Key Points
- Swallowing is organized by a network of swallowing-related neurons that form the central swallowing pattern generator (SPG) located in the medulla oblongata.
- Microelectrode recordings have shown that the swallowing network includes two main groups of interneurons: (1) a dorsal swallowing group (DSG) in the nucleus tractus solitarii (NTS) of the dorsomedial medulla; and (2) a ventral swallowing group (VSG), located in the ventrolateral medulla.
- The DSG contains the generator neurons involved in triggering, shaping, and timing the sequential or rhythmic swallowing pattern, and the VSG contains switching neurons that distribute the swallowing drive to the various pools of motoneurons involved in the motor activity.
- The location of the DSG within the NTS, which is the primary sensory relay, is convenient for peripheral input to shape the output of the network so that the swallowing movements correspond to the swallowed bolus.
- The sequential firing of the swallowing neurons depends on the neuronal circuitry, as well as on the cellular properties of neurons. Within the network, inhibitory connections are thought to play a crucial role in the sequential firing of the neurons.
- The VSG forms premotor neurons for oral and pharyngeal motor neurons, but esophageal premotor neurons may be located within the DSG. Both the DSG and VSG receive cortical input that regulates their output.
- There is considerable plasticity in the activity of the swallowing network
Introduction
Swallowing is a complex fundamental motor activity that subserves, in mammals, an alimentary function and the protection of the upper respiratory tract. It constitutes one of the most elaborate motor functions, even in humans, because it requires the coordination of an extraordinary bilateral sequence of activation and inhibition of more than 25 pairs of muscles in the mouth, pharynx, and larynx, plus the esophagus.1, 2, 3, 4 A striking characteristic of swallowing is that the whole motor sequence can be readily initiated by stimulating a nerve, namely the internal branch of the superior laryngeal nerve (SLN).5, 6, 7 Interestingly, long-lasting repetitive stimulation of the SLN can elicit a pattern of rhythmic motor activities of swallowing.3, 6, 8
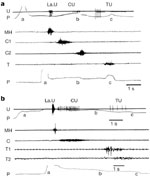
Neurophysiologic studies, performed in animals, have generally considered swallowing as having two phases: an oropharyngeal phase that constitutes an irreversible motor event, followed by an esophageal phase, corresponding to the primary peristalsis of the esophagus.1, 2, 4, 9, 10 In mammals, all muscles involved in the oropharyngeal stage are striated and therefore are driven by several pools of motoneurons located mainly in various cranial motor nuclei in the brainstem.1, 3, 4 In some species, the esophageal muscle is entirely composed of striated fibers and is therefore also controlled by cranial motoneurons (Figure 1a). In other species, however, for example, cats, opossums, and primates, a variable portion of the lower esophagus is composed of smooth muscle fibers, controlled by central preganglionic neurons and peripheral neurons of the enteric nervous system (Figure 1b).2, 10, 11, 12
Figure 1: Swallowing motor pattern and sequential activity of vagal motor fibers in species with striated (a, sheep) or striated and smooth muscle (b, baboon) esophagus.
a,b: Motor or preganglionic fibers (U) and electromyographic (EMG) recordings were obtained from different animals. U: Records of the discharge of different vagal fibers (nerve suture technique) that originally supplied the larynx (LaU), the cervical (CU), and the thoracic esophagus (TU) P: Pressure variations recorded by an intraluminal balloon propelled during swallowing (a, balloon distention; b,c, balloon entering the thoracic esophagus or the stomach, respectively). MH, C, C1, C2, T, T1, T2: EMG activity recorded, during deglutition, in mylohyoideus muscle and at different levels of the cervical or thoracic esophagus. (Source: Adapted from Roman123, Roman and Gonella 10 and Roman and Tieffenbach.53, with permission)
It is now clearly established, as originally postulated in the pioneer work by Meltzer,13 that the sequential and rhythmic patterns of swallowing are formed and organized by a central pattern generator (CPG). The CPG was previously described as a swallowing center, which can be subdivided into three systems: an afferent system corresponding to the central and peripheral inputs to the center; an efferent system corresponding to the outputs from the center, consisting of the various motoneuron pools involved in swallowing; and an organizing system, located in the medulla oblongata, corresponding to the interneuronal network that programs the motor pattern.1, 14
The present review focuses on the brainstem CPG, dealing mainly with the functional studies of the various pools of neurons, that is, motoneurons and interneurons, involved in the motor activity. It is noteworthy, however, that swallowing has received less attention than other fundamental motor activities such as locomotion, mastication, or respiration.15 This is probably owing to the complexity of the motor pattern, along with the great number of muscles and cranial nerves involved, which renders neurophysiologic studies difficult.
Activity and Localization of Brainstem Swallowing Neurons
It was on the basis of microelectrode recordings that the swallowing-related neurons were identified, and the structures where they are located were mainly established, providing a general picture of the organization and functional principles of the swallowing CPG (Figure 2).6, 16, 17, 18, 19, 20, 21 In addition, lesion experiments, electrical brain stimulations, in situ microinjections of putative transmitters, anatomic tract tracing techniques, and more recently identification of c-fos expression in neurons have generally yielded fairly concordant results as regards the swallowing CPG.1, 21, 22, 23, 24, 25, 26, 27, 28, 29
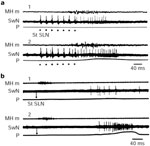
Figure 2: Brainstem swallowing sites and activity of swallowing neurons.
a: Drawings of coronal hemisections of the brainstem, at the level of the intermediate subpostremal part of the nucleus tractus solitarii (NTS), in rat and sheep. Dots indicate the localization of active points, stimulation of which induces swallowing, of swallowing neurons in rat or sheep (microelectrode recordings), and of glutamate injection sites inducing deglutition. b: Diagram showing the typical swallowing sequence of different medullary NTS interneurons [vertical lines, superior laryngeal nerve (SLN) stimulation; black rectangle, mylohyoideus contraction (MH m); gray and white rectangles, discharge of oropharyngeal or esophageal neurons]. c: Oropharyngeal and esophageal neurons (SwN) recorded before (1) and after (2) motor paralysis (gallamine 2 mg/kg). Note that the neuronal discharge remained unaltered after motor paralysis. (Source: Adapted from Jean.6, 109 Jean et al. 110, Kessler and Jean 21, Kessler et al. 27. with permission )
Electrophysiologic data have been obtained on several species, such as the cat, dog, monkey, rat, and sheep,6, 21, 30, 31, 32, 33, 34 but, to date, the most extensive studies have been carried out on anesthetized sheep.6, 16, 18, 19, 20, 35, 36, 37, 38, 39 Swallowing-related neurons, also referred to as swallowing neurons, are either (1) normally silent phasic neurons, which present a burst of spikes, called "swallowing activity,"6 occurring in a constant temporal relationship with the swallowing motor activity; or (2) spontaneously active neurons, which exhibit a transient increase in their discharge frequency, or a phasic inhibition of their spontaneous discharge during swallowing.6, 21, 34 Neurons have been classified into two main categories: oropharyngeal neurons, which fire before or during the oropharyngeal phase of swallowing, and esophageal neurons, which discharge during the esophageal peristalsis (Figure 2b).3, 6 In addition, brainstem swallowing neurons can be subdivided into motoneurons or preganglionic neurons, which provide innervation to the striated muscles or the esophageal smooth muscle, respectively, and interneurons forming the organizing system of the network that generates the sequential or rhythmic pattern of swallowing.
