Key Points
- Development of the lung as a derivative of the upper gastrointestinal tract.
- Predisposition of anatomy of the lungs to aspiration of food, saliva, and vomitus.
- Development of four-chambered heart to ensure full oxygenation of arterial blood.
- Progressive development of respiratory tract in fetus.
- Movement of inhaled air through progressively smaller "conductive" airways.
- Exchange of gases though attenuated membranes of alveoli with capillary blood.
- Pulmonary vasculature is a low-pressure circulation that serves to exchange respiratory gases and acts as a filter that protects the systemic circulation from emboli.
- Pulmonary vasculature metabolizes chemical mediators traversing the lungs.
- Pulmonary function tests permit assessment of disease severity and distinction between restrictive and obstructive lung disorders.
Relationship of the Lungs to the Gastrointestinal Tract
The intimate relationship between the pulmonary and gastrointestinal systems cannot be considered an unmixed blessing. Direct connections between the mouth, esophagus, and stomach put the lungs at risk for aspiration during swallowing and regurgitation, and excess gas can be swallowed (Figure 1). The pharynx and mouth are used in common for eating, vomiting, and breathing, and food and liquids entering the mouth must be diverted away from the lungs by the epiglottis to avoid flow into the lungs. Major and minor episodes of aspiration contribute to the terminal stages of many diseases, and aspiration appears to play a role in a variety of chronic disorders, such as cough, bronchial asthma, bronchiectasis, and pulmonary fibrosis. Protection of the lungs from oral, nasal, and gastric contaminants is provided by a complex and not always competent set of reflexes that can be disrupted by structural and functional abnormalities, many of which are associated with aging and neurologic disorders.
Figure 1: Intersection of respiratory and gastrointestinal (GI) tracts.
The proximity of respiratory and GI tract has resulted in a complex set of morphologic and reflex adaptations that permit separation of respiratory and GI functions in the aerodigestive tract. For example, breathing requires airflow between the nose or mouth and trachea. On the other hand, the lungs must be protected from aspiration during swallowing or vomiting by closing the epiglottis and larynx, and movement of fluid out the nose is prevented by sealing the soft palate.
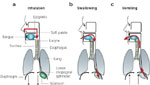
This unfavorable anatomic arrangement can only be understood in terms of the evolution of the lungs as an appendage of the gastrointestinal tract. Gas exchange in single-cell organisms is based on simple diffusion with the environment. The need for specific respiratory organs arose with the emergence of multicellular animals during the Precambrian period, approximately 0.5 billion to 1.0 billion years ago. Gills were presumably the first respiratory organs, and their remnants can still be seen in human embryos and occasionally in adults in the form of branchial cleft cysts. Gills represent evaginations of tissue that allow exchange of gases between water and blood (Figure 2). The subsequent development of lungs as invaginations of the upper gastrointestinal tract can be understood in terms of current terrestrial fish that maintain gas exchange by swallowing air. This enables them to crawl on land and even into trees in their pursuit of insects. Lungs are much more suitable for life on land than gills, which collapse and desiccate when exposed to air, making gas exchange impossible. The evolution of lungs provided an auxiliary mechanism for exchanging respiratory gases. In most fishes, the lungs were subsequently converted into single, midline "swim bladders" that enable fish to stabilize their vertical position in water.
Figure 2: Comparison of gills and lungs.
Gases diffuse between water and capillaries in gills, which are immersed in the flow of water. In contrast, diffusion of gases between inhaled air entering the lungs and capillaries occurs within the lungs. Circulation in the fish is accomplished by a two-chambered heart, whereas a four-chambered heart is used to separate the systemic and pulmonary circulations in mammals.
The phylogenetic and embryologic origins of lungs from the gastrointestinal tract have been associated with a number of serious problems: unlike the gills, which are positioned in series between the heart and peripheral tissues (Figure 2), early gastrointestinal lungs were arranged in parallel with the remainder of the systemic tissues and oxygenated blood emerging from these lungs was mixed with poorly oxygenated blood from the rest of the body. A return to full saturation of arterial blood with oxygen was not achieved until the development of a four-chambered heart, which is characteristic of mammals and birds. Oxygen needs in these "endotherms" are much higher than those in their cold-blooded "exothermic" ancestors. The openings of the respiratory and gastrointestinal tracts remain inextricably linked. Because the lungs are fundamentally invaginations, ventilation is "tidal" in mammals, and a certain proportion of the inhaled air never actually reaches the gas exchange regions of the lungs, making the process less efficient. Presumably because of the demand for high rates of metabolism during flying and reduced oxygen tensions at altitude, avian lungs are fundamentally more efficient than those of mammals: inhaled air passes through avian lungs, which are not inflated but conduct air to large sacs in the abdomen and bones. The inhaled air is subsequently routed from the body cavities by different portions of the airways, thereby minimizing rebreathing of exhaled air.
