Key Points
- Swallowing is an essential gastrointestinal (GI) function that is under strong cerebral control.
- Swallowing is bilaterally but asymmetrically represented in the human motor cortex.
- Dysphagia after stroke may be a consequence of damage to the "dominant" swallowing hemisphere.
- Recovery of swallowing after dysphagic stroke appears to relate to compensation of function in the undamaged hemisphere.
- Therapies that can accelerate this compensatory process may help in the future to restore swallowing function in acute stroke.
Introduction
The process of swallowing is a complex neuromuscular activity that allows the safe transport of material from the mouth to the stomach for digestion without compromising the airway.1 Of course, this is a fairly simplistic description, as the act of swallowing requires a sophisticated integration of both central control and anatomic structures to produce the sensorimotor output that we call the swallow. This review focuses specifically on the role of the cerebral cortex in the regulations of swallowing, highlighting evidence from neurophysiologic studies in animals and humans, including recent advances in brain functional imaging. In addition, it examines inferential observations from lesions to the cortex that cause swallowing problems (dysphagia), and explores newer directions in the rehabilitation of swallowing after stroke.
Neurophysiologic Observations in Animals
The cortex has been strongly implicated in the control of swallowing. Numerous investigators have observed that stimulation of the cerebral cortex in different animal species is able to evoke the full swallowing sequence.2, 3 For example, in anesthetized sheep, swallowing can be evoked from a rostral region in the orbitofrontal cortex,4 whereas in primates the main cortical areas for eliciting a swallow appear to be dorsolateral and anterolateral frontal cortex, including an area known as the cortical masticatory area.5 Furthermore, it appears that, in animals, swallowing can be evoked by stimulation of both hemispheres,6 suggesting a bilateral, and possible equi-hemispheric contribution to cortical swallowing control. In addition, the corticofugal pathways to the brainstem have been mapped,7 demonstrating a definite route from cortex to the swallowing center in the brainstem. Damage to these fibers does not abolish the swallowing response completely, although marked dysphagia can occur. Indeed, anencephalic fetuses can still swallow,8 and lesions above the obex do not disturb the sequence of firing of the central pattern generator when evoked following superior laryngeal nerve (SLN) stimulation. However, these observation are based on experiments where SLN triggered swallowing has been studied in the artificial setting of the anesthetized animal. It is, therefore, likely that the cortex has a significant modulatory role in the control of the brainstem swallowing center and may have an initiating responsibility in the development of a voluntary swallow.
Functional Imaging of the Cerebral Cortex and Human Swallowing
The recent technologic advances in functional imaging of human brain have revolutionized our understanding of how the cerebral cortex operates in processing sensory and motor information. In particular, functional magnetic resonance imaging (fMRI), positron emission tomography (PET), magnetoencephalography (MEG), and transcranial magnetic stimulation (TMS) have become established as useful methods for exploring the neuroanatomy of swallowing, within both cortical and subcortical structures (Table 1). There are a number of advantages and disadvantages associated with each technique. For example, fMRI allows a detailed investigation of the functional neuroanatomy of the human brain with a spatial resolution of  2 mm or less. Moreover, fMRI can be performed, unlike PET, using a single-event-related approach, which correlates cerebral activity with each task trial. The result is that improved functional information can be derived from such paradigms with potentially reduced motion-induced artifact. This may be advantageous for studying swallowing, where physiologic information about the complete functional swallow is desirable, and where motion-related problems inherent to the task are particularly likely during block trial designs using fMRI. Consequently, it seems likely that fMRI will supersede PET as the main functional imaging method for studying cerebral function as related to blood flow.
2 mm or less. Moreover, fMRI can be performed, unlike PET, using a single-event-related approach, which correlates cerebral activity with each task trial. The result is that improved functional information can be derived from such paradigms with potentially reduced motion-induced artifact. This may be advantageous for studying swallowing, where physiologic information about the complete functional swallow is desirable, and where motion-related problems inherent to the task are particularly likely during block trial designs using fMRI. Consequently, it seems likely that fMRI will supersede PET as the main functional imaging method for studying cerebral function as related to blood flow.
