Q1. What is the mostly likely diagnosis and differential diagnosis?1, 2, 3, 4
Barium swallow and esophageal motility studies were obtained.
A provisional diagnosis of achalasia was made.
The primary differential diagnosis for someone with difficulty swallowing both liquids and solids includes esophageal motility disorders and oropharyngeal dysphagia. Infrequently, cases with very tight esophageal strictures can present with dysphagia for liquids and solids, especially if a solid food bolus causes a transient food impaction. The localization of this patient's symptoms to the upper sternum points toward the esophageal motility disorder rather than pharyngeal problem such as a Zenker's diverticulum that generally localizes to the neck.
Q2. Is the selection of these tests appropriate?
The barium swallow is shown in Figure 1.
Primary care physicians will commonly evaluate a complaint of dysphagia with a barium esophagogram, whereas gastroenterologists typically start with an endoscopy. However, in patients with achalasia barium studies provide greater sensitivity for the diagnosis of achalasia than endoscopy. As many as 40% of upper endoscopies can be normal, particularly during the early course of the disease. Given the likelihood of a motility disorder a motility study was also scheduled.
Q3. What is your interpretation of the barium swallow?
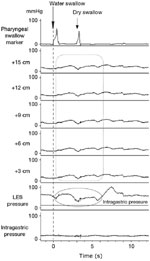
The esophageal motility study is shown in Figure 2.
The barium esophagram depicts a mildly dilated, tortuous esophagus with a symmetrical tapering constriction at the level of the gastroesophageal junction. This appearance is highly suggestive of achalasia.
Q4. What is your interpretation of the esophageal motility study?
This demonstrates pharyngeal contractions with wet or dry swallows. The distal 15 cm of the esophageal body showed no contractile activity with swallows. Furthermore, deglutitive relaxation of the lower esophageal sphincter (LES) is absent. When the gastric baseline is accounted for, there also exists an esophageal to gastric pressure gradient. This gradient is the reverse of the pressure differential present in normal subjects and supports the finding of the functional obstruction at the level of the LES. These manometric findings are characteristic of achalasia
Q5. What is your differential diagnosis after these studies?
A diagnosis of achalasia is made. However, the etiology of achalasia is not defined by these studies. Table 1 summarizes various etiologies of achalasia. The vast majority of cases in the United States have an unknown cause and are referred to as primary achalasia. There is a long list of secondary causes of achalasia. In South America, infection with Trypanosoma cruzi (Chagas' disease) is a common cause of achalasia. Some physicians might consider a diagnosis of Chagas' disease because of the patient's history of a trip to Mexico before experiencing the symptoms. Although T. cruzi is certainly endemic to certain parts of Mexico, Chagastic achalasia develops as a result of a chronic, probably autoimmune-mediated, destruction of the enteric neurons that takes several years, and in some cases decades, to manifest. Therefore, the 1-month time frame makes Chagas' disease highly improbable.
Another important cause of secondary achalasia is carcinoma. Carcinomas of the gastroesophageal junction may cause achalasia by local invasion of the myenteric plexus. However, some distant cancers, particularly small cell lung carcinoma, may also cause achalasia due to myenteric neuronal dysfunction as a part of paraneoplastic syndrome. An esophagogastroduodenoscopy (EGD) needs to be done to exclude tumor of the gastroesophageal junction. If the clinical suspicion for cancer associated achalasia is high, diagnostic testing beyond endoscopy with computed tomography (CT) scanning and endoscopic ultrasonography may be indicated. In the case under discussion, however, the 5-year antecedent history of dysphagia makes a cancer unlikely.
Q6. Explain the pathophysiology of this condition.
See the article Pathophysiology of achalasia and diffuse esophageal spasm for a discussion of the pathophysiology.
Q7. What are possible explanations for the episodes of chest pain?
Paroxysms of chest pain occur in up to 60% of patients with achalasia and are not always temporally linked to the esophageal dilation from retained food following meals. The mechanisms of the painful episodes are not fully understood. In some cases, pain may be associated with tertiary esophageal body contractions. In other cases, it has been speculated that the pain may be neurogenic in origin. Acid reflux paradoxically can sometimes occur in these patients. If reflux occurs, it cannot be cleared effectively owing to the lack of peristalsis and functional obstruction at the LES. Poor esophageal emptying also promotes bacterial overgrowth with lactic acid formation that can acidify the esophageal lumen and may produce chest pain.
