Key Points
- Brainstem structures forming the swallowing pattern generator (SPG) are intimately associated with the nucleus tractus solitarius (NTS). Distinct but interconnected neural circuits are involved in oral, pharyngeal, and esophageal stage swallowing.
- Oral-stage subcircuitry includes the rostral solitary complex, the rhombencephalic parvicellular reticular formation (RFpc), and its projections to trigeminal (Vm), facial (VIIm), and hypoglossal (XIIm) motor nuclei.
- Pharyngeal stage subcircuitry includes premotor neurons in the intermediate, interstitial, and ventral NTS subnuclei (NTSim, NTSis, NTSv) and motoneurons in the nucleus ambiguus.
- Esophageal stage subcircuitry includes NTSce, primary sensory vagal neurons from the esophagus and motoneurons of the compact formation of the nucleus ambiguus that innervate the striated muscle of the esophagus.
- Connections between NTS subnuclei and rhombencephalic RF enable the SPG to produce sequential inhibition and excitation of motoneurons and ensure the bilateral coordination of the swallowing "half-centers". Connections between NTS and dorsal motor nucleus of the vagus nerve (DMV) provide a mechanism for the regulation of activity in the smooth muscle portion of the esophagus and the lower esophageal sphincter. These connections also provide for functional coupling of the buccopharyngeal and esophageal stages of swallowing.
- Fast information transfer in SPG networks utilizes excitatory amino acidergic transmission via several glutamate receptor subtypes and release from tonic
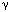 -aminobutyric acid (GABA)-ergic inhibition provides a mechanism for triggering SPG activity.
-aminobutyric acid (GABA)-ergic inhibition provides a mechanism for triggering SPG activity.
- Brainstem cholinergic mechanisms are implicated in coupling the pharyngeal and esophageal stage and in generating rhythmic or propulsive esophageal motility.
Introduction
To achieve the complex task of coordinating the activity of several dozen paired muscle groups, swallowing (deglutition) requires neural control mechanisms involving levels of the neuraxis extending from the spinal cord to the cortex. The brainstem has long been thought to harbor the central network generating the basic spatiotemporal pattern of deglutitive neuromuscular activity. As illustrated by the work of Bidder,1 Mosso,2 and Miller and Sherrington,3 much of the pioneering research on swallowing dealt with its properties as a brainstem reflex effecting bolus transport through the upper alimentary tract, along with protection of the airway and the middle ear. This view lingers on in clinical thinking to this day, but has undergone substantial evolution in contemporary experimental work.
Almost 30 years ago, Doty4 outlined a now classical but still relevant concept of the swallowing center that envisaged four salient features: (1) an intrinsic organization; (2) "peremptory" control over deglutitive motoneurons; (3) a mechanism for decoding afferent inputs; and (4) responsiveness to "central excitatory states." Thanks to recent advances in neuroanatomic, neurochemical, and pharmacologic methodology, a model of circuitry representing a structurally defined brainstem swallowing pattern generator (SPG) has evolved, opening up new avenues toward elucidation of the underlying cellular mechanisms. The term swallowing pattern generator reflects the major conceptual reorientation toward the intrinsic operations of this system as an autonomous network. Although Doty's postulates have been validated in part, the need for further refinements has become evident, particularly with regard to the organization of sensory inputs and the neurochemical correlates of central network functions.
Although autonomous in its intrinsic operations, the SPG depends on sensory input from the periphery via primary afferent neurons for both the initiation of swallowing and accommodation of the motor pattern to size, consistency, and texture of the bolus. Afferents coursing in branches of cranial nerves V, VII, IX, and X transmit low threshold mechanical (tactile, proprioceptive, tension), thermal, and chemical (gustatory) information to their brainstem relay nuclei of the trigeminal sensory and solitary complexes.5 Moreover, IXth nerve afferents from sinoaortic chemoreceptors may convey excitatory drive to the SPG6 As these second-order neurons also mediate a variety of reflexes and motor patterns other than swallowing, specific properties of the afferent signal (e.g., impulse frequency and origin in a particular peripheral structure) or their central processing must determine an appropriate and specific motor outcome (i.e., swallowing instead of retching or gagging). Remarkably, afferent signals from both the periphery and suprabulbar structures are capable of activating the SPG. Thus, a problem common to transfer of sensory information to motor networks, namely the decoding of coded neuronal information,7 as well as the integration of central commands, appears to be solved by the SPG in the same way as in other motor networks. Precisely how this task is accomplished is unknown at present; however, second-order sensory neurons, in particular in the NTS, as well as higher order solitarial and reticular neurons providing neuromodulatory inputs are thought to play a key role.
Pharmacologic approaches have provided first insights into the nature and potential diversity of chemical signals utilized by deglutitive neural circuits.5, 8 Thus, Doty's hypothesized "central excitatory states" can now be related to neuromodulatory inputs into the SPG. Certain of these involve neuronal substrates and neurotransmitter systems extrinsic to the SPG and may operate as links in deglutitive pathways descending from the forebrain and diencephalon. On the other hand, neurons intrinsic to the SPG largely depend on excitatory amino acidergic transmission for fast information transfer but are also endowed with other messenger substances acting as cotransmitters.
This review surveys the current progress made in studies of deglutitive motor control in laboratory animals, particularly rodents, as these have yielded the most detailed neuroanatomical information to date. A book chapter by Miller et al.5 , a more recent review by Jean9 and his review in this work may be consulted for broader coverage of the relevant literature and background information on suprabulbar and peripheral deglutitive pathways not dealt with here. The brainstem structures forming the SPG and its associated circuits will be considered under the broad headings of (1) neuroanatomic organization, (2) in vivo mapping studies, and (3) transmitter mechanisms.
Neuroanatomic Organization
General Features
The principal components of the SPG are defined in terms of both afferent and efferent connectivity. The former comprises primary sensory inputs that are capable of evoking either the deglutitive motor sequence (oral, pharyngeal, and esophageal stage) or secondary (bolus-induced) esophageal peristalsis; the latter represents the motoneuron pools innervating the muscle layers of the aerodigestive tube (upper alimentary tract, UAT). Each of the three successive stages—(1) bolus formation and presentation to pharynx, (2) pharyngeal propulsion and transit with airway closure, and (3) peristaltic transport through the esophagus—can be enacted quasi-independently, implying discrete neuroanatomic substrates of control or a capability of the SPG to function selectively in different subcircuits. The bilateral symmetry of afferent and efferent neural circuits imposes a functional "half-center" organization, represented on both sides of the brainstem.10
The circuit diagrams presented below should be consulted extensively so as to enable the reader to navigate with greater ease through the maze of anatomic detail. For each deglutitive stage, the underlying organization will be discussed in terms of three integral components: viscerosensory afferents (input), premotoneuronal network (interneuronal throughput), and visceromotor efferents (output). In essence, the concept of stage-specific circuits derives from evidence demonstrating that the NTS subdivisions receiving viscerosensory afferent input from the UAT maintain viscerotopically organized connections with motoneurons that innervate muscle tunics in the corresponding pharyngeal and esophageal portions of the UAT. Interneuronal throughput in these subcircuits, especially that controlling the esophagus, is simpler than initially supposed, as NTS second-order sensory neurons engage the associated motoneuron pools with monosynaptic connections, in addition to relaying in the medullary reticular formation. This direct linkage implies a mandatory (i.e., peremptory) mode of motor control by at least some components of the SPG. Remarkably, the tightness of these premotor connections progresses from one deglutitive stage to the next.
