NW (ed). The Natural History of Inbreeding and Outbreeding. University of Chicago Press: London. pp 352–374.
Laikre L, Ryman N, Lundh NG (1997). Estimated inbreeding in a small, wild muskox Ovibos moschatus population and its possible effects on population reproduction. Biol Conserv 79: 197–204.
Lande R, Schemske DW (1985). The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 39: 24–40.
Latter BDH (1998). Mutant alleles of small effect are primarily responsible for the loss of fitness with slow inbreeding in Drosophila melanogaster. Genetics 148: 1143–1158.
Li ZK, Luo LJ, Mei HW, Wang DL, Shu QY, Tabien R et al. (2001). Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. I. Biomass and grain yield. Genetics 158: 1737–1753.
Liptak T (1958). On the combination of independent tests. Magyar Tud Akad Mat Kutato Int Kozl 3: 171–197.
Marshall TC, Sunnucks P, Spalton JA, Greth A, Pemberton JM (1999). Use of genetic data for conservation management: the case of the Arabian oryx. Anim Conserv 2: 269–278.
Miller PS, Hedrick PW (2001). Purging of inbreeding depression and fitness decline in bottlenecked populations of Drosophila melanogaster. J Evol Biol 14: 595–601.
Morton NE, Crow JF, Muller HJ (1956). An estimate of the mutational damage in man from data on consanguineous marriages. Proc Natl Acad Sci USA 42: 855–863.
Mosteller F, Bush RR (1954). Selected quantitative techniques. In: Lindzey G (ed). Handbook of Social Psychology. Addison-Wesley: Cambridge, MA.
Ohta T (1971). Associative overdominance caused by linked detrimental mutations. Genet Res (Cambr) 18: 277–286.
Packer C, Pusey AE, Rowley H, Gilbert DA, Martenson J, O'Brien SJ (1991). Case study of a population bottleneck: Lions of the Ngorougoro Crater. Conserv Biol 5: 219–230.
Ralls K, Ballou JD (1983). Extinction: lessons from zoos. In: Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas L (eds). Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations. Benjamin Cummings: Menlo Park, CA. pp 164–183.
Ralls K, Ballou JD (1986). Captive breeding programs for populations with a small number of founders. Trends Ecol Evol 1: 19–22.
Ralls K, Ballou JD, Templeton AR (1988). Estimates of lethal equivalents and the cost of inbreeding in mammals. Conserv Biol 2: 185–193.
Ralls K, Brugger K, Ballou JD (1979). Inbreeding and juvenile mortality in small populations of ungulates. Science 206: 1101–1103.
Reed DH, Lowe EH, Briscoe DA, Frankham R (2003). Inbreeding and extinction: effects of rate of inbreeding. Conserv Genet 4: 405–410.
Reid JM, Arcese P, Keller LF (2003). Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc Roy Soc London Ser B 270: 2151–2157.
Ribble DO, Millar JS (1992). Inbreeding effects among inbred and outbred laboratory colonies of Peromyscus maniculatus. Can J Zool 70: 820–824.
Richardson DS, Komdeur J, Burke T (2004). Inbreeding in the Seychelles warbler: environment-dependent maternal effects. Evolution 58: 2037–2048.
Rumball W, Franklin IR, Frankham R, Sheldon BL (1994). Decline in heterozygosity under full-sibs and double first-cousin inbreeding in Drosophila melanogaster. Genetics 136: 1039–1049.
Saccheri IJ, Brakefield PM, Nichols RA (1996). Severe inbreeding depression and rapid fitness rebound in the butterfly Bicyclus anynana (Satyridae). Evolution 50: 2000–2013.
Sausman KA (1984). Survival of captive-born Ovis canadensis in North American zoos. Zoo Biol 3: 111–121.
Scobie P (1997). Single Population Animal Records Keeping System. International Species Information System (ISIS): St Paul, MN.