Motoneurons and Preganglionic Neurons
As revealed by neuroanatomic studies, swallowing motoneurons and preganglionic neurons are localized within the trigeminal (V), facial (VII), and hypoglossal (XII) motor nuclei, the nucleus ambiguus (IX, X) and the dorsal motor nucleus of the vagus (X), and at the cervical spinal level between C1 and C3.1, 3, 4, 14 It is noteworthy, however, that the main motor nuclei involved in the motor activity are the XII motor nucleus and the nucleus ambiguus. Indeed, most, if not all, of the motoneurons within these nuclei participate in swallowing.1, 4 Data have shown the existence of a myotopic pattern of organization within each motor nucleus,40 in particular within the nucleus ambiguus with the well-known rostrocaudal pattern of organization of motoneurons innervating the esophagus, pharynx, and larynx.41, 42, 43 As regards the innervation of the smooth muscle esophagus, the majority of the preganglionic neurons are located within the X dorsal motor nucleus (DMX). They consist of two separate groups, one located in the rostral part of the DMX and the other in its caudal portion, providing, respectively, excitatory and inhibitory inputs to the esophageal smooth muscle and lower esophageal sphincter.44, 45
Extracellular recordings have shown that within V and XII motor nuclei and the nucleus ambiguus,6, 19, 21, 35, 36, 46, 47 the oropharyngeal motoneurons exhibit a short burst (in the 50- to 200-ms range) of low frequency (40–50 Hz) spikes during swallowing. Depending on the neuron, the bursting discharge can precede the beginning of swallowing by a few milliseconds or be delayed from the onset of the sequence, with an important overlap in the firing of motoneurons involved in the leading complex, which invariably initiates the act of swallowing.48 Intracellular recordings on hypoglossal and nucleus ambiguus motoneurons49, 50 have shown that, during swallowing, the firing of motoneurons was superimposed on a bell-shaped membrane depolarization. In some cases, however, complex depolarizing-hyperpolarizing or hyperpolarizing-depolarizing waves of membrane potential are evoked in motoneurons during swallowing. This indicates that in addition to an excitatory drive, these motoneurons may also receive inhibitory inputs or have complex intrinsic properties that are activated by the swallowing drive.
The firing behavior of esophageal motoneurons is somewhat different.19, 50, 51 The bursting discharge has a longer duration (up to 800 ms) and a very low frequency (10–20 Hz), and a variable phase lag depending on the portion of the esophagus innervated. No central recordings have been performed so far on the activity of the preganglionic vagal neurons that innervate the smooth muscle esophagus. The data, obtained in cross-innervation experiments or by directly recording the activity of vagal fibers,52, 53 show that these neurons are activated sequentially during swallowing. The burst firing of the preganglionic neurons has a long duration (in the 1-second range) and a very low frequency (3–8 Hz) (Figure 1b).53 Recordings of the vagal efferent fibers innervating the opossum esophagus and the dog lower esophageal sphincter, both of which are composed of smooth muscles, indicate that both the excitatory and inhibitory vagal pathways are involved in swallowing.52, 54
Therefore, electrophysiologic studies show that (1) the later the neuron becomes active during swallowing, the longer it will fire and the lower its discharge frequency will be; and (2) a central drive does exist for the smooth muscle esophagus.
Interneurons
Extensive microelectrode recordings, first performed on sheep6, 17, 20, 35, 36, 37, 46 and subsequently on other species such as rat and cat,21, 33, 34, 55, 56, 57 have shown that the swallowing neurons are located in two main brainstem areas: (1) in the dorsal medulla within the nucleus tractus solitarii (NTS) and in the adjacent reticular formation, where they form the dorsal swallowing group (DSG); and (2) in the ventrolateral medulla, just above the nucleus ambiguus, where they form the ventral swallowing group (VSG).
Oropharyngeal Interneurons
Within the NTS, oropharyngeal neurons6, 14 exhibit a typical sequential firing pattern that parallels the sequential or the rhythmic motor pattern of deglutition (Figure 2b). Because these neurons are still active during fictive swallowing elicited in paralyzed animals, their bursting discharge cannot be attributed to peripheral afferent inputs generated by the muscular contraction, and it actually corresponds to a central swallowing activity (Figure 2c).6, 21, 57 Therefore, NTS oropharyngeal neurons are premotor neurons of the network that generates swallowing.
The firing pattern of these neurons is characterized by a short burst of spikes (100–300 ms) with a high frequency, in the range of 100 Hz, and an instantaneous frequency that can be as high as 400 Hz (Figure 3a).6, 21 Considerable overlaps occur between the sequential burst firing of the various neurons. Intracellular studies on cats have shown that the bursting activity of oropharyngeal NTS neurons is superimposed on a high-amplitude depolarizing wave, between 15 and 20 mV, which indicates that a strong central drive is exerted during swallowing.58
Figure 3: Neuronal swallowing patterns.
a: Activity of an oropharyngeal neuron (SwN) recorded within the sheep NTS during swallowing. Single-pulse stimulation to the ipsilateral SLN elicited an initial synaptic response in the form of one short latency spike, followed by the swallowing burst firing when swallowing occurred (mylohyoideus muscle EMG: MH m). b: Neuronal firing (SwN) elicited in an oropharyngeal neuron with preswallowing discharge, in response to stimulation of the ipsilateral SLN (recorded from the sheep NTS). Note that the neuron exhibited only the preswallowing discharge when no swallowing was elicited. (Source: Adapted from Ciampini and Jean37 and Jean.6,with permission)
Interestingly, some oropharyngeal neurons exhibit a particular pattern of firing, starting long before the onset of the motor sequence. This continuous discharge, called "preswallowing activity," decreases and stops quite rapidly when no swallowing occurs, but continues and increases, turning into a bursting swallowing activity, when swallowing is initiated (Figure 3b). This pattern of discharge suggests that these neurons are involved in the initiation of swallowing, and it has been postulated that they may constitute the trigger neurons in deglutition.6, 37
In the ventrolateral medulla above the nucleus ambiguus, there is also a large population of oropharyngeal swallowing neurons, forming the VSG.6, 14, 21, 30, 34, 55, 59, 60 These neurons have been identified as interneurons, on the basis of criteria such as the lack of antidromic activation by vagus nerve stimulation, a high discharge frequency, and a bursting discharge that starts before most of the motoneurons in the nucleus ambiguus become active. The burst firing behavior of the VSG neurons is very similar to that of the DSG neurons both in terms of sequential firing pattern and of discharge duration and frequency.6, 21 The VSG neurons are still active during fictive swallowing and, like the DSG neurons, they belong to the neuronal network that generates swallowing.