The principal pools of deglutitive motoneurons innervating striated musculature of the UAT reside in cranial nerve motor nuclei V, VII, IX/X, and XII, including the accessory portions of V and VII.5 Those providing preganglionic innervation for smooth muscle layers of the esophageal body are contained in the DMV, where they make up a rostral contingent projecting to excitatory, and a caudal group projecting to inhibitory, myenteric neurons.11, 12, 13 Neck muscle motoneurons are located in the ventral horn of cervical spinal cord segments 1 to 3, in case of the spinal accessory nucleus down to segment C7.14, 15, 16, 17 Motor axons supplying these facultative swallowing muscles reach their targets via the ansa cervicalis, accessory nerve, and short branches of cervical spinal nerves.
Motoneurons activated during deglutition participate in a wide range of other motor programs; that is, they are inherently multifunctional. At first glance, the topography of UAT special visceral efferents in the cranial motor nuclei V, VII, IX to XI, and XII does not reveal a deglutitive pattern; however, the myotopic organization and dendroarchitecture of the ambiguus complex18, 19, 20 (Figure 1) exhibit features that may facilitate integration and coordination of deglutitive motor output. The existence of presumptive gap junctions between about 30% of esophagomotor neurons in the rat nucleus ambiguus compact formation suggests an additional mechanism for synchronizing motor output.21 In addition to cholinergic markers, esophageal motoneurons of the ambigual compact formation express other putative mediators with as yet unknown functions, including calcitonin gene-related peptide (CGRP), galanin, N-acetylaspartylglutamate, and brain natriuretic peptide,22 as well as nitric oxide synthase (NOS).19
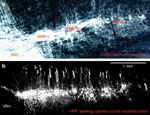
Figure 1: Cytodendroarchitecture of the rat ambiguus complex.
Immunohistochemical staining for heat shock protein 27 (HSP27, panel a) reveals contiguous columns of motoneurons that line up in an oblique sagittal plane spanning the length of the ventral rhombencephalon (photomicrograph courtesy of Dr. D.A. Hopkins). The serial reconstruction (b) shows the same neuronal groupings after retrograde labeling with horse radish peroxidase HRP. Three main divisions are recognizable: a rostral compact formation of esophagomotor neurons (AMB-c), a caudal loose formation (AMB-l) of laryngomotor neurons, and an interposed semicompact formation of chiefly pharyngomotor neurons. The AMB-sc column continues into the rostral tip of the ambiguus complex overlying the facial motor nucleus (VIIm). Note dendritic radiations and bundling. Additional ventral most cell groups (b) represent the AMB external formation of vagal preganglionic neurons. (Source: Adapted from Bieger and Hopkins,18 with permission of J. Wiley & Sons.)
Additional intricacies of esophageal motor control merit attention. Thus, all mammalian species, including rodents with a striated muscle esophagus, possess a full-length inner smooth muscle tube or tunica muscularis mucosae whose functional role remains to be clarified. The complex innervation of this structure by the myenteric plexus,22 the sympathetic nervous system,5 and spinal afferents,22, 23 along with the inferred inputs from the dorsal vagal/glossopharyngeal complex, are consistent with a supportive role in bolus transport.
Primary Afferents and Their Relay Nuclei
Transganglionic tracing studies have revealed the detailed somatotopy of primary afferent terminal fields in brainstem and spinal cord. Besides the well-known areas in the principal and spinal trigeminal nuclei, sensory neurons of the trigeminal ganglion also project to the NTS, particularly its lateral subnuclei.24, 25 Proprioceptive neurons of the mesencephalic trigeminal nucleus send their axons to the trigeminal motor nucleus (Vm) and, via Probst's bundle, densely to the rhombencephalic parvicellular reticular formation (RFpc).26, 27 Somatosensory, general and special visceral (gustatory) afferents of the IXth and Xth cranial nerves terminate in specific subnuclei of the NTS with only little overlap, and in the paratrigeminal nucleus (PTN).28, 29, 30, 31 Specifically, afferents from the soft palate, pharynx, and larynx terminate in NTSis, NTSim and in the PTN. Afferent terminals traced anterogradely from the superior laryngeal nerve (SLN), the major route of swallowing reflex afferents, are shown in Figure 2.
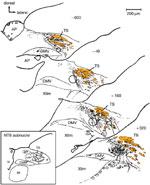
Figure 2: Subnuclear divisions of rat nucleus tractus solitarii (boxed insert) and distribution of central terminals of afferents coursing in the superior laryngeal nerve (SLN).
Besides various afferents unrelated to deglutitive function, the SLN contains the majority of swallowing reflex afferents. The intrasolitarial distribution of anterogradely labeled terminals illustrated in this set of transverse sections overlaps with that revealed after tracer injections into the pharynx, larynx or upper esophagus. Location of NTS subnucleus centralis (core portion) is marked by dotted outline. Numbers indicate anterior-posterior distance (micrometers,  m) from rostral edge of area postrema. Fascicles of the solitary tract are shown in color. AP, area postrema; DMV, dorsal motor nucleus of the vagus nerve; D, NTS, nucleus tractus solitarii; cen, central; dl, dorsolateral; gel, gelatinous; int, intermediate; is, interstitial; v, ventral, vl, ventrolateral subnucleus; D, dorsal; L, lateral; TS, tractus solitarius; IV, fourth ventricle; XII, hypoglossal motor nucleus. (Source: Adapted from Altschuler et al.,30 with permission of J. Wiley & Sons).
m) from rostral edge of area postrema. Fascicles of the solitary tract are shown in color. AP, area postrema; DMV, dorsal motor nucleus of the vagus nerve; D, NTS, nucleus tractus solitarii; cen, central; dl, dorsolateral; gel, gelatinous; int, intermediate; is, interstitial; v, ventral, vl, ventrolateral subnucleus; D, dorsal; L, lateral; TS, tractus solitarius; IV, fourth ventricle; XII, hypoglossal motor nucleus. (Source: Adapted from Altschuler et al.,30 with permission of J. Wiley & Sons).
Esophageal afferents are clustered in the NTSce.30 In general, afferents from cervical segments are represented rostral to those from thoracic and abdominal levels. Mucosal afferents from the upper cervical esophagus travel via the SLN to terminate in NTSis and NTSim, whereas muscular afferents coursing in the recurrent laryngeal nerve (RLN) reach the NTSce, similar to their counterparts from thoracic and abdominal esophagus.32 The RLN also contains some afferents terminating in NTSis and NTSim, which may originate in the trachea.33
Although there are monosynaptic connections between vagal primary afferents and general visceral efferent neurons of the dorsal motor nucleus of the vagus nerve,34, 35, 36 it is unknown if these include also esophageal or other swallowing-relevant afferents.
Functional studies in arterially perfused rat brainstem preparations suggest that stimulation of sinoaortic IXth nerve chemoreceptor afferents can also elicit swallowing. This response may be mediated by neurons in the NTSim that receive convergent inputs from both pharyngoesophageal and chemoreceptor afferents.6, 37 At first glance, this finding may be surprising, unless one takes into account that such a mechanism may constitute a defensive reflex for safeguarding airway patency.