Human brain imaging techniques such as PET and fMRI reflect changes in cortical function that are secondary consequences to alterations in regional cerebral blood flow, and have limited temporal resolution. Magnetoencephalography is a newer brain imaging modality that has started to resolve some of these limitations. It detects the postsynaptic magnetic fields generated by active populations of cortical neurons with millisecond temporal resolution and has a comparable spatial resolution to PET and fMRI. Nevertheless, until now, technical limitations in MEG data acquisition and analysis have restricted its use in exploring the cortical activation patterns during complex sensorimotor tasks such as swallowing. Finally, TMS is a non-invasive stimulation-based approach to studying central nervous system (CNS) function. Unlike the other brain imaging techniques, TMS does not rely on a task being performed; rather, it probes cortical pathways from motor cortex to muscles, via electromyography (EMG) recordings, building up a "map" of the cortical representation of a particular muscle or group of muscles. One advantage in the study of swallowing is that TMS can be used in patients with swallowing problems, because swallowing itself does not need to be performed, which is difficult in a scanner.
Functional Magnetic Resonance Imaging
Functional magnetic resonance imaging (fMRI) has been extensively used to investigate swallowing, and has good spatial resolution, of at least 2 mm, but the temporal resolution of a few seconds limits its use when studying deglutition. It was originally fraught with difficulties when used to assess brain signals associated with swallowing, because of large movement artifacts due to the swallow itself, especially in the inferior regions of the brain. Newer analysis techniques have allowed us to overcome these problems and obtain useful images.9 Traditional block trial studies require repeated swallows within short spaces of time, and this can induce inhibition of esophageal peristalsis, which may then mask some of the cortical activity that may otherwise be seen. Functional magnetic resonance imaging, unlike some of the other functional imaging modalities, may also be used with a single-event-related approach where the cerebral activity is correlated with each specific task. Event-related studies have advantages over block trial designs, especially with swallowing, as they can provide better functional information and are less likely to produce artifacts due to motion.
The simplest studies using functional imaging have looked at the areas of increased cortical activation during voluntary swallowing of either saliva or water, prompted by cues. Other studies have used a number of different techniques to investigate swallowing more thoroughly and to try to isolate the various neural components controlling each phase of deglutition, for example, using comparisons with tongue or hand movements and non-cued, involuntary swallowing (Figure 1). Although multiple regions are activated during swallowing, there appears to be some variability between subjects and between studies. During volitional swallowing the most consistent cortical activation is seen in the primary motor cortices and primary somatosensory cortices.10, 11, 12, 13, 14, 15 The greatest area of fMRI activation in the primary motor cortex is over the midinferior lateral portion of the precentral gyrus that corresponds with what is already known from direct cortical stimulation to be a region representing the face, tongue, and pharynx. This activation in the lateral precentral gyrus during swallowing tasks occurs dorsocaudally to the hand area.12
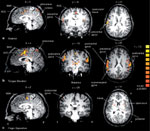
Figure 1: Voxelwise analysis of BOLD responses.
Responses are shown for a group of 14 subjects to (a) voluntary saliva swallow, (b) tongue elevation, and (c) voluntary finger-thumb opposition. Regions of significant activation are displayed on sagittal (left), coronal (middle), and axial (right) brain slices. Talairach-Tournoux plane coordinates are displayed above each image. (Source: Martin et al.11 with permission from the American Physiological Society.)