The evolution of mammalian lungs was closely linked with the parallel development of surfactant. This mixture of phospholipids, neutral lipids, and proteins reduces the surface tension of the air–fluid interfaces of the lung. Surfactant facilitates distention of air spaces and also inhibits the tendency of small units to empty into larger units. This tendency is related to the fact that the surface tension of a bubble is inversely proportionate to the radius of the bubble. Because surfactant is more active in smaller than larger alveoli, it serves to make surface tension of the alveoli more uniform. As alveoli are compressed during exhalation, unsaturated phospholipids and cholesterol are squeezed out of the surface film, which becomes relatively enriched with dipalmitoyl cholesterol and tends to exclude water from the surface, thereby reducing surface tension.
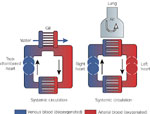
The development of the diaphragm in mammals made it possible to exchange air much more efficiently than in either amphibians or reptiles (Figure 3). Living reptiles depend on expansion and contraction of the rib cage and buccal swallowing of air for moving air in and out of lungs, which are septated and more complex in reptiles than amphibians, but still much simpler in structure than in mammals. Respiration is also facilitated in crocodiles by the movement of the liver, which is attached to the pubis by a strand of muscle fibers that acts to expand the lungs. Unlike amphibian nostrils, reptilian nostrils are connected to the airways, but there is no hard palate and reptiles must stop breathing when there is food in the mouth.
Figure 3: Schematic diagram of lung anatomy with cross-sections of bronchi, bronchioles alveolar ducts, and alveoli.
a: Gross anatomy of lung and thorax. b: Microscopic anatomy of bronchial wall. C: View of terminal airway and alveoli. d: Alveolar structure.
Development of the Lungs
Human lungs first appear as evaginations of the primitive gut, which invade the surrounding mesenchyma at day 26. Two buds form on the left and three on the right, representing the precursors of the mainstem bronchi and lobes in the fully formed lungs. This is referred to as the "embryonic" stage of lung development, and by the end of this period, the major airways are accompanied by the pulmonary vasculature. The early histology of the next phase of development is "pseudoglandular" with columnar cells containing glycogen lining the airways. This pseudoglandular stage lasts from the 6th to 16th week of gestation and is associated with progressive and complete division of the airways into smaller branches and formation of the diaphragm. This is followed by the "canalicular" stage, which lasts from the 16th to 26th weeks. During this period, pulmonary acini develop with capillary proliferation and attenuation of the pulmonary epithelium. Premature babies can sometimes survive at the end of the canalicular stage. The "saccular" stage lasts from the 28th to 38th weeks. It is characterized by widening of the air spaces into saccules, which in turn generate alveolar ducts and sacs. The epithelial cells differentiate into flat type I cells and larger type II cells after the 26th week. The type II cells secrete a mixture of lipids and proteins called surfactant during the final weeks of gestation. As indicated above, surfactant is essential to reduce the surface tension of the fluid, and some of the proteins in surfactant also have antimicrobial properties. During the last 2 weeks of gestation ("alveolar stage"), alveolar formation commences and at the time of birth, several million alveolar like structures are present. Septation of the air spaces is enhanced by secretion of thyroxine, whereas steroids may inhibit septation, though it accelerates thinning of the airway walls. Alveoli continue to develop following birth, until the child reaches about 8 years of age. In all, a total of about 300 million alveoli are formed with a total surface area of about 70 m2. At the time of birth, the lung converts from a fluid-secreting organ to one that absorbs fluid. Breathing movements appear prior to birth and are responsible for the aspiration of some amniotic fluid.