As regards the location of DSG neurons, most of the relevant data were obtained before the cytoarchitectonic subdivisions of the NTS were established on the basis of anatomic studies.61, 62, 63, 64 Therefore, their location has not been specified in relation to these subdivisions. However, in all the species studied, it has emerged that the oropharyngeal neurons are situated rostrocaudally at the level of the intermediate subpostremal portion of the NTS,62, 63 within the medial part of the lateral NTS, which overlaps the interstitial, intermediate, ventral, and to some extent, the ventrolateral subdivisions of the nucleus.3, 6, 21 Interestingly, anatomic results have shown that both laryngeal and pharyngeal afferent fibers project mainly to the interstitial and intermediate subdivisions of the NTS in all the species studied.61, 65 Moreover, recent experiments, using c-fos immunohistochemistry, also showed that these two subnuclei are mainly concerned with the oropharyngeal phase of swallowing (Table 1).28, 29 It is noteworthy, however, that some neuroanatomic studies failed to clearly identify VSG neurons.22, 23, 66
Esophageal Interneurons
Microelectrode recordings have shown that, within the dorsal medulla, there are also premotor neurons that fire during the esophageal phase of swallowing. Depending on the esophageal neuron, the swallowing discharge occurs with a variable phase lag after the onset of the motor activity.19, 56, 67 The duration of the bursts of spikes is long (200 ms to 1 second) and the firing frequency is low, because it does not exceed 40 Hz and may be as low as 10 to 20 Hz. Therefore, as regards the interneuronal activity, one can observe an increase in the duration of the discharge, and a decrease in the firing frequency when the neuron is active later during swallowing (Figure 4). To date, no central recordings have been performed on esophageal interneurons in species with a smooth muscle esophagus.
Figure 4: Diagram showing the opposite gradients in the firing frequency and the burst duration of the different types of swallowing neurons.
Ins, interneurons; Mns, motoneurons; Pgglns, preganglionic neurons.
The existence of a large population of esophageal interneurons in the ventrolateral medulla is less clear. Bursting discharges in phase with esophageal peristalsis have been recorded in the medullary region above the nucleus ambiguus.19 However, in the case of esophageal neurons, applying stimulation to the vagus nerve systematically induces an antidromic field potential. Without any intracellular evidence, it is therefore not possible to clearly distinguish between motoneurons and actual interneurons.3, 19
The DSG esophageal neurons are also situated at the level of the intermediate subpostremal part of the NTS, between the tractus solitarius and the DMX,6 a region that probably corresponds to the centralis subdivision of the NTS in the rat.56, 61 It is noteworthy that anatomic studies have shown that the esophageal afferent fibers end within this specific NTS subdivision,1 and that the subnucleus centralis is the main NTS subnucleus concerned with the esophageal phase of swallowing.28, 29
Connections and Function of Brainstem Groups of Swallowing Neurons
Although the detailed connections between functionally identified neurons within the CPG still remain to be mapped, results of electrophysiologic and anatomic experiments have provided some information about the connections between the various groups of swallowing neurons.16, 17, 18, 68
Oropharyngeal Circuitry
In addition to the swallowing burst, the oropharyngeal neurons can exhibit, by stimulating the afferent fibers in the ipsilateral SLN, a short-latency synaptic response, in the form of a single spike (Figure 3). The synaptic response of the DSG neurons may occur with a very short, stable latency of 1 to 2 ms, indicating that at least some of these neurons are monosynaptically connected to afferent fibers.6, 21, 32, 57, 69 As regards VSG neurons and motoneurons, several pulses are generally required to initiate the synaptic spike, the latency of which is visibly longer (7–12 ms) and variable, suggesting the existence of a polysynaptic pathway.6, 21, 35, 36, 46, 47 Interestingly, a synaptic response can also be initiated in oropharyngeal neurons by stimulating a specific cortical area, which induces swallowing, with a latency shorter in the DSG (5–8 ms) than in the VSG neurons (10–16 ms) (20). These results suggest that the neurons of the VSG are probably activated via neurons of the DSG. Indeed, regardless of the stimulated afferent pathway, the initial response of the VSG neurons is abolished after lesion of the DSG.14, 20 Although no direct evidence is available, at the single cell level, that a connection of this kind exists between the DSG and VSG, several anatomic experiments have shown connections between the NTS region and the ventrolateral reticular formation surrounding the nucleus ambiguus, where swallowing neurons are located.70, 71, 72, 73
Electrophysiologic experiments have shown that only oropharyngeal neurons within the brainstem VSG could be antidromically activated by stimulating the swallowing region of V or XII motor nuclei where swallowing motoneurons are located, whereas none of the neurons within the DSG exhibited any antidromic activation under the same conditions.16, 17 In addition, other studies have established that, within the VSG, the same identified oropharyngeal neuron can project to more than one motor nucleus, that is, to V and XII motor nuclei, to the XII motor nucleus and the nucleus ambiguus, or to V and XII motor nuclei and to the nucleus ambiguus,18, 55 which are all motor nuclei involved in swallowing. These electrophysiologic data fit in well with those coming from anatomic studies, showing that the ventrolateral medullary region, which contains oropharyngeal neurons, is connected to the homologous contralateral medullary region and to V, VII, X, and XII motor nuclei, all of which are also involved in swallowing.68, 74, 75, 76, 77 Other anatomic studies indicate that a direct connection may exist between swallowing regions in the NTS, such as the interstitial and the intermediate subnuclei, and motoneurons in the nucleus ambiguus, at least in the rat.22, 23, 66, 78 It is puzzling, however, that the oropharyngeal population of interneurons within the ventrolateral medulla, that is, the VSG, was not determined in these anatomic studies, because all the results available to date on functionally identified swallowing neurons have shown that motoneurons are driven by oropharyngeal neurons within the VSG.
In any case, the electrophysiologic data suggest the existence, within the swallowing network, of a circuit linking together the afferent fibers, the oropharyngeal neurons in the DSG, the VSG, and the motor nuclei (Figure 5). This kind of trisynaptic circuit is probably a basic element in the functioning of the CPG, although more complex connections do exist. Interestingly, it has been shown that the excitatory amino acids and their receptors are strongly involved at each synapse of this circuit, that is, between the afferent fibers and DSG neurons and the DSG and VSG neurons.25, 27, 79, 80, 81, 82, 83, 84 Nitrergic transmission also has been shown to facilitate this circuitry.85
Figure 5: Diagram of the oropharyngeal and esophageal circuits.