Ultrastructural studies demonstrate that terminals of afferents from the esophagus and the larynx form asymmetric synapses on dendrites of second-order neurons in the NTSce and NTSis.38, 39 They may contact several dendrites, thus forming synaptic glomeruli. Primary afferents utilize glutamate as their main transmitter but apparently do not release nitric oxide (NO) from their central terminals. However, they often synapse on dendrites of nitrergic second-order neurons.40 The NTSce neurons comprising the core region express neuronal nitric oxide synthase nNOS, leu-enkephalin, and somatostatin22, 41, 42 and, based on these markers, fall into different subpopulations. Noradrenergic neurons are found in its shell region.43
The paucity of inhibitory synapses within the NTSce region would seem counterintuitive in light of functional evidence pointing to the existence of inhibitory processes operating at this level.44 Moreover, the identification in NTSce neurons of 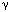 -aminobutyric acid (GABA)
-aminobutyric acid (GABA)  1-messenger RNA (mRNA) subunit appears relevant in this context.45
1-messenger RNA (mRNA) subunit appears relevant in this context.45
As noted above, connections between vagal second-order sensory neurons in the NTS and motoneurons are particularly tight in the case of ambigual neurons innervating muscles of the pharynx, larynx, and esophagus, the former two groups receiving monosynaptic inputs from interstitial and intermediate subnuclei and the latter from the central subnucleus. Ultrastructural analysis of synaptic contacts in the compact, semicompact, and loose formations of the nucleus ambiguus, partly combined with tracer injections into the nucleus tractus solitarii, has revealed significant differences in the location of synapses on perikarya and dendrites, respectively, and types of synaptic vesicle.46, 47, 48, 49, 50 In the compact formation, almost all synapses are found on dendrites and contain small round clear vesicles (Gray's type I, presumptive excitatory). Pharyngeal and laryngeal motoneurons in the semicompact and loose formations, respectively, receive Gray's type I and type II (symmetric, pleomorphic vesicles, presumptive inhibitory) at about equal numbers on both dendrites and somata. Synapses identified by anterograde tracing from the NTSce, NTSim, and NTSis concur with this general pattern. Solitario-ambigual neurons project also to small and medium-size non–motoneurons of the nucleus ambiguus.47, 48 Some of these neurons may project back to the NTS.26, 51 Thus, premotor neuronal structures have mainly excitatory influences on the esophagus, whereas those for the pharynx and larynx are compatible with balanced excitatory/inhibitory inputs.
Moreover, there are significant projections from trigeminal (spinal and mesencephalic nuclei) and solitary complexes to the rhombencephalic RFpc,26, 52, 53 including Probst's nucleus and the intermediate reticular formation. It is reasonable to assume that premotor neurons in these reticular nuclei participate in afferent decoding and information processing.
Except for a few aberrant trigeminal and vagal fibers, primary afferents do not appear to project to the (rostral) reticular formation. However, second-order sensory projections from the NTS, spinal V, and paratrigeminal nucleus54 reach the parabrachial area. In particular, the NTSim, but not the NTSce, sends a substantial projection to the parabrachial complex.55 This pathway may account for the swallowing-associated activity described in the sheep dorsal pons9 (see Pontine Interneurons, below).
Oral-Stage Subcircuit
Consisting of volitional and reflexive components (preparation, formation, and lingual propulsion of bolus), the oral stage5 presents a less well identified motor pattern as compared with the subsequent stages of swallowing. The network responsible for coordinating the oral stage provides integration with circuitry controlling mastication or drinking. Neuroanatomic evidence detailed below suggests a highly complex connectivity that overlaps only in some aspects with subcircuits thought to control the subsequent parts of the deglutitive sequence.
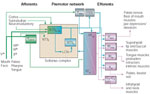
A proposed circuit model of the oral stage network is presented in Figure 3. As revealed by combined retrograde and anterograde tract tracing in the rat,52 the presumptive components of this subcircuit comprise portions of the rostral solitary complex, the rhombencephalic parvicellular reticular formation (RFpc), and its projections to trigeminal (Vm), facial (VIIm), and hypoglossal (XIIm) motor nuclei. The RFpc subjacent to the dorsal vagal complex and lateral to the hypoglossal motor nucleus is implicated in deglutitive control because this region contains neurons that are active during the pharyngeal stage of deglutition (see below) and are likely to receive afferents from parts of the NTS receiving sensory inputs from the oral cavity.52 The RFpc at the level of the rhombencephalon forms a sheet-like continuum extending in a ventro-rostro-lateral orientation toward the ventral vagal complex. Ventromedially it is flanked by the intermediate parvicellular reticular zone (RFiz), which includes a group of cholinergic neurons located just dorsomedial to the compact formation of the nucleus ambiguus.56, 57
Figure 3: Proposed network circuit controlling the oral stage of swallowing.
C1–C3 – cervical spinal cord first to third segments; LCN – local circuit neuron; NTS – Nucleus tractus solitarii; NTSis – interstitial subnucleus; NTSint – intermediate subnucleus; NTS1 – lateral subnucleus; NTSv - ventral subnucleus; PBN – parabrachial nuclei; RFiz – reticular formation intermediate zone; RFpc – reticular formation, pars parvicellularis; VH – ventral horn; Vth – trigeminal nerve; Vm/a – motor/accessory nucleus of trigeminal nerve; Vmes – mesencephalic nucleus of trigeminal nerve; Vsens – sensory nuclei of trigeminal nerve; VIIth – facial nerve; VIIm/a – motor/accessory nucleus of facial nerve; IXth -glossopharyngeal nerve; Xth – vagus nerve; Xm – nucleus ambiguus;
The projections from the RFpc52 are bilateral and distributed preferentially to (1) jaw opener motoneurons in ventral Vm, (2) facial motoneurons in the dorsal intermediate subdivision innervating deep oral musculature (stylohyoid, posterior digastric), and (3) both protruder and retractor motoneurons in XIIm (ventral and dorsal subdivision, respectively). A mainly crossed projection to jaw closer motoneurons (dorsal division of Vm) originates from a group of large multipolar cells embedded in the RFpc, corresponding to the nucleus of Probst's tract. A defining feature of the RFpc neurons may be the formation of axon collaterals.58, 59, 60, 61
The inferred role in the control of oral-stage swallowing activity of connections between the RFpc and the rostral NTS62 remains to be established. The same holds true for the presumed linkage with NTS subnuclei believed to form part of the SPG proper.
In the rat, Vm, VIIm, and XIIm also receive input from the NTS. However, these solitarial efferents, unlike those to the ambiguus complex, are relatively sparse,52, 61 although a preferential distribution to Vm jaw opener and accessory VIIm neurons is evident. In contrast, in the sheep, such connections are believed to be absent.9 Instead, groups of interneurons in the ventral medullary reticular formation (putative "switching neurons" of Jean) are thought to operate as links between deglutitive NTS interneurons and motor neuron pools of the rostral rhombencephalon other than those projecting via the glossopharyngeal and vagus nerves. Anterograde52, 63, 64 and retrograde51, 52, 65 tracing studies in the rat implicate the periambigual parvicellular reticular formation as the presumptive location of ventral "switching" interneurons. A putative function of these premotor elements is coordination of deglutitive motor output within and between each half of the medulla.
Pharyngeal Stage Subcircuit
(Figure 4)
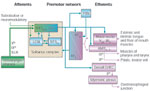
Figure 4: Proposed network circuit controlling the pharyngeal stage of swallowing.