Much has been learned in recent years about signaling mechanisms in the morphogenesis of the lungs. The interaction between the endoderm of the gut and the mesenchyma is essential for the subsequent development of the lungs. The pulmonary vasculature, septal fibrous network, smooth muscle, and cartilage are all derivatives of the mesenchyma, and mesenchymal cells secrete growth factors, which drive the development of airways and air spaces. A multitude of factors secreted by the underlying mesenchyma play a critical role in the budding of the airways. Of these, several families deserve mention. The fibroblast growth factors (FGFs) are a large family of at least 24 proteins, including six that are found in the developing lung, of which FGF10 appears to be the most essential. It is secreted by the mesenchyme and induces growth and differentiation of nearby epithelium. Four receptors for the FGF molecules have been described in the respiratory epithelium and the absence of these receptors blocks lung formation
The sonic hedgehog protein (first described in fruit flies and named after a video game character) plays an important role in directing the formation of numerous organs including the brains and limbs. It is required for regulating branching in the airways. Other proteins include the "WNT" growth factors, "sprouty", and the bone morphogenetic protein 4. Development of the pulmonary vasculature depends on a complex interaction of growth factors, including transforming growth factor- (TGF-
(TGF- ), vascular endothelial growth factor (VEGF), forkhead box (FOX) transcription factors, integrins, and caveolin. Alveolar formation is relatively late, and is dependent on local tissue factors as well as glucocorticoids.
), vascular endothelial growth factor (VEGF), forkhead box (FOX) transcription factors, integrins, and caveolin. Alveolar formation is relatively late, and is dependent on local tissue factors as well as glucocorticoids.
During embryogenesis, the diaphragm descends with the phrenic nerve from the C3-C5 level to the lower thoracic vertebrae. The cervical origin of these structures explains the radiation of pain to the shoulders associated with stimulation of the sensory fibers of the esophagus.
Anatomy of the Adult Lungs
Airways
The mammalian lungs are designed to optimize exposure of blood to oxygen (Figure 3). The normal human lungs weigh about 1 kg, of which 40% to 50% is blood. These lungs contain about 2.5 L of air at end expiration and 6 L of air at full inflation. They are usually divided into three lobes on the right and two lobes on the left by pleural membranes. Air is inhaled through the nose and mouth through the "airways," passing from the larynx and trachea and thence into a rapidly dividing series of about 16 generations of conductive bronchi and bronchioles. The bronchi are kept relatively rigid by a series of cartilaginous rings over the first generations. These cover the anterior of the trachea and are open in the back where the bronchi are covered with a membrane. Thereafter, the airways are not invested with cartilage and are referred to as bronchioles.
Nearly 50 distinct types of cells have been identified in the lungs, of which at least 12 can be found in the airways. Mucus is secreted onto the bronchial surfaces by submucous glands and by "goblet cells" that are present in abundance on the bronchial surface. Thick islands of mucus rest on a thin layer of an electrolyte solution that is generated in part by the submucous glands. Ciliated columnar cells are also present in the airway membrane, and the coordinated movement of the cilia aid in the transport of mucus covering the airways toward the mouth, where approximately 500 mL of airway fluid is swallowed each day. It is this "mucociliary elevator" which is largely responsible for removing foreign material, including organisms that land on the bronchial surfaces. Below the larynx, the airways are sterile in normal subjects. Injury to these ciliated cells is characteristic in smokers, who are likely to develop chronic bronchitis, with colonization of the trachea and bronchi by oral organisms and production of excessive amounts of mucus. Patients with congenital abnormalities of ciliary function (Kartagener's syndrome) must raise mucus from the airways by coughing. They are prone to develop respiratory infections and dilation of the bronchi, referred to as bronchiectasis. Abnormalities in mucus viscosity appear to play a role in the pathogenesis of cystic fibrosis, which is characterized by recurrent infections and the progressive development of bronchiectasis.
Most of the resistance of the lungs to air movement is normally located in the bronchi, which tend to maintain some muscular tone. Excess bronchoconstriction of small bronchi between 2 and 5 mm in diameter appears to be responsible for increased airway resistance in most patients with asthma.
Alveoli
Alveoli begin to appear in the walls of the 17th generation of bronchioles, which are therefore referred to as respiratory bronchioles (Figure 3). By the 20th generation of airways, the entire wall of the airway is composed of alveoli, and these structures are referred to as alveolar ducts. Alveolar ducts end at about the 23rd generation in blind sacs, which are lined with alveoli and are referred to as alveolar sacs. There are approximately 300 million alveoli within the lungs and these provide a surface area of exchange estimated to be about 90 m2, or about the size of a tennis court. The barrier separating the pulmonary capillaries from the alveolar air is composed of endothelial cells, an attenuated interstitial space, and pulmonary epithelial cells (pneumocytes). There are two types of pneumocytes. The type I cells are very flat (0.2  m in diameter over much of their surface) and cover most of the alveolar surface. The type II cells are more irregularly shaped and contain lamellar bodies, which are secreted as surfactant. These cells can divide and give rise to both type I cells and more type II cells.
m in diameter over much of their surface) and cover most of the alveolar surface. The type II cells are more irregularly shaped and contain lamellar bodies, which are secreted as surfactant. These cells can divide and give rise to both type I cells and more type II cells.