Most studies have also shown increased brain signal as measured using the Blood-Oxygen-Level-Dependent (BOLD) method in the majority of subjects during volitional swallowing in the anterior cingulate cortex (Brodmann Area (BA) 32,33), anterior insular cortex (BA 16), frontal operculum (BA 44), anteromedial temporal cortex (BA 21,22), superior premotor cortex (BA 6,8), and the precuneus. There is less consistently increased activation across subjects in the cerebellum, supplementary motor area, prefrontal cortex, posterior parietal cortex (BA 5), middle and inferior frontal gyri, cuneus, parietal opercula, motor association areas, sensorimotor integration areas, posterior cingulate (BA 23,31), basal ganglia, thalamus, and internal capsule.10, 11, 12, 13, 14, 15, 16 When fMRI is used specifically to look at the cerebellum and basal ganglia during volitional saliva swallowing, there is evidence of bilateral cerebellar activity more on the left, bilateral putamen, globus pallidus, and substantia nigra activation.15
Volitional swallowing results in activation of areas involved not only in the organization of a swallow itself, but also in the cue recognition and response and planning of the event. One method of differentiating these components is to study reflexive swallowing, which can be stimulated by injecting tiny amounts of water directly into the pharynx. Different swallowing tasks produce slightly different cortical maps. The areas most consistently and prominently activated in all tasks are the lateral precentral gyri, postcentral gyri, supplementary motor area, and insular cortex.12, 13, 14, 15, 16, 17 Reflexive swallowing induces bilateral activation concentrated in the primary somatosensory and motor cortices (BA 4,6), which is present in all subjects.16 When automatic saliva swallowing is compared to volitional saliva swallowing, there is more activation of the caudal anterior cingulate cortex during the reflexive swallow.17 Using visually cued go/no-go swallowing tasks during fMRI shows marked activation in the pericentral gyri and anterior cingulate cortex during the go condition, suggesting these areas are specifically involved in the act of swallowing. However, there is activation in the cuneus and precuneus during both go and no-go tasks, supporting the view that these areas are more concerned with processing of cues.18
By comparing the volitional movement of individual muscles involved in the swallow, such as the tongue, with those associated with the full swallow sequence, it may be possible to identify areas related more to the control of swallowing itself, rather than those linked more to motor planning. Tongue contraction alone produces bilateral signal increases within the sensorimotor cortex and operculum, as well as some activation in the supplementary motor area, putamen, thalamus, and cerebellum; there is also activation within the medulla corresponding to the regions known to contain the hypoglossal nuclei.19 Tongue movement and swallowing both produce activation of left lateral pre- and postcentral cortices, the anterior parietal cortex, and the anterior cingulate cortex. Tongue movements in these areas show a greater overall activation than that seen with volitional swallowing, perhaps because of a larger volitional component to tongue movement tasks compared with swallowing where much of the processing may be within the brainstem. Movements of the tongue also produce more activation than swallowing in the right pericentral gyri, supplementary motor area, premotor cortex, right putamen, and thalamus. Superimposed mapping of areas with increased activity suggests that only swallowing activates the most lateral extent of left pericentral and anterior parietal cortex, the rostral anterior cingulate cortex, precuneus, cuneus, middle frontal gyrus, and right parietal operculum. However, the main areas, where activation is stronger in swallowing than tongue elevation, are the precuneus and cuneus.11 Cortical activation in volitional swallowing also shares many regions with jaw clenching, lip pursing, and tongue rolling. Activation during all of the above functions can be found in anterior cingulate cortex, motor, and premotor cortices and occipital/parietal regions (BA 7,19,31).20 There is little agreement among studies about the activation of the insula, some having found it predominantly during swallowing tasks and others during both swallowing and tongue movement tasks.11, 16, 19
During volitional swallowing, the activation is usually bilateral, but with some areas having hemispheric dominance, 63% of subjects show left hemisphere dominance. This may be why some studies in stroke patients have shown a preponderance of left hemispheric lesions causing dysphagia.21 Most subjects show asymmetry of the postcentral gyrus activation (BA 4).11, 21 There also appears to be strong lateralization of increased activity in the midlateral precentral gyrus and somatosensory cortices (BA 1,2,3), supplementary motor area (BA 6), prefrontal cortex (BA 9,10), transverse temporal gyrus (BA 42), insula, operculum, cingulate gyrus, internal capsule, and premotor cortices.14, 15, 21 This dominance of one hemisphere is most often on the left, but if it is dominant on the right, then the lateralization is stronger. The asymmetry of activation may also vary depending on the type of swallow performed.21 In another study, of right-handed subjects, all had significantly more activation in the right insular than in the left.17
The cerebral regions that are most activated during dry swallowing in adults are similarly active in the brains of children. The main activation is seen in pre- and postcentral gyri (BA 3,4), superior motor cortex (BA 24), insula, inferior frontal cortex (BA 44,45), Heschl gyrus (BA4 1,42), putamen, globus pallidus, superior temporal gyrus (BA 38), and the nucleus ambiguus in the brainstem. Even in those children who have never eaten orally and where quite different patterns of activation or diminished signal may be expected, the areas of increased activity within the brain remain the same during dry swallows.22
Positron Emission Tomography
Positron emission tomography (PET) is a more established brain imaging modality, although its temporal resolution is inferior to that of fMRI and it provides better spatial resolution only when imaging subcortical regions. It also exposes the participants to ionizing radiation, making repeat studies undesirable.