AMBsc,l – semicompact and loose formation of nucleus ambiguus; GVEC – general visceral efferent column; LCN – local circuit neurons; NTS – nucleus tractus solitarii; NTSce – central subnucleus; NTSint/is – intermediate and interstitial subnuclei; NTSv – ventral subnucleus; PBN – parabrachial nuclei; RFiz – reticular formation intermediate zone; RFpc – reticular formation pars parvicellularis; SLN – superior laryngeal nerve; V(a) – accessory motor nucleus of trigeminal nerve; IXth – glossopharyngeal nerve; Xth – vagus nerve; XIIm – hypoglossal nucleus
Based on anterograde52, 63, 64 and retrograde transsynaptic66, 67 tracing experiments, the solitarial interneurons projecting to buccopharyngeal stage motoneurons, that is, pharyngeal and laryngeal motoneurons in the nucleus ambiguus, have been located in the intermediate, interstitial, and ventral NTS subnuclei (NTSim, NTSis, NTSv). Neurons in the NTSim are thought to provide premotor input to pharyngeal constrictor motoneurons in the semicompact formation18 of the nucleus ambiguus. In addition, solitarial projections to facial, trigeminal, and hypoglossal motoneurons have been described in the rat (see above). Retrograde transneuronal tracer studies using pseudorabies virus injections into rat pharyngeal muscles do not make specific mention of ventral reticular formation neurons.66 It remains to be determined if all three NTS subnuclei play overlapping or distinct roles in deglutitive motor programming. For instance, chemostimulation maps (see Mapping Studies, below) do not unequivocally include the interstitial subnucleus as a site where swallowing responses can be triggered. Remarkably also, the NTSv is poorly supplied with UAT primary afferents.30
Interconnections between these NTS subnuclei and the rhombencephalic RF probably enable the DMPG to produce sequential inhibition and excitation of motoneurons and to ensure the bilateral coordination of the swallowing "half-centers." Connections, via NTSce or directly, with the dorsal general visceral efferent column of parasympathetic preganglionic neurons would represent the central link for swallowing-induced relaxation of the gastroesophageal sphincter,68 sometimes regarded as the final stage of the swallowing sequence.10
Esophageal Stage Subcircuit
(Figure 5)
Figure 5: Proposed network circuit controlling the esophageal stage of swallowing.
Ach – acetylcholine; AMBc – compact formation of nucleus ambiguus; GABA - 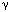 -aminobutyric acid; GEJ – gastroesophageal junction; IGLEs – intraganglionic laminar endings; LCNs – local circuit neurons; LMM – lamina muscularis mucosae; NTS – nucleus tractus solitarii; NTSce – central subnucleus; NTSint/is – intermediate and interstitial subnucleus; RFiz – reticular formation intermediate zone; RLN – recurrent laryngeal nerve; SLN – superior laryngeal nerve; TMP – tunica muscularis propria; Xth – vagus nerve
-aminobutyric acid; GEJ – gastroesophageal junction; IGLEs – intraganglionic laminar endings; LCNs – local circuit neurons; LMM – lamina muscularis mucosae; NTS – nucleus tractus solitarii; NTSce – central subnucleus; NTSint/is – intermediate and interstitial subnucleus; RFiz – reticular formation intermediate zone; RLN – recurrent laryngeal nerve; SLN – superior laryngeal nerve; TMP – tunica muscularis propria; Xth – vagus nerve
Because esophageal peristalsis is held in abeyance during rapidly repeated oropharyngeal stage activity, or occurs independently, as in secondary (bolus-induced) peristalsis, it must be governed by a separate neural control circuit. Evidence from retrograde and anterograde neuroanatomic pathway tracing studies supports this basic concept. Thus, the NTS subnucleus centralis receives a dense terminal projection from primary sensory vagal neurons innervating low-threshold mechanoreceptors in the esophagus30 and, in turn, sends a massive projection to motoneurons of the compact formation of the nucleus ambiguus,41, 51, 67, 69 the source of special visceral efferents to the striated muscle tunic of the esophagus.18 By comparison, projections from esophageal response loci in the NTS central subnucleus to the medullary reticular formation seem relatively sparse,63, 64 in agreement with anatomic anterograde tracing studies.52 However, collateral projections to the RFiz were noted in another study using neurobiotin for extra- or juxtacellular labeling of functionally identified NTSce neurons.12
The crucial question of whether the esophageal and the buccopharyngeal subcircuits are synaptically linked appears to have been resolved by viral tracing studies that demonstrate a projection from intermediate and interstitial subnuclei to the central subnucleus.20, 67 However, the extent or strength of this linkage remains unclear. Thus, functional coupling between the buccopharyngeal and esophageal stages of swallowing may involve additional groups of NTS interneurons. Among these, cholinergic and GABA-ergic neurons are prime candidates (see Transmitter Mechanisms, below). In the cat, a newly defined NTS subnucleus (see c-fos/Fos Mapping, below) is now proposed as another possibility.70 It is again worth noting that, in viral transneuronal retrograde tracing studies,67 NTSce neurons are visualized before neurons in the medullary RFpc and other brainstem structures (nuclei of the spinal trigeminal tract, area postrema, locus ceruleus, subceruleus area, raphe nuclei, and midbrain central gray).
At present, information is incomplete regarding the connections between the solitarial subnuclei and vagal preganglionic neurons that control the motility of UAT smooth musculature. A group of noradrenergic neurons forming the shell region of the NTSce has been implicated in esophagogastric reflex paths that trigger gastric relaxation during esophageal distension. These neurons reportedly send extensive projections to preganglionic neurons in the dorsal motor nucleus of the vagus nerve,12, 43 the source of general visceral efferents to UAT smooth musculature.
However, another anterograde tracing study of NTS efferents in the rat52 suggests a paucity of connections with the DMV, but in view of contrary evidence from functional mapping studies,12, 43, 64 it seems reasonable to infer that the sites selected for tracer deposits missed some of the critical NTS subnuclei harboring deglutitive premotor neurons, including esophageal premotor neurons of the NTSce. This conclusion is further strengthened by data from "microchemostimulation" mapping studies (see Mapping Studies, below).
The current view of solitarial neurons as internuncial elements linking sensory input from and motoneuronal output to the UAT is no doubt somewhat simplistic. For example, intracellular staining of neurons in the NTS subnuclei intermedialis and centralis (Lu71; D.Bieger, unpublished observations) shows that, apart from their main axons projecting to the ambiguus complex, cells in these regions emit multiple axon collaterals that form extensive intrasolitary projections. Similarly, Rogers et al.12, 43 have demonstrated the extensive dendritic arborizations of NTSce cells, which reach the ependyma overlying the NTS. In addition, other connections include crossed intersolitary projections,63 involving NTS subnuclei other than those receiving vagal input from the UAT, thereby forming additional local circuits. It is tempting to speculate that these intrasolitary networks play a role in coupling of the deglutitive stages, as well as in swallow-induced (deglutitive) and bolus-induced (distal) esophageal inhibition. Projections to the rostral parts of the general visceral efferent column GVEC of VIIth, IXth, and Xth nerve parasympathetic preganglionic neurons would provide a possible mechanism for coordinated activation of salivary and UAT mucous glands.
Functional Maps of Brainstem Deglutitive Pathways
Mapping of swallow-activated neuronal activity has been achieved by various means, including in vivo microelectrode extracellular recording, as well as by Fos immunocytochemistry. Deglutitive neural substrates have also been delineated by means of chemical microstimulation; for pertinent evidence see Transmitter Mechanisms, below. Apart from differences in sensitivity and specificity, these techniques have in common at least three limitations or caveats that need to be taken into account: (1) Neurons displaying activity during any particular stage of the swallowing sequence may not necessarily be involved in generating the deglutitive motor program but instead act as relays to other neural circuits serving different functions (e.g., breathing, coughing, vomiting, retching, as well as a variety of associated esophageal reflexes). (2) Deglutitive activation may be due to reafferent signals from the aerodigestive tube. (3) Metabolic neuronal markers such as the c-fos signal cannot be detected in neurons undergoing synaptic inhibition, a process likely to play a significant role in deglutitive motor programming.