Pulmonary Interstitium
The tissues separating the endothelial and epithelium of the lungs contain abundant fibroblasts, elastic and collagen fibers that give structural integrity and elasticity to the pulmonary tissues. When the chest cavity is opened, the elasticity of the lungs acts to expel all of the air remaining in the lungs, which then collapse (atelectasis). Loss of elastic fibers plays an important role in the failure of the lungs to contract adequately in patients who have emphysema. On the other hand, overgrowth of fibrous tissues in the lungs in patients with pulmonary fibrosis is responsible for the difficulty that they experience during inhalation.
Pulmonary Vasculature
Hydrostatic pressures are normally maintained at much lower levels in the pulmonary vasculature than in the systemic arteries. Pulmonary artery systolic pressures average about 15% those in systemic arteries. The relatively thin walls of the right ventricle and pulmonary vessels reflect this difference. Unlike systemic arterioles, smooth muscle fibers are attenuated in the pulmonary arterioles, which are difficult to distinguish from venules in microscopic sections, other than the fact that the arterioles tend to be associated with the bronchi. Pulmonary arterial pressures fall soon after birth because the pulmonary arteries are dilated by high oxygen tensions. Local vasoconstriction in hypoxic areas of the lungs directs blood flow toward well-ventilated regions without causing significant increases in the pressures of the relatively compliant pulmonary vascular bed. However, severe hypoxia can lead to significant vasoconstriction and pulmonary artery hypertension that can result in right-sided heart failure (cor pulmonale). Administration of inhaled oxygen is used to avoid this complication in patients with significant lung disease.
Low pressures in the pulmonary circulation also reflect what might be considered the second most important function of these vessels, which act as a filter that prevents emboli from the systemic veins, frequently in the lower extremities, from reaching the brain, heart, and other critical arterial beds. Congenital and acquired shunts that bypass the pulmonary microvascular filter can be associated with serious systemic emboli. Large emboli can cause an increase in pulmonary artery pressure in normal lungs, but smaller emboli are associated with little change in hemodynamics unless many vessels are occluded by emboli. The pulmonary vasculature also serves to modify vasoactive amines such as angiotensin I and bradykinin derived from extrapulmonary tissues.
Pleura
The lungs are enveloped by a thin layer of mesothelial cells referred to as the pleura (Figure 3). This membrane is analogous to the pericardium, which covers the heart, and the peritoneum, which covers the intraabdominal organs. The "visceral" layer covering the lungs is continuous with the "parietal" layer that covers the inner surface of the chest cavity. These two layers of pleura are separated by a thin layer of fluid, which amounts to less than 10 mL in the normal adult lungs. This fluid contains a population of mesothelial cells and significant concentrations of mucopolysaccharides, which acts as a lubricant for the smooth movement of the pleural layers against one another. Expansion of the chest wall is transmitted to the lung surface through the pleural surface, but this process can be interrupted if air or excess fluid enters the pleural compartment, thereby separating the visceral and parietal layers. A pneumothorax can be produced by air entering the pleural cavity from a hole in the lungs or the chest wall. Pleural fluid can reflect an imbalance in hydrostatic pressure (e.g., heart failure) or protein oncotic pressure (e.g., cirrhosis or nephritic syndrome), which results in the formation of a transudate. Alternatively, it can be caused by an increase in the permeability of the pulmonary capillaries, with the formation of an exudate (e.g., in inflammatory or neoplastic involvement of the pleural spaces).
Diaphragm and Chest Wall
The mammalian diaphragm separates the thoracic and abdominal cavities. It incorporates two anatomically and physiologically distinct portions. The costal portion is attached to the ribs and is responsible for ventilation. During exhalation, the lateral portions of the diaphragm are in contact with the sides of the rib cage, and become separated from the ribs during inhalation, resulting in a piston-like movement that expands the lungs. The crural fibers surround the esophagus. They also contract during inhalation but have a relatively minor effect on respiration. The crural portions of the diaphragm relax when food is swallowed, regardless of contraction of the costal diaphragm. Furthermore, the crural diaphragm also relaxes during vomiting, during which time the costal diaphragm contracts with the abdominal musculature to increase intraabdominal pressures. The crural fibers appear to act in concert with the smooth muscle of the esophagus to prevent reflux of food and gastric fluid into the esophagus.