Most work on swallowing has been done in healthy subjects using H215O injection to estimate regional blood flow (Figure 2). Voluntary swallowing of saliva produces strong bilateral increases in cortical blood flow, particularly in the inferior precentral gyrus, extending into primary somatosensory cortex (BA 43), the right insula, and the left cerebellum; there is also some activation in the putamen and thalamus, although less consistently.23 Volitional water swallows produce a similar picture of increased blood flow in both caudolateral sensorimotor cortices (BA 3,4,6) and the superomedial cerebellum. There is also a less consistent activation in the temporopolar cortices (BA 38), right anterior insula, right orbitofrontal cortex (BA 11), left mesial premotor areas (BA 6,24), dorsal brainstem, and amygdala.24 In both of these types of swallowing, there is bilateral, but often asymmetrical, increased cerebral blood flow within the sensorimotor cortex. In water swallowing most subjects were strongly lateralized, whereas in saliva swallows only 25% showed significant asymmetry; this dominance is unrelated to handedness and correlates well with data on the same subjects using transcranial magnetic stimulation to assess asymmetry of swallowing motor cortex.23, 24 Swallowing of infused water causes an associated decrease of blood flow within the posterior parietal cortex, right anterior occipital cortex, left superior frontal cortex, right prefrontal cortex, and superomedial temporal cortex.24
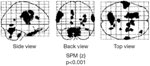
Figure 2: Statistical parametric mapping maps.
The group mean statistical parametric mapping (SPMz) maps of the areas of increased regional cerebral blood flow (rCBF) associated with swallowing are shown as three orthogonal projections through sagittal (side view), coronal (back view), and transverse (top view) views of the brain. A threshold of p =.001 was applied. A number of areas are activated, including regions corresponding to sensorimotor cortex, bilaterally, right insula, left cerebellum, left mesial frontal cortex, temporopolar cortex, and dorsal brain stem. (Source: Hamdy,24 with permission from the American Physiological Society.)
Fluorine 18 (F18)-labelled fluorodeoxyglucose (FDG) PET may be used as an alternative to water PET. It uses a glucose analogue to quantify regional glucose metabolism rather than blood flow, and this gives a better spatial resolution. It also allows tasks to be performed outside the scanner as FDG-6-phosphate, the metabolic product of FDG, which remains trapped within brain tissue for an extended period of time. This allows swallowing to be performed in the more physiologic sitting position before being scanned supine. When volitional swallowing is assessed using FDG PET, there is evidence of increased glucose metabolism in left sensorimotor cortex (BA 3,4,6), right prefrontal cortex (BA 10), bilateral lateral postcentral gyri (BA 43), right temporal cortex (BA 39), cerebellum, thalamus, precuneus (BA 31), anterior insula, and bilateral occipital cortex (BA 18,19).25 Decreased activity is seen in right premotor cortex, bilateral sensory and motor association cortices, left posterior insula, and left cerebellum.
Magnetoencephalography
Magnetoencephalography (MEG) is able to give very good temporal resolution (milliseconds) of brain activation as it detects the postsynaptic magnetic fields generated by active cortical neurons. Thus, it is particularly useful when studying dynamic functions such as swallowing. The basic MEG instrument contains magnetic detection coils called a superconducting quantum interference device (SQUID), which is placed over the head and records magnetic field changes. The original equipment was unable to provide useful data on dynamic functions such as tongue movement or swallowing as they produced EMG signals and that masked any cortical activity.26 More advanced analysis techniques are now available, which allow investigation of MEG signals during motor tasks. Magnetoencephalography has more recently been used with synthetic aperture magnetometry analysis that provides much better resolution of the resulting images.27, 28, 29 This high temporal resolution compared to other functional imaging techniques allows some analysis of the sequence in which central activation occurs during swallowing in addition to localization of active regions (Figure 3).
Figure 3: Magnetoencephalography data co-registered with magnetic resonance images.
Figure shows activation during progression of the swallowing sequence (a), and (b) during water infusion into the mouth, (c) tongue movement, and (d) swallowing. (Source: Hamdy, unpublished data, with permission.)