The oropharyngeal circuit includes two main groups of brainstem neurons: a dorsal swallowing group (DSG) located within the NTS, and a ventral swallowing group (VSG) located in the ventrolateral medulla. The DSG contains the generator neurons involved in triggering, shaping, and timing the sequential or rhythmic swallowing pattern. The VSG contains the switching neurons, which distribute the swallowing drive to the various pools of motoneurons involved in swallowing. The esophageal circuit may involve a DSG, a VSG, and the motor or preganglionic nuclei. However, the circuit may be simpler, with a direct connection between the DSG and motor nuclei. Both circuits may receive information from the periphery, from the cerebral cortex, and from various supramedullary structures. DMX, dorsal motor nucleus of the vagus, NA, nucleus ambiguus.
Esophageal Circuitry
A synaptic response can also be elicited in esophageal neurons by stimulating the afferent fibers in the SLN or the vagus nerve, depending on the linking of the neuronal firing with the contraction of the upper or lower esophagus, respectively. The latency of the synaptic response is variable, and clearly longer for esophageal neurons in the ventrolateral medulla (interneurons and/or motoneurons) than for those of the DSG. These data suggest the existence of a simple esophageal circuit linking together the afferent fibers, the neurons in the DSG, and the motor nuclei (nucleus ambiguus or DMX), directly or via VSG neurons (Figure 5).6, 19, 50
Neuroanatomic studies have shown that NTS neurons, which are supposed to be esophageal neurons because they are located in the subnucleus centralis where esophageal afferent fibers project, send axon terminals in the rostral compact formation of the nucleus ambiguus where esophageal motoneurons are situated. A connection between the NTS subnucleus centralis and the DMX has also been shown to exist.66, 86 Therefore, a direct connection between NTS esophageal neurons and esophageal motoneurons may exist. It has been shown that glutamatergic and somatostatinergic transmission may be involved in the connection between NTS subnucleus centralis and the rostral compact formation of the nucleus ambiguus.25, 87, 88, 89, 90, 91, 92 In addition, the NTS subnucleus centralis contains nitrinergic and catecholaminergic neurons, which may be involved in the control of preganglionic vagal neurons.89, 93, 94, 95, 96, 97
Connections Between Oropharyngeal and Esophageal Circuits
During swallowing, the oropharyngeal and esophageal circuits are functionally linked in order to shape the entire motor sequence. There is no direct evidence available so far about connections between identified swallowing neurons belonging to the oropharyngeal and esophageal circuits. However, anatomic data, obtained with tract tracing techniques, show that such connections may exist between neurons located in the interstitial and centralis subnuclei of the NTS, where oropharyngeal and esophageal DSG neurons are located, respectively.66 Interestingly, pharmacologic experiments suggest that 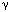 -aminobutyric acid (GABA) ergic and cholinergic transmission may be involved in the coupling of oropharyngeal and esophageal phases of swallowing.87, 98, 99, 100, 101, 102
-aminobutyric acid (GABA) ergic and cholinergic transmission may be involved in the coupling of oropharyngeal and esophageal phases of swallowing.87, 98, 99, 100, 101, 102
Synchronization of the Two Swallowing Hemi–Central Pattern Generators
It is noteworthy that the swallowing CPG consists of two hemi-CPGs, each located on one side of the medulla,1, 3, 26, 103 which have to be tightly synchronized to organize the coordinated contraction of the bilateral muscles of the oropharyngeal region and of the esophagus. Microelectrode recordings have shown that in each case, a particular swallowing neuron produces a swallowing bursting discharge in response to stimulation of the ipsilateral as well as to that of the contralateral afferent fibers, regardless of the type of swallowing neuron tested. This indicates that at each step of the network operation, the entire population of neurons within the DSG, the VSG, or the motoneuronal pools is active.
However, the mechanisms underlying the synchronization of the two hemi-CPGs are not known, and this matter has not been well documented yet. It is likely that the peripheral afferent fibers do not play a crucial role, because lesion experiments have shown that splitting the medulla caudal to the obex, which interrupts the vagal afferent fibers crossing the midline through the solitary tract, does not affect swallowing.1, 103 In addition, the short latency synaptic response of neurons, within either the DSG or the VSG, is induced only by stimulating the ipsilateral SLN. Therefore, the swallowing neurons receive a direct input only via the ipsilateral afferent fibers.
There are probably connections between central neurons, situated on each side of the brainstem, that play a key role in the coordination of the two hemi-CPGs. Anatomic connections mediated by fibers crossing the midline have been found to exist between the two medullary regions where swallowing neurons are located, that is, the DSG and the VSG.68, 104, 105 Electrophysiologic and neuroanatomic data suggest that swallowing interneurons, identified within motor nuclei or in their close vicinity, might be involved in the bilateral coordination of the motoneuronal pools.16, 17, 21, 35, 36, 68, 106, 107 The neuronal population within the NTS seems also to play an important role in the synchronization processes. The results of unilateral lesion experiments performed on the NTS esophageal neuronal population have shown that upon stimulating the ipsilateral SLN, only an oropharyngeal stage of swallowing is elicited, whereas upon stimulating the contralateral nerve, a complete process of deglutition including the esophageal stage is initiated.26 These results suggest, in addition, that under ipsilateral stimulation conditions, the swallowing motor sequence is mainly generated in the ipsilateral hemi-CPG and that this CPG transfers the swallowing premotor signal to the contralateral CPG.6, 26 Further experiments are required to elucidate the mechanisms underlying the synchronization of the two hemi-CPGs. The lesion and electrophysiologic data available indicate, however, that swallowing NTS neurons play a crucial role in these synchronization processes.