Q8. Discuss the treatment options available.4, 5, 6, 7, 8, 9, 10, 11, 12, 13
After detailed discussions on the various treatment options, the patient undergoes an uneventful laparoscopic Heller myotomy with Dor fundoplasty. He is discharged on postoperative day 1. On follow-up 4 weeks later, the patient reports substantial improvements in his dysphagia but does note some delay in the transit of solid food in his lower chest region. The food usually passes after several seconds and is aided by drinking liquid. He denies heartburn although he has been taking a proton pump inhibitor since the surgery. The chest pain episodes remain unchanged in terms of frequency or severity. His nocturnal coughing and postprandial regurgitation have completely resolved and he has been gradually gaining weight.
Briefly, pharmacologic agents that relax the LES can be prescribed while waiting for more definitive treatment. Such agents include nitrates and calcium channel blockers. These agents should be administered by the sublingual route. Overall limited effectiveness, inconvenience of dosing and side effects of headaches and hypotension limit the utility of this approach.
Endoscopic injection of botulinum toxin provides symptomatic relief of dysphagia in about 60% of patients at 6 months. Predictors of response include age >50 and the presence of vigorous achalasia. Objective measures of esophageal function do not improve to the same degree as seen with pneumatic dilation. Moreover, the response to botulinum toxin wanes with time, and repeated injections have been associated with a decrease in the number of patient responders. Therefore, botulinum toxin is recommended for patients who are poor candidates for more invasive therapies owing to medical comorbidities.
Many centers perform pneumatic dilation as first-line therapy for achalasia. Pneumatic dilation using the Rigiflex balloon is an accepted first-line therapy for achalasia, with reported response rates of 85% at 1 to 2 years and perforation rates averaging 2.6%. Predictors of response include larger balloon diameter, lower postdilation LES pressure and age >40. A graded approach is recommended that starts with a 3-cm balloon with incremental balloon diameters reserved for nonresponders. Long-term follow-up studies suggest that the effectiveness of pneumatic dilation diminishes over time, with approximately 40% remission rates at 5 years even with repeated dilations.
Laparoscopic Heller myotomy has been a significant advance in the management of achalasia. Postoperative recovery is short and averages less than 1 day in most series. Uncontrolled observational studies suggest that the effectiveness is superior to pneumatic dilation. Potential complications include gastroesophageal reflux disease that can lead to development of symptomatic peptic strictures. Combining the myotomy with a fundoplication is commonly done, but the optimal type of fundoplication is debated.
For a fuller discussion of the treatment options, see the article Pathophysiology of achalasia and diffuse esophageal spasm.
Q9. Why is empiric therapy with a proton pump inhibitor a reasonable recommendation for the patient?
A follow-up barium swallow is performed and demonstrates free passage of barium through a patent gastroesophageal junction with intact fundoplasty. The patient is reassured and scheduled for follow-up in 1 year.
Ten years later, the patient is referred again by his primary care physician with complaints of increasing dysphagia, predominantly for solid food. His symptoms have been worsening over the past few months. His prior chest pain is now very infrequent and of lesser severity. He denies weight loss.
Reflux is a common complication of both endoscopic and surgical therapies that seek to obliterate LES pressure in the setting of an aperistaltic esophagus. In one of the larger surgical series, reflux was documented by pH testing in 17% of patients following laparoscopic myotomy with most of these subjects not reporting heartburn. Successful treatment of achalasia effectively transforms the manometric picture from achalasia to one of a "scleroderma" esophagus with the concomitant risks of regurgitation and acid reflux disease. Complications of Barrett's esophagus and peptic stricture have been documented in several reported series following Heller myotomy. Reflux and its complications are of particular concern in achalasia patients who appear to have diminished visceral sensation and may not complain of typical heartburn symptoms.3
Q10. What are the causes of postoperative dysphagia?
Early postoperative dysphagia can be caused by incomplete myotomy, periesophageal scarring, underlying esophageal dysmotility, massive esophageal distention with sigmoid deformity, or mechanical obstruction by fundoplication, paraesophageal hernia, or crural diaphragmatic hiatus repair. It is important that patients recognize that some degree of dysphagia is anticipated even after successful myotomy, given the esophageal aperistalsis that characterizes the disease, as it is uncommon for esophageal peristalsis to recover following therapy directed at the LES.
Late postoperative dysphagia is most commonly caused by the development of either a recurrent high-pressure zone at the LES or a peptic stricture complicating acid reflux. Progressive esophageal dilation and sigmoid deformity can occur. Less commonly, an obstructed fundoplication or development of esophageal cancer can manifest. The recurrent high-pressure zone may be attributed to the development of scarring of the myotomy site that leads to connection of the cut edges of the muscularis propria, thus reestablishing the circular integrity of the LES. In cases of postoperative dysphagia owing to either an incomplete myotomy or recurrent high-pressure zone, pneumatic dilation can be employed as an alternative to redoing surgery.