At 1000 to 1500 milliseconds (ms) before volitional water swallowing, activation can be identified in the anterior cingulate gyrus (BA 24,25,32,33) and supplementary motor areas of both hemispheres.30 The insula and inferior frontal gyrus (BA 44) are also particularly active prior to the onset of motor swallowing activity, and there is also some simultaneous lesser activity in the posterior cingulate, middle frontal gyrus, and premotor and motor areas (BA 8,9).27, 31 Activity starts within the insula and cingulate cortex and then progresses to the frontal gyri. The activation within the cingulate cortex is relatively short and mostly prior to the swallow. It is therefore thought to be involved in the initiation of voluntary swallowing and its cognitive processing. In comparison, the activation of the frontal gyri and insula are longer lasting and continue into the motor phase of swallowing.31 It should also be noted that the activation within the insula and cingulate cortex is specific to deglutition. When it is compared with MEG during finger movements, there is no comparable activity in these areas.31 During the execution of volitional water swallows, the frontal operculum is activated along with the continued activation of the left insula, sensorimotor cortices, and integration areas.27 The pericentral gyri and inferior parietal lobule show sustained decreases in power throughout water infusion, during tongue thrusting, and at the initiation of swallowing, but this disappears immediately following the volitional phase of swallowing. During the swallow, activity moves from the caudal pericentral cortex rostrally to the superior postcentral gyri and paracentral lobule.28
The planning and processing of volitional swallowing has been studied by comparing it to reflexive swallowing, where tiny amounts of fluid are infused into the pharynx in order to directly stimulate a swallow. These swallows differ in that no cue is required and there is usually little or no oral phase to the swallow. The primary sensorimotor cortices (BA 1,2,3) become bilaterally activated prior to the volitional movements of both swallowing and tongue movement, but there is no activation prior to reflexive swallowing.27 Prior to volitional swallowing, there is also some lesser activation of the sensorimotor integration areas (BA 5,7) and primary motor cortex (BA 4). During the volitional swallowing and tongue movements, bilateral midlateral sensorimotor cortices (BA 4,3,1,2) and sensorimotor integration areas are activated, whereas during the reflexive swallowing movements there is more medial activation of the primary sensorimotor cortex.27
The degree of lateralization of activation within the primary sensorimotor cortex (BA 1,2,3) seen with MEG appears to relate to the particular movement. It is most lateralized to the left during volitional water swallows, less strongly lateralized during reflexive swallowing, and not lateralized during tongue movements.27 The areas of activation within a region may also vary depending on the task performed. Oral water infusion preferentially activates the caudolateral sensorimotor cortex, whereas volitional swallowing and tongue movement activate the superior sensorimotor cortex. Sensory input from the tongue simultaneously activates caudolateral sensorimotor and primary gustatory cortex.28
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) uses an electromagnetic field to generate electric currents in the neural tissue beneath the stimulator site. The site of stimulation is not as well localized as with direct current electrodes placed on or in the brain tissue itself, but TMS has the advantage of being noninvasive.32 Using TMS, maps can be created of the cortical areas devoted to motor representation of swallowing by plotting the areas that elicit an EMG potential in the oropharyngeal muscles. Motor excitability, another marker of the strength of the cortical motor projection, can be measured by the size of the EMG responses resulting from stimulation of these areas.