Microelectrode Studies
For detailed information on the functional typology and firing characteristics of dorsal and ventral swallowing group (DSG and VSG) neurons the reader should consult the review by Jean. Here, only the most salient points will be covered. The majority of data on swallowing-related neurons in the mammalian brainstem come from extracellular recordings obtained in anesthetized or decerebrated animals,5, 9 including mainly the sheep, as well as the cat, rat, dog, and monkey. Intracellular recordings have been reported only recently and deal mainly with motoneurons (see below), except for two studies describing activity of interneurons in the cat72 and rat NTS.6 The most commonly used method for eliciting deglutition in these investigations is electrical stimulation of the SLN, resulting in reflexive activity involving chiefly the pharyngeal stage, with variable participation (or sometimes inhibition) of the esophageal stage. Based on these data, the preeminent role of the NTS and the periambigual reticular formation in deglutitive motor pattern generation was first established,9 thus providing a frame of reference for subsequent neuroanatomical investigations.
General features of swallowing neurons include (1) usual absence of a tonic (or resting) discharge and (2) a spike burst temporally correlated with deglutitive motor output. Other deglutitive neurons may show an accelerated resting discharge or phasic inhibition coinciding with swallow. Deglutitive neurons are furthermore excited by distention of that part of the UAT that contracts in phase with the swallowing neuronal discharge. Their general localization in the medulla oblongata to some extent overlaps with neurons involved in cardiorespiratory regulation.37
Interneurons
These elements are characterized by two criteria: lack of antidromic activation with electrical stimulation of appropriate motor nerves, and a high spike rate.9 Their two principal locations are (1) the NTS and subjacent RFpc; and (2) the ventrolateral reticular formation adjoining the nucleus ambiguus, representing the DSG and VSG of neurophysiologists. Additional loci are found in the immediate vicinity of motor nuclei of cranial nerves V, VII, and XII and of the principal sensory nucleus of the trigeminal nerve.
Specific NTS subnuclear localizations of DSG neurons were not determined in the original studies.9, 73 However, their location in general overlaps with that of NTSis, NTSim, NTSv, and adjacent NTSvl. Burst discharges persist in the presence of neuromuscular paralysis (curarization), implying their central origin. Presumptive DSG interneurons of the rat NTS exhibit a predominantly excitatory convergence of inputs from pharyngoesophageal mechanoreceptors and IXth nerve sinoaortic chemoreceptors.6
The DSG neurons related to the esophageal stage of swallowing in the rat make up the main portion of the NTSce. More is known about their behavior during secondary peristalsis evoked by esophageal distention12, 44, 74, 75 than during swallowing. Reflecting the potential diversity or complexity of the esophageal premotor control, responses to localized esophageal balloon distention reveal that at least three different types of neurons can be distinguished in the NTSce region.44 One of these types would qualify as a local circuit neuron generating distal inhibition in the esophageal body or the stomach; the other types show either rhythmic or nonrhythmic discharges temporally correlated with distention-induced esophagomotor activity.
The VSG neurons have thus far only been identified for the oropharyngeal stage of swallowing. Compared with DSG neurons, they display longer synaptic latencies and lower instantaneous spike rates but have similar firing characteristics.9, 76 Deglutitive interneurons are also located in or near trigeminal and hypoglossal motor nuclei.
Pontine Interneurons
Pontine interneurons are located in a region extending "rostrally to the VIIth nerve exit, above Vm at the level of the trigeminal principal sensory nucleus."9 Upon SLN stimulation, these spontaneously active cells produce a short-latency [1.5—4 millisecond (ms)] synaptic response. However, unlike that of DSG or VSG neurons, the deglutitive discharge of these cells is abolished by neuromuscular paralysis and, hence, likely to represent a response to sensory feedback from oropharyngeal receptors. Because these neurons can be antidromically driven from the ventral posteromedial nucleus of the thalamus, they would appear to form part of an ascending sensory pathway relaying in the parabrachial complex.54, 55
Motoneurons
Information on the deglutitive activity of motoneurons comes mostly from extracellular recording experiments in sheep and rat.9, 73 Motoneurons active during the pharyngeal stage produce low-frequency spike bursts at durations in the range of 50 to 200 ms and lack spontaneous activity. Short-latency (7–12 ms) activation is seen in pharyngeal stage motoneurons on stimulation of the ipsilateral afferent fibers in the SLN or IXth nerve, implying mediation via oligosynaptic pathways. In sheep, XIIm neurons produce deglutitive burst discharges at spike frequencies of 10 to 70 Hz, but only a minority of cells appears to receive oligosynaptic input via the SLN or lingual nerve.77
Firing characteristics of esophageal stage motoneurons have been studied by (1) recording from the nucleus ambiguus,9 from which efferents to esophageal striated muscle arise; and (2) by means of single-fiber unit recordings from preganglionic vagal axons78, 79 supplying the smooth muscle portions of the esophagus. Burst discharges of nucleus ambiguus neurons exhibit a long duration (150—800 ms) with a low spike rate (10—40 Hz). Both parameters seem to correlate with the timing of the discharge; with increasing delay, burst duration lengthens and spike frequency decreases. In contrast, preganglionic fibers from the DMV to esophageal smooth muscle have discharge rates of 3 to 8 Hz with burst durations in the range of 1 s. Rat DMV neurons are either excited or inhibited by esophageal distention, depending on their localization12 (medial-rostral: inhibition; laterocaudal: excitation).
Intracellular recordings in the sheep rostral nucleus ambiguus have revealed a marked hyperpolarization of the membrane potential preceding the deglutitive spike burst; the apparent synaptic inhibition coincides with the oropharyngeal stage.80 Intracellular recordings in cat XIIm styloglossal (tongue retractor) and genioglossal (tongue protruder) motoneurons81 demonstrated simple excitatory postsynaptic potential (EPSPs) and complex EPSP–inhibitory postsynaptic potential (IPSP) sequences, respectively, during deglutition. Corresponding evidence for a centrally coordinated synaptic inhibition was first obtained in electromyography (EMG) studies showing a cessation of activity in certain "leading complex" motoneuronal pools preceding and following the deglutitive discharge ("bracketing inhibition").10 However, this inhibition does not involve all pharyngeal stage motoneurons.
c-fos/Fos Mapping
At present, the number of studies designed to visualize the deglutitive neural network by means of this metabolic marker technique is limited, and the data reported in the rat,82 mouse,83 and cat70 do not present a coherent picture. Similar problems, inconsistencies, or apparent lack of specificity are found in studies of other functions (e.g., cardiovascular systems). The first study using this method82 was carried out in ketamine/xylazine-anesthetized rats (n = 3) subjected to prolonged stimulation of the recurrent laryngeal nerve. Based on this report, putative swallow-associated cell activation in the rhombencephalic reticular formation and solitary complex exhibits a rather poorly defined diffuse pattern. Moreover, the c-fos signal was absent in neurons supplying efferents to UAT striated or smooth musculature. These data conflict with neurophysiologic evidence and pharmacologic investigations considered below. It is thus doubtful that they can be interpreted as confirming earlier localizations of a "swallowing center" in the rostroventral medullary reticular formation,10, 84 which thus far has eluded detection by microelectrode recording.