Function of the Various Interneuronal Groups in the Central Pattern Generator
It has been established in several networks involved in fundamental motor behavior that, within a given CPG, all the neurons are not equal because some of them play a preeminent role.108 As regards swallowing, data already obtained suggest that neurons in the DSG are likely candidates to act as generator neurons in the initiation and organization of the sequential or rhythmic motor pattern.14, 109, 110 The swallowing network in mammals, therefore, provides a unique example of neurons located within a primary sensory relay, that is, the NTS, which nevertheless play the role of generator neurons. Several lines of evidence support the "major" role of NTS neurons in swallowing. The NTS neurons exhibit a sequential or rhythmic firing pattern that parallels the motor pattern.6, 21 As this firing remains unaltered after complete motor paralysis, it is clear that it is an actual premotor activity centrally generated. Moreover, most, if not all, of the neurons having preswallowing activity are located within the NTS.6, 21, 57 In addition, it has been shown, by electrophysiologic exploration of the brainstem with concentric bipolar electrodes and pharmacologic experiments using fine microinjections of excitatory amino acids, that the active points that trigger deglutition are situated only in the region of the solitary complex (Figure 2a, b).7, 21, 24, 25, 27, 80 Furthermore, electrolytic lesion of the NTS results in the abolition, not only of the swallowing elicited by SLN stimulation, but also of that elicited by stimulating the swallowing cortical area.20 Lastly, fine lesions performed on sheep in the NTS region, which contains esophageal motility-controlling neurons, abolished the esophageal phase of swallowing without affecting the oropharyngeal phase, which indicates that some of the neurons actually involved in the generation of esophageal motility had been destroyed within the NTS.26 Recent data, obtained with c-fos immunohistochemistry, confirm the prime role of NTS neurons in both the oropharyngeal and esophageal phases of swallowing.28, 29
As regards the swallowing neurons in the ventrolateral medulla, the results available are consistent with the view that during swallowing, these neurons are driven by NTS neurons. As neurons of the VSG are connected to motoneurons, one of their functions probably consists in activating the motoneuronal pools during swallowing. The existence of neurons with collaterals to several pools of motoneurons also suggests that they may also participate in the coordination of the motoneuronal pools during swallowing.18, 55, 68 Within the swallowing CPG, the ventral swallowing neurons act therefore very likely as switching neurons that distribute and coordinate the sequential or rhythmic drive generated in the dorsal group to the various pools of motoneurons involved in swallowing.14, 109
It must be pointed out that this pattern of organization does not mean that the connections between the dorsal group and the ventral group, and those between the ventral group and the motoneurons, include only excitatory connections. Indeed, results obtained from intracellular studies on swallowing motoneurons in XII motor nucleus and in the nucleus ambiguus49, 50 have suggested that during the functioning of the network, both excitatory and inhibitory drives can be exerted along the anatomic pathways.
Activation and Modulation of Brainstem Neuronal Activity
Sensory Inputs
Sensory inputs are essential, either to induce the whole swallowing motor sequence or parts of this motor sequence such as esophageal peristalsis,1, 3, 4, 111 but also to modulate the central network activity.112
The afferent fibers involved in the initiation of swallowing are those running within the maxillary branch of the trigeminal nerve, the glossopharyngeal nerve, and the vagus nerve, especially its superior laryngeal branch (SLN).1, 3, 4 Electrophysiologic results, however, have shown that SLN afferent fibers constitute the main afferent pathway involved in the initiation of swallowing.5, 32, 37, 38, 113, 114 Therefore, the solitary tract and the NTS, particularly its intermediate subpostremal part, which receives vagal afferent fibers including those from the SLN, constitute the main afferent central structures involved in deglutition.1, 3
In all the species studied, results obtained on oropharyngeal neurons have shown that almost all the NTS neurons are synaptically activated by the SLN.6, 21, 32, 34 In most cases, the oropharyngeal neurons receive a direct, that is, monosynaptic, input from SLN afferent fibers,6, 21, 32, 57 and the morphologic arrangement of the SLN terminals in the interstitial NTS subnucleus suggests that laryngeal afferent fibers may exert strong synaptic effects on postsynaptic elements,65 supporting the idea that SLN afferent fibers play a major role in the initiation of swallowing. It has been observed, in sheep and cats, that IX afferent fibers activated around 90% of the oropharyngeal neurons studied. However, it seems most likely that the connection is not monosynaptic, judging from the conduction distance and the variable latency,32, 37, 69 resulting in a weaker impact of IX fibers on the swallowing neurons. These findings probably explain why the IX afferent fibers are relatively inefficient at initiating swallowing.
Although the swallowing motor sequence is centrally organized, it may change as a result of peripheral afferent information.112 Studies on the swallowing motor sequence have strongly suggested that it is under the control of a peripheral feedback mechanism.115, 116, 117 Direct evidence that sensory feedback intervenes during swallowing has also been provided by afferent nerve recordings.118, 119, 120, 121, 122 All the results suggest that continuous sensory feedback may influence the neurons of the CPG and thus modulate the central program.112, 120 Data obtained on swallowing neurons have shown that applying continuous stimulation to peripheral receptors by means of an inflated balloon can either induce a permanent activity in the neurons that are active during swallowing, or modify the bursting activity occurring during swallowing.6 To be efficient, the distention must be performed more and more distally as the neuronal discharge occurs later and later during swallowing. Results showed that the burst firing activity of the neuron increases both in duration and frequency (Figure 6). The changes resulting from peripheral stimulation are more striking in esophageal than in oropharyngeal neurons; depending on the volume of the balloon inserted, a twofold increase in the duration and a fivefold increase in the frequency can be observed in the former (Figure 6b).3, 112 The activation of peripheral receptors during swallowing therefore results in a decrease in the velocity of the peristalsis, which makes the duration of the whole sequence longer, and the muscular contraction more powerful. Sensory feedback can therefore be assumed to modify the central program, by adjusting the motor outputs depending on the contents of the tract.112
Figure 6: Effect of sensory inputs on the burst discharge of swallowing neurons.
Swallowing is triggered by stimulations of the SLN. a: Effect of a slight pharyngeal distention on the activity of an oropharyngeal neuron. The balloon is deflated (1) and inflated with 10 mL air (2). b: Esophageal distention effect on swallowing activity of an esophageal neuron. The balloon is deflated (1) and inflated (2). Note the increase in duration and frequency of neuronal discharges when the balloon is inflated at the level of the tract corresponding to the neurons. MH m, mylohyoideus EMG; SwN, discharge of the medullary interneurons; P, recordings of intrapharyngeal (a) or intraesophageal (b) pressure. (Source: Adapted from Jean.6, 112, with permission from Elsevier.)
In addition to the excitatory phenomena, the sensory inputs can also trigger inhibitory effects via central connections, as suggested by electromyographic data and vagal motor fiber recordings.2, 10, 52, 123 The occurrence of these inhibitory phenomena has been fully confirmed by microelectrode recordings showing that all esophageal neurons are strongly inhibited during the oropharyngeal stage of swallowing, the so-called deglutitive inhibition, or during a pharyngeal distention that stimulated the peripheral receptors (Figure 7a).6, 112, 124 In addition, the activity of the esophageal neurons that fire during the contraction of the lower esophagus is also inhibited during an esophageal distention that stimulates receptors of the upper esophagus (Figure 7b). The strength of the inhibition is variable depending on the size of the inflated balloon.112 These data indicate that the swallowing neurons controlling the distal regions of the swallowing tract are inhibited when neurons controlling the more proximal regions are excited. They support the idea that there may exist a rostrocaudal inhibition within the swallowing network, as suggested by the blockade of the esophageal peristalsis, which occurs during rhythmic swallowing (3).
Figure 7: Inhibitory effects on the burst firing of swallowing neurons.