It has been shown that magnetic stimulation over the motor and premotor cortex of either cerebral hemisphere is able to produce EMG responses in the pharyngeal and esophageal muscles, suggesting that swallowing is bilaterally represented. In most people these pharyngeal responses are larger in one hemisphere than the other, and this lateralization is unrelated to handedness.33 This contrasts with the oral musculature, which is thought to be symmetrically represented.33, 34 The site of cortical control of swallowing in humans was originally localized to an area anterocaudal to the face area of primary motor cortex35 using direct electrical stimulation, and this is confirmed by magnetic stimulation studies. The mapping is also able to show the arrangement of cortical projections to the different groups of swallowing muscles. Esophageal, mylohyoid, and pharyngeal muscles are all discretely represented within the motor cortex, with mylohyoid being more anterolateral than the pharyngeal muscles, which in turn are more anterolateral than the esophageal muscles.33 If both hemispheres are stimulated almost simultaneously, then the size of the pharyngeal response is larger and the latency shorter than if either hemisphere is stimulated alone. This suggests that summation occurs, perhaps within the brainstem where input from both hemispheres could converge on shared interneurons.36
Lesional Studies: Observations from Computed Tomography and Magnetic Resonance Imaging
Strokes are able to provide useful information in the study of swallowing due to the discrete nature of the lesions in a wide variety of locations. Much work has been done trying to correlate the site of the lesions with resulting deficits, in order to try and predict the type of difficulties in swallowing that patients may present with. Originally, the only methods of determining the function of particular cerebral areas was by looking at animal studies, using direct cortical stimulation in humans, for example, during neurosurgical operations or by awaiting pathologic results following death. One of the first descriptions of stroke causing dysphagia was by Bastian37 in 1898 when he described a man who "before admission to hospital had fallen down without loss of consciousness and... at first he had difficulty in deglutition and putting out his tongue, but these symptoms passed away in a few days." On necropsy he was found to have two limited lesions within the left hemisphere. Since that time there have been long-held assumptions that swallowing has bilateral central control and that only bilateral cortical lesions or brainstem lesions were able to cause dysphagia. The advent of computed tomography (CT) and magnetic resonance imaging (MRI) has allowed non-invasive study of stroke lesions and their relationship to dysphagia, as well as allowing progress to be assessed during recovery. It is important to note, however, that many of the earlier studies relied on neurologic examination rather than imaging findings to determine lesion location,38, 39, 40 and since then many studies have used only CT or a combination of CT and MRI,41 despite the fact that MRI has been shown to be more sensitive in detecting smaller, more acute lesions.42, 43, 44 Therefore, it is possible that patients originally diagnosed as having single lesions may have also had further unidentified lesions bilaterally or at other sites within the same hemisphere.
There is great variability in the site and size of lesions that can cause swallowing problems. Most of the current data available regarding the localization of human swallowing centers are from lesion studies following cerebral injury. These provide a very varied picture of the areas that are important in addition to the primary motor areas and brainstem. For example, the thalamus and cerebellum,45 basal ganglia,45, 46 pyramidal tracts,47, 48 frontal operculum,49 and insula50 have all been associated with swallowing problems when damaged. In general, studies have failed to identify any single lesion site on MRI analysis to account for aspiration, and often lesions of a similar size and in identical locations may show different results on swallowing assessments.45 One of the more consistent findings is that the larger the lesion, the more likely a patient is to develop swallowing problems.51, 52, 53 Those with brainstem54 or posterior circulation strokes are more likely to aspirate,55 as are those with bilateral lesions.55 It has also been shown that patients with hemorrhagic stroke are significantly more likely to have swallowing problems than those with ischemic stroke, irrespective of lesion site, having an incidence of dysphagia of 49% compared with 32%.52
Traditionally swallowing difficulties following stroke have been associated with brainstem damage or bilateral cortical lesions, but later studies have shown that dysphagia is also common in patients with unilateral hemispheric lesions.40, 48, 49 The cerebral cortices, although probably the main contributor to volitional swallowing, are also associated with parts of the more automatic pharyngeal and esophageal phases. Hemispheric lesions have been noted to delay the pharyngeal phase of swallowing,46, 49 increase pharyngeal transit time,55 impair coordination of movement,46, 47, 49 and reduce peristalsis.46
Although some studies report a slightly greater proportion of left hemisphere strokes compared to right in dysphagic stroke patients, there appears to be no significant correlation between which hemisphere is damaged and the chance of dysphagia.38, 39 There has been much debate about the role of each hemisphere and there are many inconsistencies between the studies trying to associate lesion site with particular swallowing abnormalities. Some studies have associated oral phase problems with CT-identified left hemispheric lesions and impairment of the pharyngeal phase to right hemispheric lesions,47, 48, 49, 56, 57, 58 whereas others have not confirmed these findings and found no correlation between site and swallowing characteristics, suggesting that control is more complex.45, 46, 53, 54, 55, 58, 59, 60 Even very similar lesions seen on MRI can produce a variety of swallowing abnormalities.45, 55 Ischemic lesions in the territory of the left middle cerebral artery have been shown to cause impaired oral stage, problems with labial, lingual, and mandibular coordination, apraxia, and a prolonged pharyngeal transit time. Right hemispheric damage appears to significantly affect all aspects of deglutition, including all the pharyngeal-stage durations, and increase aspiration, penetration, and pharyngeal pooling.47, 48