Solitarius Complex
Pharyngeal stage–associated increases in Fos-positive neurons were reported in the feline70NTSim, NTSis, and NTSvm, but appeared to be absent in NTSv and NTSvl. As already noted, the latter two display deglutitive neural activity in sheep and rat. In the mouse, pharyngeal stage–associated elevations in c-fos expression were essentially confined to the NTSis and NTSim.83 Conversely, in the cat,70 esophageal stage–associated c-fos elevations occurred in more widespread subnuclear divisions of the NTS (NTSce, NTSv, NTSdl, and NTSvl) than would be expected on the basis of either extracellular recording studies in rats and sheep or tract tracing studies in the rat (cf. Neuroanatomic Organization, above). In contrast, c-fos studies in the mouse83 suggest a more restricted distribution of swallow- or esophageal stage–activated neurons in the interstitial, intermediate, and central subnuclei, even though the experimental protocol entailed electrical stimulation of the SLN, a procedure expected to activate also various vagal afferents involved in functions other than deglutition. Activation of the NTSce core region was not evident at intensities of SLN stimulation below the threshold for elicitation of primary peristalsis. In the rat,82 putative swallow-activated neurons showed a widespread relatively sparse distribution without a clear subnuclear pattern. In addition, both control and experimental animals exhibited activity in NTS areas overlapping rostrally with the subnucleus centralis, but erroneously identified as such at more caudal NTS levels. In rats subjected to repetitive esophageal distention, a c-fos signal was reported in group CA-2 noradrenergic neurons surrounding the NTSce and, to a lesser extent, in NTSce core neurons negative for nNOS.43 A more diffuse activation was present in adjacent NTS subnuclei.43
Dorsal Motor Nucleus of the Vagus Nerve (DMV)
Pharyngeal stage–associated c-fos elevations were noted in the cat throughout the rostrocaudal extent of the nucleus, with preponderance in the dorsal aspect of the DMV and preferential involvement of small-sized presumptive interneurons.70 Similarly, in the mouse,83 neurons activated by SLN electrical stimulation were located in both rostral and caudal parts of the nucleus; however, rostrally activation occurred only at a stimulus frequency sufficient to elicit swallowing. No data were reported in the rat studies.82
Ambiguus Complex
Evidence of deglutitive c-fos activation was found in the dorsal subdivision of the cat nucleus ambiguus in association with pharyngeal stage activity, and in the ventral subdivision (at both caudal and rostral levels) following prolonged esophageal stage activity.70 Direct interspecies comparison of these viscerotopic patterns is hampered by the divergent nomenclature applied to the nucleus ambiguus subdivisions in the cat and rat or mouse. However, it seems reasonable to infer that the feline ventral subdivision (so-called retrofacial nucleus) corresponds to the esophagomotor compact division of rodents, whereas the pharyngomotor semicompact subdivision of rodents is the homologue of the dorsal subdivision in the cat (cf. Grélot et al.85). The pattern of Fos-activated neurons reported in the mouse ambiguus complex83 agrees with the viscerotopic representation of the upper alimentary tract motoneurons revealed by neuroanatomic tracer studies. In the rat,82 presumptive swallow-activated cells had a localization overlapping with that of the external formation of the nucleus ambiguus and the ventral respiratory group.
Rhombencephalic Reticular Formation (RF)
As evidenced by c-fos activation following repetitive swallowing activity in the mouse,83 the RFpc of the medulla oblongata would appear to receive deglutitive input through the appropriate NTS subnuclei. In the rat,82 a more widespread distribution of activated cells appeared to be evident in the RFpc, RFiz, periambigual RF, and centromedial RFgc. It should be noted, however, that "chemomicrostimulation" maps based on the use of excitatory amino acids in the rat do not support a role of neurons in these regions in generating deglutitive motor output (see next section).
Transmitter Mechanisms
Recent neuropharmacologic advances have provided a plethora of tools for probing the SPG, including agonists and antagonists acting at receptors for neurotransmitters or neuromediators. As evidenced by the elicitation of rhythmic (repetitive) swallowing in the anesthetized rat,8 the SPG responds with sustained excitation to a diversity of centrally acting pharmacologic stimuli. The stimulant efficacy of certain receptor agonists applied systemically or intraarterially approaches that of electrical stimulation of the superior laryngeal nerve, the chief afferent route for elicitation of the swallowing reflex. In particular, "fictive" swallowing ensues upon localized chemostimulation of NTS areas receiving deglutitive reflex afferent input. Because primary afferent vagal input in both deglutitive subcircuits most probably utilizes glutamatergic transmission,75, 86, 87, 88 the effects of excitatory amino acids such as glutamate and certain of its analogues [kainate, quisqualate, and N-methyl-D-aspartate (NMDA)] should mimic those of glutamate (or aspartate) released endogenously either from peripheral vagal reflex afferent terminals in the solitarius complex or central afferents originating from rostral levels of the neuraxis (e.g., basal forebrain, hypothalamus, cortex). On the other hand, the excitatory actions of the monoamines and certain peptides would point to the involvement of suprabulbar neuromodulatory inputs converging on the SPG. Neuromodulatory inputs such as those mediated by serotonin and noradrenaline may operate at the NTS level to regulate the gain of synaptic responses to primary afferent input.8, 12
Excitatory Amino Acids (EAA)
Deglutitive excitant effects are obtained with EAA agonists acting at kainate,  -amino-3-hydroxy-5-methyl-4-isoxazole propionic acid AMPA, NMDA and the metabotropic receptor subtypes.86, 89, 90, 91, 92, 93 Thus, all types of glutamate (EAA) receptor agonists appear capable of exciting the SPG at the NTS level. Because of their discrete neuroanatomic localization, organized nature, and potential rhythmicity, these responses have yielded important insights into the mode of operation of the SPG.
-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid AMPA, NMDA and the metabotropic receptor subtypes.86, 89, 90, 91, 92, 93 Thus, all types of glutamate (EAA) receptor agonists appear capable of exciting the SPG at the NTS level. Because of their discrete neuroanatomic localization, organized nature, and potential rhythmicity, these responses have yielded important insights into the mode of operation of the SPG.
Mapping Studies
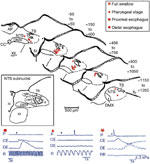
Due to their high efficacy, EAA agonists, including glutamate itself, can be used for constructing chemomicrostimulation maps of deglutitive functional organization within the solitarius complex (Figure 6). Depending on the NTS locus being stimulated, a diversity of responses can be elicited, including the complete sequence, its pharyngeal or esophageal components, or fractions of the latter.64, 89, 90, 93 In keeping with electrophysiologic evidence,73 deglutitive response loci controlling the complete swallowing sequence or its buccopharyngeal stage are clustered in an area coextensive with the intermediate and ventral subnuclei of the NTS.89, 90 Other work,94 employing L-glutamate pulses in 100 to 500 times larger ejectate volumes, reported swallow response loci extending laterally into the interstitial and ventrolateral subnuclei. Esophageal response loci are clustered in the NTSce,75, 89, 90 with sites mediating relaxation of the gastroesophageal junction lying in close proximity.93 These observations strengthen the idea that the DMPG network consists of spatially discrete, hierarchically organized modules.
Figure 6: Map of deglutitive response loci in the rat NTS as determined by pressure pulse microejection of L-glutamate or excitatory amino acid agonists.
Sites included in this analysis typically yield stable highly reproducible responses with repeated stimulation and are distinctly localized. In the rostrocaudal series of transverse sections through the NTS, the distance from the obex is indicated in micrometers ( m). The shaded region outlines the caudal dense portion of the central subnucleus.
m). The shaded region outlines the caudal dense portion of the central subnucleus.