Signer EN, Schmidt CR, Jeffreys AJ (1994). DNA variability and parentage testing in captive Waldrapp ibises. Mol Ecol 3: 291–300.
Simmons MJ, Crow JF (1977). Mutations affecting fitness in Drosophila populations. Annu Rev Genet 11: 49–78.
Spielman D, Brook BW, Frankham R (2004). Most species are not driven to extinction before genetic factors impact them. Proc Natl Acad Sci USA 101: 15261–15264.
Swanson-Wagner RA, Jia Y, DeCook R, Borsuk LA, Nettleton D, Schnable PS (2006). All possible modes of gene action are observed in a global comparison of gene expression in a maize F1 hybrid and its inbred parents. Proc Natl Acad Sci USA 103: 6805–6810.
Swindell WR, Bouzat JL (2006). Ancestral inbreeding reduces the magnitude of inbreeding depression in Drosophila melanogaster. Evolution 60: 762–767.
Templeton AR, Hemmer H, Mace G, Seal US, Shields WM, Woodruff DS (1986). Local adaptation, coadaptation and population boundaries. Zoo Biol 5: 115–125.
Templeton AR, Read B (1983). The elimination of inbreeding depression in a captive herd of Speke's gazelle. In: Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas WL (eds). Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations. Benjamin Cummings: Menlo Park, CA. pp 241–261.
Templeton AR, Read B (1984). Factors eliminating inbreeding depression in a captive herd of Speke's Gazelle (Gazella spekei). Zoo Biol 3: 177–199.
Thevenon S, Couvet D (2002). The impact of inbreeding depression on population survival depending on demographic parameters. Anim Conserv 5: 53–60.
Venables WN, Ripley BD (1999). Modern Applied Statistics with S-Plus, 3rd edn. Springer: New York.
Wang J (2000). Effects of population structures and selection strategies on the purging of inbreeding depression due to deleterious mutations. Genet Res (Cambr) 76: 75–86.
Wang J, Hill WG, Charlesworth D, Charlesworth B (1999). Dynamics of inbreeding depression due to deleterious mutations in small populations: mutation parameters and inbreeding rate. Genet Res (Cambr) 74: 165–178.
Willis JH (1999). The role of genes of large effect on inbreeding depression in Mimulus guttatus. Evolution 53: 1678–1691.
Willis K, Wiese RJ (1997). Elimination of inbreeding depression from captive populations: Speke's gazelle revisited. Zoo Biol 16: 9–16.
Zschokke S, Baur B (2002). Inbreeding, outbreeding, infant growth, and size dimorphism in captive Indian rhinoceros. Can J Zool 80: 2014–2023.
Acknowledgements
We thank all of the studbook keepers, both past and present, who have contributed to the collation of the data sets, Laurie Bingaman Lackey, Sarah Christie, Olivia Walter and Nick Lindsay for their advice regarding the pedigree data and Georgina Mace and two anonymous referees for commenting on the manuscript. The work was supported by a BBSRC studentship (02/A1/G/08089) awarded to EHB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Heredity website (http://www.nature.com/hdy)
Rights and permissions
About this article
Cite this article
Boakes, E., Wang, J. & Amos, W. An investigation of inbreeding depression and purging in captive pedigreed populations. Heredity 98, 172–182 (2007). https://doi.org/10.1038/sj.hdy.6800923
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800923
Keywords
This article is cited by
-
Ex situ versus in situ Eurasian lynx populations: implications for successful breeding and genetic rescue
Conservation Genetics (2023)
-
Multiple life-stage inbreeding depression impacts demography and extinction risk in an extinct-in-the-wild species
Scientific Reports (2021)
-
Long-term exhaustion of the inbreeding load in Drosophila melanogaster
Heredity (2021)
-
Genetic purging in captive endangered ungulates with extremely low effective population sizes
Heredity (2021)
-
Offspring survival changes over generations of captive breeding
Nature Communications (2021)