Swallowing is triggered by stimulations of the SLN. a: Very slight inflation (3 mL) of a balloon in the pharyngeal cavity does not modify the swallowing neuronal discharge (1). When the intrapharyngeal balloon is inflated with 20 mL, the neuronal activity is completely inhibited (2). b: Inhibition of an esophageal neuron corresponding to the thoracic esophagus during distention of the cervical esophagus. The balloon is deflated and several swallows are induced before and after motor paralysis (1). When the balloon is inflated (20 mL, between arrows) the swallowing discharge is suppressed (2). MH m, mylohyoideus EMG; SwN, discharge of the medullary interneurons; P, recordings of intrapharyngeal (a) or intraesophageal (b) pressure. (Source: Adapted from Jean.6, 112, with permission from Elsevier.)
Supramedullary Influences on Brainstem Swallowing Neurons
The fact that an individual can swallow voluntarily shows that the medullary swallowing network can be activated by inputs from the cerebral cortex.1, 4, 20, 125, 126, 127, 128 In addition, several clinical reports have indicated that various cortical dysfunctions may result in dysphagia, swallowing impairments, or affect esophageal peristalsis.128, 129, 130, 131 These observations point out the involvement of supramedullary influences, because the peripheral afferent pathway and the CPG, which is localized in the caudal brainstem, seem to remain unaltered in these patients. Moreover, the numerous results obtained with new approaches such as cortical evoked potentials, transcranial magnetic stimulations, magnetoencephalographic recordings, and functional brain imaging techniques, performed in animals and humans, show that several supramedullary structures may be responsible for various effects on swallowing, such as initiating the motor activity, or modulating the swallowing reflex.127, 132, 133, 134, 135, 136, 137, 138, 139
In fact, very few studies have dealt with these central influences on the neurons of the CPG. As far as the supramedullary influences on swallowing and their action on brainstem neurons are concerned, all the results available so far have been obtained in studies on the cortical influences on swallowing.140 Results obtained on sheep20 indicate that oropharyngeal neurons in the DSG were cortically activated with a shorter latency than those in the VSG (5–8 ms versus 10–16 ms). In addition, within the DSG, all the swallowing neurons with preswallowing activity tested and 79% of the remaining oropharyngeal neurons studied can be activated by cortical stimulation, whereas only 32% of all VSG neurons tested were cortically activated. The esophageal neurons in the DSG that fired during the contraction of the upper esophagus also responded to cortical stimulation, but in small numbers (38%) and with a longer latency of 10 to 12 ms. None of the esophageal neurons in the DSG, firing later during the contraction of the lower esophagus, nor the esophageal neurons in the VSG, were activated by cortical stimulation.3, 20 Although a direct pathway from the cortex to motoneuronal pools involved in swallowing has been mapped, using tracing techniques,141, 142 these results indicate that the cortical input to identified swallowing neurons mainly focuses on swallowing neurons in the DSG and involves the same circuit as the peripheral afferent fibers do, at least in sheep (Figure 5). The DSG neurons, therefore, receive convergent information from both cortical and peripheral inputs that trigger swallowing.
In sheep, a population of sensory relay neurons exhibiting burst firing in phase with the oropharyngeal stage of swallowing has been found to exist more rostrally in the pons.39, 143 These neurons are thought to be involved in providing information from the oropharyngeal receptors to the higher nervous centres.39 Based on this finding, that during swallowing sensory feedback is conveyed to the cortical area via a first relay in the pons, the swallowing cortical area may have further functions.39, 143, 144, 145 Cortical neurons may belong to a pontocorticomedullary loop, so that upon receiving sensory information, they might control the activity of the CPG swallowing neurons as they fire successively, just as peripheral afferent fibers do.3 It has been shown that cortical neurons in the swallowing cortical area of sheep are activated or inhibited during swallowing (Figure 8).146 More recent findings on monkeys indicate that cortical areas associated with feeding behavior are involved in swallowing.128, 147, 148 However, changes in the firing activity of cortical neurons during swallowing have been shown to depend on sensory feedback, because they are abolished in paralyzed animals. Because only oropharyngeal and a few esophageal neurons respond to cortical stimulation, the control of swallowing may be restricted to the oropharyngeal phase and to the beginning of the esophageal phase. Therefore, the cortical swallowing area may serve mainly to trigger deglutition and control the beginning of the motor sequence, after which the sequence might be carried out without any further cortical control.3, 20
Figure 8: Activity of cortical neurons during swallowing.
a: Repetitive stimulation of ipsilateral SLN induced rhythmic swallowing (MH m) (1). Note that the burst firing is elicited at each swallow. Activation of the same neuron during spontaneous swallowing (2). b: Discharge of a cortical cell activated by SLN stimulation (dots). Each SLN stimulation induced a short latency response of the cortical neuron (cN), which also fired during swallowing. Note the blockade of the synaptic response during the burst firing (collision). (Source: Adapted from Car.146, with permission)
Neural Mechanisms of Pattern Generation
Network and Cellular Properties
The central mechanisms that generate the bursting activity of swallowing neurons and their sequential or rhythmic firing behavior are still unknown. All the results already obtained indicate, however, that among the various groups of swallowing neurons, local mechanisms within the DSG, involving the connectivity and the synaptic interactions between NTS neurons, and their intrinsic properties,108, 149, 150, 151, 152, 153 play a key role in pattern generation.
As regards the network organization, the swallowing CPG can be viewed as a linear-like chain of neurons based on the rostrocaudal anatomy of the swallowing tract (Figure 9a). Because neurons of the NTS fire sequentially during swallowing, each neuron or group of neurons in this chain may control, through excitatory connections, more and more distal regions of the swallowing canal, and be responsible for the successive firing behavior via increasingly numerous polysynaptic connections.3, 112 The decrease in the discharge frequency of neurons firing later and later during swallowing suggests, however, that the excitatory inputs are less powerful as they progress along the chain. Interestingly, the discharge pattern of preswallowing neurons suggests that reexcitation loops may be involved in burst generation of the neurons.