Representative examples of swallowing response types (below, left to right) elicited by glutamate chemomicrostimulation (downward blue arrowheads). Manometric traces show intraluminal pressure signals recorded from pharynx (P), cervical esophagus (CE), and distal esophagus (DE), along with intratracheal pressure (R) or respiratory rate (RR). ( ) An isolated propagated pressure wave in esophagus evoked from midportion of central subnucleus. (
) An isolated propagated pressure wave in esophagus evoked from midportion of central subnucleus. ( ) Nonpropagated pharyngeal response evoked from NTSim/v region. (*) A complete swallow sequence triggered from site in ventral NTSim (all traces courtesy of M.A. Hashim). (Source: Adapted from Bieger,8 with permission from Springer Science and Business Media.)
) Nonpropagated pharyngeal response evoked from NTSim/v region. (*) A complete swallow sequence triggered from site in ventral NTSim (all traces courtesy of M.A. Hashim). (Source: Adapted from Bieger,8 with permission from Springer Science and Business Media.)
Focal application of EAA agonists to the rat rostral nucleus ambiguus complex and adjacent ventral reticular formation typically elicits a single contraction of the esophagus, most probably reflecting direct excitation of motoneurons. Depending on the intraambigual site, the response may be stationary, more or less restricted to a particular segment, or propulsive and accompanied by a transient pharyngeal relaxation.89, 95 The latter features suggest involvement of ventral swallowing group interneurons closely adjacent to motoneurons.
Interestingly, esophagomotor responses produced by application of ionotropic excitatory amino acid agonists to the NTSce region are nonrhythmic in nature,90 even though afferent stimulation at the level of the distal esophagus may result in rhythmic peristalsis.44, 74 The NTSce neurons have been shown to express mRNA for the NMDA-1 receptor.96 The EAA agonist potency rank orders90 are consistent with a prominent role for this receptor type in generating NTSce neuronal responses to synaptic input from peripheral afferents.75
However, some evidence suggests that activation of metabotropic glutamate receptors (mGluRs) produces rhythmic responses.92 This difference would be explained if afferent input via ionotropic EAA receptors were to initiate activation of higher order sensory interneurons, in particular cholinergic afferents to the NTS originating in the RFiz or the NTs itself, which would permit or facilitate repetitive activity of NTSce neurons. Alternatively, nonspecific EAA receptor stimulation may cause collateral activation of other transmitter systems with inhibitory (e.g., GABA91) or as yet undefined (e.g., neurokinins97) actions. Presynaptic mGluRs differentially modulate release of GABA at NTS second-order sensory neurons.98
 -Aminobutyric Acid
-Aminobutyric Acid
Studies using immunohistochemistry for glutamic acid decarboxylase (GAD), GABA, glycine, and, more recently, in situ hybridization for GAD67 and glycine transporter-2 have revealed widespread distribution of GABA-ergic neuronal cell bodies and terminals, which often colocalize glycine in the NTS of rat, cat, rabbit, and sheep.99, 100, 101, 102, 103, 104 Most of the immunoreactive neurons were found in subnuclei surrounding the solitary tract and in NTSis. Ultrastructural examination demonstrated mostly symmetric axodendritic synapses and the rare axoaxonic contact.
Enhancement of GABA-ergic transmission by systemic pharmacologic means results in inhibition of reflex deglutition in the anesthetized cat, an action persisting after decerebration and reversed by GABA antagonists.105 In the rat, localized blockade of NTS GABAA receptors leads to overt excitation of the SPG. This mechanism of disinhibition accounts for the deglutitive stimulant action of the GABAA receptor blocker bicuculline and GABA chloride channel blocker picrotoxin.44, 91 The underlying process of autoexcitation appears to involve NMDA receptors and cholinergic input to the NTSce. Thus, in subthreshold amounts delivered to this region, bicuculline converts S-glutamate-induced monophasic pharyngeal or esophageal responses into rhythmic ones, and promotes primary peristalsis.91 With suprathreshold amounts, peristalsis-like activity ensues that is abolished by blockade of NTS muscarinic acetylcholine receptors.91
Evidently, therefore, local GABA-ergic control provides a tonic background inhibition of the SPG that maintains both pharyngeal and esophageal stage premotor neurons in a quiescent state. Removal of this inhibition leads to sustained autoexcitation, with resultant rhythmic patterned motor output under control by these premotor neurons. It seems reasonable to infer that the underlying processes entail pacemaker-like oscillations of membrane potential in the constituent neuronal population.43, 106 In pharyngeal stage interneurons these are probably driven by NMDA receptors, and in esophageal stage interneurons by coactivation of NMDA and muscarinic acetylcholine receptors.71, 93
Furthermore, a GABAA receptor–mediated mechanism has been shown to operate at the NTS level in distal inhibition of the rat esophagus,44 that is, the cessation of peristalsis in aboral segments triggered by distention of the proximal esophagus.
Acetylcholine
Cholinergic transmission is required not only for translating motoneuronal impulses into deglutitive muscle activity but also for generating or shaping the central motor pattern of esophageal peristalsis. In the rat, both muscarinic (mAChR) and nicotinic receptors have been implicated, the former in NTS premotor control and the latter at the motoneuronal level. Stimulation of central nicotine receptors in the cat reportedly evokes full-length esophageal peristalsis107; however, the precise nature of this response and its neural substrates remain to be defined. In the rat, nicotine evokes rhythmic swallowing activity upon application to the NTS extraventricular surface (W.Y. Lu, personal communication), an action likely to result from release of other mediators, because indirect cholinergic stimulation by means of antiesterases produces inhibitory effects on rhythmic (fictive) swallowing activity.8 In this species, NTS nicotinic receptor blockade fails to affect secondary peristalsis or NTSce neuronal responses to esophageal distention.75 Although atropine is said to block esophageal peristalsis also in the sheep,9 available evidence from other species, including humans, does not clearly bear out a role of central muscarinic receptors.108, 109
The rat NTSce contains a dense terminal field of cholinergic axons57; to date, the precise connectivity of these afferents has not been identified, particularly as regards the RFiz intermediate reticular nucleus.56 One probable source is nearby choline acetyltransferase-immunoreactive neurons straddling the intermediate, interstitial, and central subnuclei.57 Cholinergic input mediated by mACh receptors plays important integrative functions of NTSce neurons, including (1) the coupling between the buccopharyngeal and esophageal stages of swallowing89, 91 and (2) the generation of premotoneuronal firing patterns appropriate for the production of both propulsive and rhythmic esophagomotor output.74, 75, 93 Coactivation of NMDA receptors appears to be essential.
More specifically, focal stimulation via mACh receptors of rat NTSce neurons69, 89, 90, 91, 93 gives rise to patterned rhythmic esophagomotor activity, resembling bolus-induced peristalsis, whereas mACh receptor blockade impairs or eliminates different types of esophageal motor responses, irrespective of their mode of elicitation (reflex afferent stimulation or central pharmacologic interventions). Because mACh receptor blockade does not disrupt activation of NTSce neurons via vagal afferents,75 cholinergic transmission at this level may serve to gate throughput from reflex afferents to motoneurons. Esophageal premotor paralysis is accompanied by a loss of rhythmicity in NTSce neuron burst firing and absent spike activity in ambiguus compact formation (AMBc) motoneurons.
Intracellular and whole cell patch recordings in medullary slice preparations have shown that AMBc neurons respond to focal muscarinic stimulation of the NTSce region with rhythmic depolarizing waves or bursts of EPSPs. This in vitro rhythm closely resembles that observed in vivo, suggesting that esophagomotor rhythm generation is a potentially intrinsic operation of the NTSce–AMBc circuitry.93 Nonetheless, in the intact system, neuromuscular paralysis (curarization) severely impairs esophagomotor rhythmogenesis.74 Reafferent feedback from vagal mechanoreceptors (esophageal intraganglionic laminar endings [IGLEs]23, 110) must therefore make a critical contribution to that process.