Aspiration has been shown to occur in both right and left hemispheric lesions, but is much more likely when the damage is bilateral or involves large vessel infarcts.41, 42, 45 Some studies suggest that most middle cerebral artery territory stroke patients who aspirate have right hemisphere strokes.48 Patients most at risk of aspiration are likely to have lesions of the periventricular white matter. Isolated lesions of the periventricular white matter are uncommon in patients with no risk of aspiration.54
When right-handed patients with first ischemic strokes identified on either MRI or CT were divided into groups of either anterior or posterior middle cerebral artery territory stroke and the lesion volume was calculated, a number of differences could be seen in specific videofluoroscopic measures. Anterior lesion subjects had significantly longer swallow durations of oral transit, stage transition, pharyngeal transit, and duration to upper esophageal sphincter opening as well as increased total swallow time. Posterior lesion patients had only longer pharyngeal transit times of liquids. In general, the anterior strokes were worse than posterior, but the anterior ones also tended to have a larger lesion volume.48 Other studies of patients with hemispheric stroke identified on CT or MRI support these findings and show there is a greater incidence of anterior lesions in those with dysphagia.54 Lesions verified on MRI to be within the frontal cortex are associated with a significant risk of prolonged dysphagia and slow recovery.52
Strokes involving the insula cortex are relatively rare, but they are caused by infarction of the middle cerebral artery territory with the anterior insular predominantly supplied by the M3 division. In one study, only four of 4800 strokes were limited to the insula, and all of these were in the posterior insula, none of which developed dysphagia.61 Other work does suggest that the insular cortex is involved in swallowing, it being the most common site of unilateral hemispheric stroke in patients with dysphagia, especially prolonged dysphagia.50, 52, 59 In particular, lesions of the anterior insular identified on CT resulted in dysphagia, whereas those of the posterior insular were not associated with swallowing difficulties.50 This would fit with known connections of the anterior insula to the motor and premotor cortices, gustatory and olfactory structures, thalamus, and nucleus tractus solitarius, all of which are important in swallowing.50 Of those stroke patients demonstrated to be at risk of aspiration, 36% have lesions involving the insular cortex and 86% of those with insular lesions have dysphagia.54
Strokes isolated to the subinsular region are also rare (0.4% of strokes). Infarcts in this region have been defined on CT or MRI as a linear subinsular lesion extending parallel and subjacent to the insular cortex for at least one third of its anteroposterior extent. The subinsular component has to be continuous with the paraventricular component, and the infarct should not primarily involve the insular cortex.62 The subinsular infarcts tend to lie in a border zone between small insular penetrating arteries and branches of the lenticulostriate arteries.63 Half of patients with subinsular lesions show evidence of dysphagia on initial admission following the stroke, but the rate of spontaneous recovery is good and most have resolution of symptoms by 1 month.62 In a study of deep cerebral infarcts extending from paraventricular white matter to the subinsular region, five of eight (63%) patients had dysphagia in combination with other symptoms.63
There is little information on swallowing difficulties following strokes involving the basal ganglia, although it has been shown that the incidence of pneumonia in patients with basal ganglia infarction is more than twice that seen in hemispheric stroke patients. If bilateral involvement of the basal ganglia occurs, then the incidence of pneumonia triples.64 This is shown to be independent of patient mobility and severity of stroke. Although there are no videofluoroscopic data available in this group, the latency of oral bolus injection to swallowing time can be analyzed by measuring submental EMG responses following injection. The swallowing latencies are longest in those demonstrated to have bilateral basal ganglia lesions on CT, followed by those with unilateral basal ganglia lesions and then those with hemispheric lesions not involving the basal ganglia. The latencies are even more delayed when measured at 1 a.m.; thus it may be the case that pneumonia in basal ganglia infarcts is related to silent aspiration during the night.64
Mechanism for Dysphagia Following Hemispheric Stroke
Although it is relatively easy to appreciate the mechanisms behind dysphagia following bilateral cortical stroke or brainstem disease, the mechanism underlying dysphagia after unilateral cerebral injury, particularly after hemispheric, has remained unclear. Speculative suggestions include occult disease in the unaffected hemisphere, cerebral edema leading to pressure on the adjacent hemisphere or brainstem, and the possibility that swallowing like speech may show significant cerebral lateralization. Indeed, in a transcranial magnetic stimulation study of the projections from both hemispheres to the swallowing musculature in a large series of pure unilateral hemispheric stroke patients, of which half had dysphagia, it was observed that although stimulation of the damaged hemisphere produced little or no response in either dysphagic or nondysphagic patients, stimulation of the undamaged hemisphere evoked much larger responses in the nondysphagic than in the dysphagic subjects.34 The conclusion from this study was that the size of the hemispheric projection of the undamaged side to swallowing muscles determined the presence or absence of dysphagia, with the implication that dysphagia would occur if damage had affected the side of the brain with the largest or dominant projection. This observation supported the concept that swallowing is lateralized within the cerebral cortex.
