Volume 29
-
No. 12 December 2022
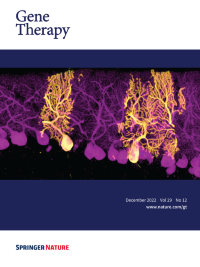
Cover Credit: Although recombinant adeno-associated viruses (rAAVs) are conquering their way as one of the most promising gene delivery systems, the lack of imaging technologies to efficiently monitor their tropism at a whole-organ level with single-cell resolution remains challenging. In this issue of Gene Therapy, a new pipeline for the biodistribution analysis of natural and new variants of AAVs by tissue clearing and light-sheet fluorescence microscopy is explored. The immunofluorescence representative image shows the preferential tropism of rAAV9 encoding EGFP for Purkinje cells.
-
No. 10-11 November 2022
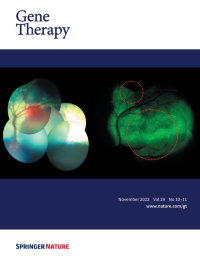
Cover Credit: Sheep fundus following subretinal injection of a modified AAV9 vector. Left: A composite image showing two subretinal blebs immediately after injection. Right: A composite image showing GFP expression 12 weeks after injection. The 7m8 modification of the GFP-carrying vector allowed radial spread of the vector, leading to coalescence of the two original blebs. Such spread has implications on the safety of the subretinal injection, as it potentially enables treating the central retina (or fovea) using a safer, more peripheral subretinal deposition of the vector.
-
No. 9 September 2022
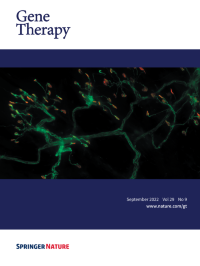
Cover Credit:Intravenous (IV) AAV-mediated gene replacement therapy is widely used for the treatment of children with the motor neuron disease Spinal Muscular Atrophy (SMA). While both IV and centrally-delivered routes of injection are being explored in clinical trials, the biodistribution of the AAV and subsequent effects on overall pathology were yet to be described in detail. The image shows diseased neuromuscular junctions (NMJs) in the transversus abdominis muscle from a mouse model of SMA, where neurons are stained in green and motor endplates are stained in red. While centrally-delivered AAV9-SMN provides better neuronal and NMJ protection than IV delivery, both treatments result in a robust rescue of survival, weight, and motor function. These results emphasized the independent contributions of peripheral organs to SMA pathology.
-
No. 7-8 August 2022
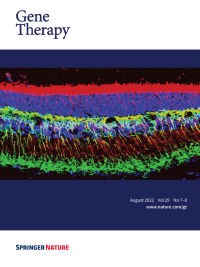
Cover Credit:AAV-mediated gene augmentation therapy holds great promise to rescue vision in retinal degenerative diseases. The image shows the retina of Rs1−/y transgenic rat model of X-linked retinoschisis rescued by retinoschisin (RS1) expressed from the AAV-RS1 vector. The retinal structures preserved are photoreceptor inner segments highlighted by RS1 (red band at the top) and synaptic contacts (yellow mesh) that are maintained between photoreceptor pre-synaptic output (RS1, red) and post-synaptic inputs to depolarizing bipolar cells (PCKa, green).
-
No. 6 June 2022
Cover Credit:Alfred Pasieka/Science Photo Library
-
No. 5 May 2022
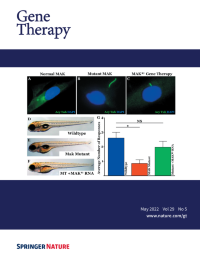
Cover Credit:Gene augmentation restores retinal MAK protein expression, rescues primary cilia length, and prevents vision loss. A-C: Immunocytochemical analysis of primary cilia length in dermal fibroblasts obtained from a normal individual (A) and a patient with MAK-associated retinitis pigmentosa (B-C). Transduction with a clinical grade gene transfer vector carrying retinal MAK rescues the ability of patient cells to regulate the length of their primary cilia (C). D-G: Generation and treatment of MAK morphant zebrafish with retinal MAK transcript restores visually guided startle responses (G). See paper by Budd A. Tucker, et al. in this issue of Gene Therapy.
-
No. 3-4 April 2022
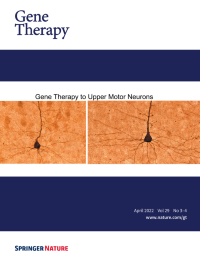
Cover Credit:Diseased upper motor neurons (UMNs) shrink in size and their apical dendrites display profound disintegration with numerous vacuoles. This phenomenon is observed both in UMNs of patients and UMNs of well-characterized disease models, regardless of species.
Upon UCHL1 gene therapy to diseased UMNs, they regain their neuronal stability, improve the integrity of their apical dendrite and soma.These results not only show that they respond to gene therapy, but also that directed gene therapy to UMNs would help restore UMN degeneration, especially in diseases that are characterized by UMN loss. See paper by Barış Genç et al. in this issue of Gene Therapy.
-
No. 1-2 February 2022
Cover Credit:Downregulation of Choline-Acetyltransferase in cholinergic interneurons by lentivirus mediated RNAi is linearly correlated with co-expression of a chemogenetic reporter.
RNA interference (RNAi) has important potential for gene-therapy application, such as downregulating neurotransmitter signaling in neuronal circuits. For use of such technologies in humans, it will be necessary to control and monitor such downregulation. In this issue of Gene Therapy,the paper by Lerchner et al. describes a method of potentially monitoring such downregulation in vivo. The image shows viral expression of RNAi and a chemogenetic reporter (green) and remaining expression of Choline Acetyltransferase (red) are inversely correlated in transduced striatal interneurons.