The nicotinic cholinoceptors present in AMBc neurons mediate a fast inward current and a spike burst discharge.95 The same receptor generates an EPSP elicited by focal stimulation of the adjacent RFiz.65 As yet the role of this input remains unclear, because blockade does not impair fictive peristalsis in vivo, nor does it alter EPSPs produced by electrical65 or mACh receptor agonist93 stimulation of the NTSce–AMBc pathway in slice preparations. Furthermore, nicotinic ACh receptor-mediated excitation of ambiguus neurons is inhibited by somatostatin.111
Other Neuromodulatory Inputs
The SPG of the rat is susceptible to excitation by several other neural messengers, such as serotonin (acting via 5-hydroxytryptamine-2A or -1C receptors), norepinephrine (acting via  1-adrenoceptors), thyroliberin, oxytocin, and vasopressin.8 Monoaminergic inputs are likely to originate from diverse sources (intra-NTS, area postrema, raphe system, locus ceruleus, and subceruleus group).5, 8 Some of the peptidergic afferents come from the paraventricular nucleus of the hypothalamus; other afferents may co-release peptides and monoamines. The diversity of these inputs reflects the range of functions (hunger, thirst, nausea, reward, sleep, etc.) that influence internal drive levels (central excitatory or inhibitory states) of the SPG. Moreover, these modulatory systems could serve as network links with other neural function generators, in particular those controlling feeding and respiration. A projection presumably subserving such integrative purposes originates from hypothalamic orexin neurons and provides virtually all NTS subnuclei relevant for deglutition with terminals.112 Intriguingly, fourth ventricle orexin infusion resulted in particularly enhanced Fos expression in NTSce.
1-adrenoceptors), thyroliberin, oxytocin, and vasopressin.8 Monoaminergic inputs are likely to originate from diverse sources (intra-NTS, area postrema, raphe system, locus ceruleus, and subceruleus group).5, 8 Some of the peptidergic afferents come from the paraventricular nucleus of the hypothalamus; other afferents may co-release peptides and monoamines. The diversity of these inputs reflects the range of functions (hunger, thirst, nausea, reward, sleep, etc.) that influence internal drive levels (central excitatory or inhibitory states) of the SPG. Moreover, these modulatory systems could serve as network links with other neural function generators, in particular those controlling feeding and respiration. A projection presumably subserving such integrative purposes originates from hypothalamic orexin neurons and provides virtually all NTS subnuclei relevant for deglutition with terminals.112 Intriguingly, fourth ventricle orexin infusion resulted in particularly enhanced Fos expression in NTSce.
Important inferences as to the cellular basis of these neuromodulatory responses can be drawn from work on rat NTS gastric second-order sensory neurons.113 As shown by in vitro imaging studies, these cells respond to  1-receptor stimulation with slow Ca2+ oscillations (
1-receptor stimulation with slow Ca2+ oscillations (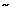 5/min) resulting from the interplay of inositol trisphosphate-mediated Ca2+ release and Ca2+–adenosine triphosphatase (ATPase) storage pumps of the endoplasmic reticulum. Cyclical elevations of cytoplasmic Ca2+ may lead to augmented responsiveness to both viscerosensory input and background excitatory drives.
5/min) resulting from the interplay of inositol trisphosphate-mediated Ca2+ release and Ca2+–adenosine triphosphatase (ATPase) storage pumps of the endoplasmic reticulum. Cyclical elevations of cytoplasmic Ca2+ may lead to augmented responsiveness to both viscerosensory input and background excitatory drives.
Subnucleus centralis neurons contain NOS19, 114, 115 and receive nitrergic afferents from both peripheral (but see Atkinson et al.40) and central116 sources. Preliminary evidence suggests that inhibition of NOS increases the responsiveness of centralis neurons to muscarinic activation92 and blocks a crossed inhibition in which rhythmic esophagomotor activity induced by NTS mACh receptor stimulation on one side is suppressed by the identical stimulus applied contralaterally.117
Information transfer from NTS esophageal premotoneurons to ambigual compact formation (AMBc) motoneurons involves a complex interplay of glutamatergic fast transmission with somatostatin118 and probably also nitric oxide. Somatostatin-mediated modulation of NMDA receptors plays a critical role in motoneuronal ESPS generation and spike production.69 The NTSce–AMBc pathway appears to carry predominantly excitatory fibers, suggesting that inhibitory inputs to esophageal motoneurons, such as that evidenced by deglutitive inhibition of esophageal peristalsis,44 arise either from other NTS subnuclei or from neurons in the bulbar reticular formation. Few, if any, of the fibers in this pathway project to the contralateral AMBc. The exact source of crossed projections from the NTS to the AMBc63 remains to be identified. Besides a cholinergic nicotinic ACh receptor–mediated input, the RFiz, is a likely source of glutamatergic EPSPs detected in AMBc neurons.65
Outlook
An important general principle to emerge from the evidence described in the preceding section is the autonomy of the deglutitive coordination from peripheral sensory input under certain experimental conditions. Evidently, a discrete neurochemical stimulus impinging on what constitutes neuroanatomically Doty's "afferent portal" of the swallowing center4, 10 can substitute for encoded sensory input to activate the complete, coordinated swallowing sequence. Because this form of afferent input does not provide specific timing cues, the central deglutitive network must have the capability of operating autonomously, that is, without or with only minimal afferent input. Clearly, however, the buccopharyngeal stage motor output is significantly modified by sensory feedback. Studies in the awake human have shown that motor timing of the initial stages of swallowing is regulated by bolus variables119 and subglottic pressure or lung volume120 as well as body posture.121 Sensory input is of paramount importance in the esophageal stage of swallowing, the rat being no exception.74 Yet certain aspects of motor programming such as rhythm generation may be controlled centrally and remain functional in brainstem slice preparations.93, 106
Some immediate challenges for future research arise from the neurochemical heterogeneity of NTSce neurons. For instance, do different neuronal phenotypes engage in specific functions in relation to (1) the regional variation in esophageal reflex motility; (2) the organization of premotor inhibitory processes; and (3) the diversity of projections within the dorsal vagal complex? A related intriguing issue is whether central commands to striated and smooth UAT musculature are channeled through the same premotor neuron pools. Similarly, it would be important to determine if some of the NTSim pharyngeal premotor neurons believed to project to esophageal premotor neurons of the NTSce are cholinergic in nature.
The nature of transmitters mediating deglutitive inhibition presents another enigma: if GABA- or glycinergic inhibition were not to contribute to intrinsic SPG operations at the NTS level, would these processes be confined to the RFpc/RFiz?
Finally, the issue of multifunctionality in swallowing interneurons,9 particularly those associated with the NTS, poses an unresolved problem. Given the potential diversity of interactions between the DMPG and other medullary function generators, a high degree of sensory convergence and divergence of efferent output would be expected to occur at the level of second-order neurons, including those forming part of the SPG. Conceivably, neuromodulatory inputs impinging on the SPG network are required for channeling network function-specific patterns of excitation and inhibition through the SPG neuropil and associated local circuit neurons. In this manner, decoding of sensory input and stabilization of functionally dedicated circuits may be achieved.
Acknowledgments
Our thanks are due to Dr. David A. Hopkins for insightful comments and the Figure 1 photomicrograph; Sylvia Ficken for preparing computer graphics; and Madonna Hawco for secretarial help.