Mechanisms of Recovery of Swallowing After Cerebral Cortex Damage
Given sufficient time, a large proportion of dysphagic stroke patients eventually recover the ability to swallow again.39 However, the mechanism for this recovery, seen in as many as 90% of the initially dysphagic stroke patients, has remained controversial. In a study of stroke using transcranial magnetic stimulation, both dysphagic and nondysphagic patients were serially mapped over several months while swallowing recovered.65 The findings of this study showed that the area of pharyngeal representation in the undamaged hemisphere increased markedly in patients who recovered, whereas there was no change in patients who had persistent dysphagia or in patients who were nondysphagic (Figure 4). Furthermore, no changes were seen in the damaged hemisphere in any of the groups of patients. These observations imply that over a period of weeks, the recovery of swallowing after stroke depends on compensatory reorganization in the undamaged hemisphere. The situation appears to differ from that in the limb muscles, where limb recovery after hemiparesis appeared more likely to result from an increase in the activity of the remaining viable cortex in the damaged hemisphere.65 In such cases, the scope for expansion of a normal connection from the undamaged part of the brain may be a limiting factor in recovery.
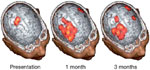
Figure 4: Transcranial magnetic stimulation (TMS) topographic maps superimposed on MRI images.
The maps show the size of pharyngeal cortical representation during recovery of a dysphagic patient from stroke. The patient had a left hemisphere cortical stroke affecting the lower sensorimotor cortex and recovered functional swallowing by 1 month. The unaffected hemisphere is shown to have an increasing representation during recovery, whereas that of the affected hemisphere exhibits little change. (Source: Hamdy et al.65 with permission from the American Gastroenterological Association.)
Pharyngeal Stimulation, the Cerebral Cortex, and Swallowing Rehabilitation After Stroke
In one study, fMRI was used to demonstrate that pharyngeal stimulation (through electrical pulses delivered by an intraluminal catheter) enhanced swallowing motor cortical excitability, and could alter the recruitment pattern of cortical activations associated with the task of swallowing.66 The same group was able to show that pharyngeal stimulation resulted in functionally stronger, bilateral, cortical (sensorimotor) activation in areas related to swallowing.
The effects of pharyngeal stimulation have been investigated in acute dysphagic stroke patients.66 The application of 10 minutes of 5 Hz of pharyngeal electrical stimulation at 75% of that maximally tolerated by the patient was used. The stimulation resulted in a long-term (60 minutes) increase in swallowing corticobulbar excitability predominantly within the undamaged, but not the damaged, hemisphere. Critically, this was strongly associated with an improvement in swallowing using videofluoroscopy, the standard marker of swallowing performance during the same time frame.66 The exciting implication from these results is that that sensory input to the human adult brain can be programmed to promote beneficial changes in plasticity that result in an improvement of GI (swallowing) function after cerebral injury. Although the more long-term (days to weeks) effects of this approach still need to be established, the observations hold great promise for future treatment strategies.
Conclusion
Swallowing is an essential GI function that is carefully regulated by the central nervous system and, in particular, by the cerebral cortex. Following cortical injury, swallowing can be dysregulated, and, in many cases, become completely dysfunctional with devastating effects for the sufferer. The application of newer neurophysiologic methods including brain functional imaging has just begun to shed light on how the cerebral cortex operates in controlling swallowing, both in health and disease. These observations also provide a window of opportunity of potential therapeutic options, including neurostimulation to rehabilitate dysfunctional swallowing, with the promise of improved clinical management of this vital function.