Figure 9: Possible mechanisms of the swallowing pattern generation.
a: The swallowing network can be viewed as a chain of neurons with excitatory (black triangles) and inhibitory (black dots) connections, and sensory feedback (broken lines). The excitatory inputs are less powerful along the chain, in contrast to the inhibitory influences, resulting in long periods of inhibition of neurons controlling more distal parts of the tract. b: The central pattern generator may be subdivided in an oropharyngeal and an esophageal network. The esophageal net is first inhibited by the oropharyngeal net (black dot). This primary inhibition is followed by an excitatory action (black triangle), rendering possible the successive activation of esophageal neurons. c: Inhibitory-excitatory sequences during swallowing. Field potential recorded at the esophageal site within the sheep NTS (1). SLN stimulation induced a single swallowing, producing a biphasic wave potential. Intracellular recording of an esophageal motoneuron within the nucleus ambiguus during swallowing (2). Note that the burst firing of the neuron occurred after a hyperpolarization of the membrane potential, indicating that the neuron has undergone inhibition during the oropharyngeal stage. (Source: Adapted from Jean3 and Zoungrana et al.50, with permission from American Physiological Society)
In addition to excitatory connections, there are also inhibitory connections between the various links in the chain, because when the neurons responsible for the beginning of the swallowing sequence fire, those controlling the more distal parts of the tract are inhibited, and their activity is delayed.3, 6 Therefore, inhibition is also successively transmitted throughout the network, resulting in longer periods of inhibition in neurons controlling more distal part of the swallowing canal.3 Several data have suggested that the inhibitory message may occur prior to the excitatory transfer of the bursting discharge and therefore it plays an important role in the central mechanisms.1, 52, 154 Recordings of field potentials, within the DSG of esophageal neurons, showed, during swallowing, a biphasic wave of potential reflecting an inhibitory-excitatory sequence.3 Intracellular studies on esophageal motoneurons have shown that a wave of hyperpolarization occurs prior to the bursting discharge of the swallowing neurons (Figure 9c).50 These inhibitory mechanisms not only may be responsible for delaying the onset of neuronal firing, but also may contribute directly to the sequential excitation of the neurons via mechanisms such as disinhibition or postinhibitory rebounds. Interestingly, disinhibition has been shown to exist within the swallowing network because blockade of GABA inhibitory transmission resulted in rhythmic oropharyngeal or esophageal motor events.101, 155
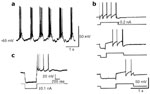
Most of the data on the intrinsic properties of neurons liable to be involved in swallowing have been obtained in studies on brainstem slices. The link between the endogenous properties of neurons studied under these in vitro conditions and their possible role in swallowing pattern generation is far from being elucidated. The most relevant data have been obtained in studies on NTS neurons, in particular those performed in the periinterstitial region of the NTS where swallowing-related neurons are situated.156, 157, 158, 159 The NTS neurons were found to have several endogenous properties, some of which seem to be relevant to swallowing pattern generation (Figure 10).110, 158 The NTS neurons possess an early transient outward potassium current (IKA), which is responsible for the delayed firing of the neurons that occurs when they are depolarized from negative membrane potentials.158, 159, 160 IKA therefore might be involved in the sequential activity of swallowing neurons. It has also been reported that some NTS neurons possess low-threshold activated calcium currents, IT(Ca), which are responsible for postinhibitory rebounds.161, 162 Therefore, inhibitory inputs on swallowing neurons may activate the IT(Ca), and participate in the bursting activity. Moreover, the responses of NTS neurons to the N-methyl-D-aspartate (NMDA) receptor activation have shown that they have pacemaker-like properties.158, 159 In addition to mechanisms such as those involving reexcitation phenomena, if swallowing neurons possess these pacemaker-like properties, it is very likely that they may participate in burst generation, like in several other CPGs.108, 163
Figure 10: Cellular properties of NTS neurons recorded in vitro on rat brainstem slices.
a: Typical rhythmic burst firing superimposed on cyclic depolarization of membrane potential, under bath application of N-methyl-D-aspartate (60 M). Note that the burst of spikes may be followed by depolarizing afterpotentials typical of pacemaker-like properties. b: Delayed excitation in an NTS neuron. A control depolarizing pulse elicited repetitive firing (top trace). When it was preceded by a conditional hyperpolarizing pulse, the first spike in the burst was delayed (middle and bottom traces). Note that the delay was longer when the duration of the hyperpolarizing pulse increased. c: Postinhibitory rebound in a NTS neuron. Note the burst-firing pattern following the hyperpolarization of the NTS neuron. (Source: Adapted from Tell and Bradley162 and Tell and Jean.158, 159, with permission.)
M). Note that the burst of spikes may be followed by depolarizing afterpotentials typical of pacemaker-like properties. b: Delayed excitation in an NTS neuron. A control depolarizing pulse elicited repetitive firing (top trace). When it was preceded by a conditional hyperpolarizing pulse, the first spike in the burst was delayed (middle and bottom traces). Note that the delay was longer when the duration of the hyperpolarizing pulse increased. c: Postinhibitory rebound in a NTS neuron. Note the burst-firing pattern following the hyperpolarization of the NTS neuron. (Source: Adapted from Tell and Bradley162 and Tell and Jean.158, 159, with permission.)
One or Two Central Pattern Generators?
In species with an entirely striated esophagus, it is now clearly established that deglutition, that is, the oropharyngeal phase followed by primary peristalsis, is a centrally patterned motor activity. In species with a smooth muscle esophagus, in addition to intrinsic peripheral mechanisms of both neural and muscular origin, which are obviously involved in the organization of the smooth muscle motor pattern,1, 2, 10, 17 several data have suggested that the central nervous system may also contribute to the organization of the sequential motor pattern.52, 53, 164, 165, 166 In fact, results indicate that unlike the all-or-none oropharyngeal sequence, the esophageal phase may show some lability, suggesting that the central program controlling this phase may be less robust than that responsible for the oropharyngeal phase. This difference is most striking in the case of secondary peristalsis of the esophagus, because peripheral afferent feedback has been shown to be essential for the propagation of the peristaltic wave.2, 3, 10, 28, 123 These data suggest that the swallowing CPG can be subdivided into two subnetworks, an oropharyngeal and an esophageal net of neurons, each mediating the patterning of the respective phase of deglutition. But they also suggest that the esophageal net is likely to have less robust central mechanisms and be more dependent on afferent inputs (Figure 9b).3
Microelectrode recordings, however, failed to detect any difference between the neurons involved in both primary and secondary peristalsis or their discharge parameters.6 This is puzzling from the patterning point of view. Why are afferent inputs more necessary during secondary peristalsis, and why is the central program ineffective? In fact, when the same neurons are active in both motor events without any difference between their discharge patterns, the sole difference between primary and secondary peristalsis is that the latter lacks an oropharyngeal phase, and consequently is not accompanied by any oropharyngeal network activity. Therefore, the activity of the oropharyngeal net might be an important factor in the central programming of primary peristalsis. That is to say that the esophageal net may program esophageal peristalsis only when triggered by the oropharyngeal net. In this way, the oropharyngeal net may serve as an intrinsic modulatory system, a mechanism that seems to play an important role in several CPGs.167 When the oropharyngeal net is activated, the esophageal net can program the peristaltic wave, whereas when it is inactive, as in the case of secondary peristalsis, the program requires peripheral influences. It may be supposed that the strong inhibition generated during the oropharyngeal stage of swallowing may be followed by long-lasting excitatory or facilitatory effects exerted by the oropharyngeal neurons on the esophageal network, resulting in the sequential discharge of the neurons. Parallel excitation and inhibition have been shown to exist at multiaction synapses. If the time course of one action is longer than the other, then the configuration of the network can lead to a temporal reversal in the sign of the synaptic action.150 In the case of the swallowing CPG, inhibition may be followed by delayed excitation. Field potential recordings support such a hypothesis, because they show that the strong inhibition of esophageal neurons during the oropharyngeal phase is followed by a wave of excitation. Interestingly, DSG neurons with such multiaction synapses might be located between the NTS and the DMX, a region crucial for linking the oropharyngeal and the esophageal phases of swallowing, which may correspond to the ventromedial NTS subnucleus in the cat.28 In addition, GABA and acetylcholine might be involved in the fast inhibitory and the delayed excitatory actions, respectively.87, 99, 101
Nature of the Swallowing Central Pattern Generator: Fixed or Flexible?
