Abstract
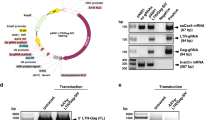
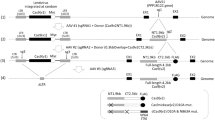
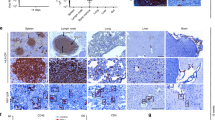
A CRISPR/Cas9 gene editing strategy has been remarkable in excising segments of integrated HIV-1 DNA sequences from the genome of latently infected human cell lines and by introducing InDel mutations, suppressing HIV-1 replication in patient-derived CD4+ T-cells, ex vivo. Here, we employed a short version of the Cas9 endonuclease, saCas9, together with a multiplex of guide RNAs (gRNAs) for targeting the viral DNA sequences within the 5′-LTR and the Gag gene for removing critically important segments of the viral DNA in transgenic mice and rats encompassing the HIV-1 genome. Tail-vein injection of transgenic mice with a recombinant Adeno-associated virus 9 (rAAV9) vector expressing saCas9 and the gRNAs, rAAV:saCas9/gRNA, resulted in the cleavage of integrated HIV-1 DNA and excision of a 978 bp DNA fragment spanning between the LTR and Gag gene in the spleen, liver, heart, lung and kidney as well as in the circulating lymphocytes. Retro-orbital inoculation of rAAV9:saCas9/gRNA in transgenic rats eliminated a targeted segment of viral DNA and substantially decreased the level of viral gene expression in circulating blood lymphocytes. The results from the proof-of-concept studies, for the first time, demonstrate the in vivo eradication of HIV-1 DNA by CRISPR/Cas9 on delivery by an rAAV9 vector in a range of cells and tissues that harbor integrated copies of viral DNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO . Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc 2015; 18: 20049.
Kulpa DA, Chomont N . HIV persistence in the setting of antiretroviral therapy: when, where and how does HIV hide? J Virus Erad 2015; 1: 59–66.
Coffin JM . HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 1995; 267: 483–489.
Coffin JM . HIV viral dynamics. AIDS 1996; 10: S75–S84.
Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR . CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 2007; 110: 4161–4164.
Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R . Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol 2005; 79: 141799–14188.
Lusic M, Giacca M . Regulation of HIV-1 latency by chromatin structure and nuclear architecture. J Mol Biol 2015; 427: 688–694.
Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA 2014; 111: 11461–11466.
Khalili K, Kaminski R, Gordon J, Cosentino L, Hu W . Genome editing strategies: potential tools for eradicating HIV-1/AIDS. J Neurovirol 2015; 21: 310–321.
Ebina H, Misawa N, Kanemura Y, Koyanagi Y . Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 2013; 3: 2510.
Liao HK, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun 2015; 6: 6413.
Zhu W, Lei R, Le Duff Y, Li J, Guo F, Wainberg MA et al. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 2015; 12: 22.
Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther 2012; 23: 377–381.
Boudreau RL, Rodriguez-Lebrón E, Davidson BL . RNAi medicine for the brain: progresses and challenges. Hum Mol Genet 2011; 20: R21–R27.
Naldini L . Gene therapy returns to centre stage. Nature 2015; 526: 351–360.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med 2012; 4: 120ra15.
Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A et al. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002; 13: 1391–1412.
Curreli S, Krishnan S, Reitz M, Lunardi-Iskandar Y, Lafferty MK, Garzino-Demo A et al. B cell lymphoma in HIV transgenic mice. Retrovirology 2013; 10: 92.
Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA 1992; 89: 1577–1581.
Santoro TJ, Bryant JL, Pellicoro J, Klotman ME, Kopp JB, Bruggeman LA et al. Growth failure and AIDS-like cachexia syndrome in HIV-1 transgenic mice. Virology 1994; 201: 147–151.
Barisoni L, Bruggeman LA, Mundel P, D'Agati VD, Klotman PE . HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 2000; 58: 173–181.
Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 1991; 185: 109–119.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015; 520: 186–191.
Doudna JA, Charpentier E . Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014; 346: 1258096.
Hsu PD, Lander ES, Zhang F . Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014; 157: 1262–1278.
White MK, Hu W, Khalili K . The CRISPR/Cas9 genome editing methodology as a weapon against human viruses. Discov Med 2015; 19: 255–262.
White MK, Khalili K . CRISPR/Cas9 and cancer targets: future possibilities and present challenges. Oncotarget 2016; 7: 12305–12317.
Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y et al. Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Sci Rep 2016; 6: 22555.
Behringer R . Manipulating the Mouse Embryo: a Laboratory Manual, 4th edn. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2014.
Acknowledgements
We wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for sharing the ideas and reagents. We thank Fang Li and Ting Zhang for technical assistance in cloning of the vector. Preparation of the rAAV9 vector was done by the Penn Vector Core, Gene Therapy Program at the University of Pennsylvania. This work was supported by: P30MH092177 (KK), R01MH106392 (RB), R01MH092371 (KK), R01DA013137 (RB), and R01NS087971 (KK, WH), R01HD043680 (RB), P01DA037830 (KK).
Author contributions
KK, JG, WH, RB conceived experiments; RK, RB, CY, JO, RB, HL performed experiments; KK, JG, WH, HEG, PF, RB analyzed data and KK, RK, JG, RB helped in preparing manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaminski, R., Bella, R., Yin, C. et al. Excision of HIV-1 DNA by gene editing: a proof-of-concept in vivo study. Gene Ther 23, 690–695 (2016). https://doi.org/10.1038/gt.2016.41
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.41
This article is cited by
-
HIV-1 mRNA knockdown with CRISPR/CAS9 enhances neurocognitive function
Journal of NeuroVirology (2024)
-
Preclinical safety and biodistribution of CRISPR targeting SIV in non-human primates
Gene Therapy (2023)
-
Optimal control of an HIV infection model with logistic growth, celluar and homural immune response, cure rate and cell-to-cell spread
Boundary Value Problems (2022)
-
Defective HIV-1 genomes and their potential impact on HIV pathogenesis
Retrovirology (2022)
-
CRISPR/Cas9: a tool to eradicate HIV-1
AIDS Research and Therapy (2022)