The fundamental act of swallowing, in particular its oropharyngeal stage, is a stereotyped motor behavior and the swallowing CPG has been classically viewed as a dedicated circuit, that is, as a specific network of neurons that is hardwired so as to produce a sequence of excitation and inhibition that is always the same (Figure 11a).1, 3, 4 However, this view is now challenged in light of numerous data obtained mainly on the central nervous system of invertebrates. In addition to being a classical dedicated network, most of the CPGs seem in fact to be either reorganizing or distributed circuits, and a single neural circuit can combine features typical of each of these different architectures,168, 169, 170 resulting in considerable functional plasticity.168 This is a particularly relevant point in the case of the swallowing CPG. The swallowing CPG is not an automatic, continuously functioning CPG, and the question arises as to whether the swallowing neurons are completely inactive when no swallowing occurs, or whether these neurons may have other functions. This question is especially interesting, because we have established that swallowing neurons include NTS neurons, and it has by now emerged that the NTS is far from being simply a sensory relay, as it is involved in numerous functions.63 Given that the NTS contains only a small population of neurons, that is, around 40,000 in the case of the rat caudal NTS,171 is each NTS neuron in this very small population devoted to one single fixed function, or is there, within the NTS, one or several populations of neurons that are flexible and participate in several functions, depending on the inputs they receive? In invertebrates, it has been observed that swallowing depends on a pattern generator that is temporarily formed, preparatory to the production of the motor activity.172, 173 That is to say that when a given stimulus is delivered, a pool of appropriate neurons is activated and forms the swallowing CPG, whereas when no swallowing activity is required, these neurons are involved in other tasks.
Figure 11: Schematic representations of the swallowing central pattern generator (CPG).
a: The swallowing (Sw) CPG may be a dedicated circuit with neurons subserving only this function. This CPG has connections with others CPGs, such as those involved in respiration, mastication, etc., to ensure functional interactions under physiologic conditions. b: The swallowing CPG may be a reorganizing circuit consisting of pools of flexible neurons, that is, neurons that can function in several CPGs involved in the organization of various kinds of motor behaviors. c: Alternatively, it is possible that the swallowing CPG may be formed temporarily by neurons belonging previously to other CPGs. Following swallowing input, these neurons contribute to the newly formed swallowing CPG. (Source: Adapted from Dickinson and Moulins168 and Jean.3, with permission)
Although there is no direct evidence, in mammals, that the swallowing CPG is flexible, recent results have shown that, within the network, some neurons may participate in activities other than swallowing-related ones (Figure 11b). It has been established that not only motoneurons, but also interneurons, can be involved in at least two different tasks, such as swallowing and respiration, swallowing and mastication, or swallowing and vocalization.30, 55, 57, 58, 59, 60, 174, 175 In addition, in vitro data on brainstem slice preparations have provided evidence that cellular properties of NTS neurons may underlie such a functional flexibility depending on the balance between excitatory and inhibitory inputs, which modulates the interactions between neuronal ionic conductances.158, 159
Whatever the case may be, the available evidence indicates that, within the mammalian brainstem, neurons do exist that might have a multifunctional role. It therefore can be postulated that at least some of the components of the swallowing network are not dedicated to swallowing alone, but can also serve some purpose in other central networks or that, like in invertebrates, the swallowing CPG is formed temporarily (Figure 11b, c).
Conclusion
Neurophysiologic studies have shown that swallowing depends on a CPG located in the medulla oblongata, which involves several brainstem motor nuclei (V, VII, IX, X, XII) and two main groups of interneurons: a dorsal swallowing group (DSG) in the NTS and a ventral swallowing group (VSG) located in the ventrolateral medulla above the nucleus ambiguus. Within the CPG, neurons in the DSG play the leading role in generating the swallowing pattern, whereas neurons in the VSG act as switching neurons, distributing the swallowing drive to the various motoneuronal pools. It is quite remarkable that a CPG for a fundamental motor activity should be located within a primary sensory nucleus, namely the NTS. As in the case of other CPGs, the functioning of the central network can be influenced by both peripheral and central inputs.
Little is known so far about the mechanisms at work in the CPG. The sequential burst firing of the swallowing neurons probably depends on the pattern of intrinsic connections within the swallowing network. Central inhibitory connections, in particular, are supposed to play a major role in neuronal sequential firing. Intrinsic cellular properties such as pacemaker-like properties, delayed excitation, and postinhibitory rebound, in particular those of NTS neurons, probably also contribute to determine the shaping and timing of the swallowing motor pattern. Interestingly, recent data indicate that the swallowing CPG may show some degree of flexibility, suggesting that at least some of the swallowing neurons may belong to pools of neurons that are common to several brainstem CPGs.
Several questions remain to be answered regarding brainstem control of swallowing. Although they are technically difficult, electrophysiologic studies dealing with clearly identified swallowing neurons will be necessary to obtain significant insights into (1) the mechanisms underlying swallowing pattern generation; (2) the synchronization between the two hemi-CPGs, which has received little attention but is essential for the coordination of the muscular contraction; and (3) the linking between the oropharyngeal and esophageal phases of swallowing. It seems likely that by focusing research on populations of neurons with multiple recordings rather than single neuron recordings, it will be possible to gain new insights into how the mammalian CPGs function. In addition to research on anesthetized or decerebrate animals, studies on other experimental models such as the isolated brainstem preparations may also be useful for this purpose.176, 177 The supramedullary influences on swallowing, which have been only sparsely documented so far, also require more thorough investigation, in particular in light of the numerous forebrain structures identified, with the functional brain imaging techniques, to be involved in swallowing. In particular, this would make it possible to specify the neuronal pathways involved. Although swallowing is a vital function, some data have suggested that its underlying mechanisms may be not fixed at birth.178, 179, 180 Therefore, studies on the postnatal development of brainstem control of swallowing would be of particular interest to identify how the central nervous system operates to establish mature networks devoted to fundamental activities in the adult brain.