The ribs are connected by two layers of intercostal muscles. The outer layer runs obliquely downward and forward from the upper to lower ribs and act to lift the chest cavity. The internal interosseous intercostals run obliquely downward and backward and assist in exhalation. The scalene muscles run from the first five vertebrae to the first two ribs and lift the chest cage during inhalation.
Physiology
Pulmonary Mechanics
Successful exchange of gases in the lungs is predicated on efficient ventilation of the lungs. Tidal respiration is based on cyclical expansion and contraction of the lungs. During inhalation, active contraction of the diaphragm and external intercostal muscles of the ribcage draw air into the lungs. Each of these muscle groups acts together to expand the lungs. During inhalation, intrathoracic pressures decrease and intraabdominal pressures increase. Intercostal contraction results in visible expansion of the chest wall, whereas diaphragmatic contraction pushes the abdominal contents downward and outward. Expansion of the chest and abdominal walls should be well coordinated. If the abdomen and chest move in opposite directions, one or the other of these systems is either weak or paralyzed. Furthermore, the left and right diaphragms should move together. Weakness of one or both diaphragms can reduce the ventilatory capacity of the lungs. This can be tested with a "sniff test": the subject is asked to sniff while the movement of the diaphragms is evaluated by fluoroscopy or ultrasound. During inspiration, downward movement of the diaphragm on the intact side increases intraabdominal pressures and decreases intrathoracic pressures, resulting in an upward movement of the paralyzed side. Diaphragmatic paralysis usually reflects injury to the phrenic nerve, which may occur anywhere along its extended length from the C3-C5 vertebrae through the thorax to the undersurface of the diaphragm. Unilateral paralysis of the diaphragm results in mild impairment of ventilation. Ventilation can be barely maintained with bilateral phrenic nerve paralysis if the intercostal and auxiliary respiratory muscles remain functional, but severe restriction of mechanical ventilation will be observed. The auxiliary muscles include the scalenus (which are active during normal inspiration) and the sternocleidomastoid muscles (which are only used with high levels of respiratory effort). The overall strength of the respiratory apparatus can be easily evaluated by measuring the maximum inspiratory and expiratory pressures (PImax and PEmax) that the patients can exert against a fixed obstruction of the mouthpiece.
Although inhalation requires active muscle contraction, exhalation is normally a passive process and is dependent on the elasticity of the pulmonary tissues. When the inward force exerted by the elastic tissues of the lungs matches the outward force of the thorax, the lung volume becomes stable at the "resting midposition" of the lungs (Figure 4). This is equivalent to the functional residual capacity (FRC) of the lungs. The rate and volume of exhalation can be increased by contracting the abdominal and internal intercostal muscles. Loss of elastic tissues within the lungs due to emphysema reduces the ability of patients with this disorder to exhale. Patients with severe intrathoracic obstruction characteristically utilize abdominal muscles to force the diaphragms upward during exhalation. However, this maneuver also reduces the diameter of the bronchi, limiting the rate at which they can exhale. More time is consequently needed during the expiratory phase and this must be kept in mind when adjusting the inspiratory/expiratory time ratios during mechanical ventilation.
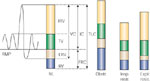
Figure 4: Lung volumes.
IRV, inspiratory reserve volume; TV, tidal volume; ERV, expiratory reserve volume; RV, residual volume; IC, inspiratory capacity; FRC, functional residual capacity; TLC, total lung capacity; VC, vital capacity; Obstr., obstructive lung diseases (e.g., asthma, chronic obstructive lung disease); Insp. Restr., inspiratory restrictive lung disease (e.g., pulmonary fibrosis, pleural fibrosis or effusion, diaphragmatic weakness); Expir. Restr., expiratory restrictive lung disease (e.g., ascites, obesity, pregnancy, weakness of abdominal musculature).
The mechanical properties of the respiratory apparatus are usually evaluated with a variety of "pulmonary function tests." These tests are primarily devised to distinguish between airway obstruction, due to such disorders as asthma and chronic obstructive lung disease and respiratory restriction associated with a wide variety of illnesses such as pulmonary fibrosis, pleural effusions, or fibrosis or muscle weakness. In principle, airway obstruction is detected by measuring the resistance (R) of the airways to the movement of air. R is defined as the ratio between the pressure increase ( P) needed to generate a flow (F) of air out of the lungs:
P) needed to generate a flow (F) of air out of the lungs:
R =  P/F
P/F
Restriction of the respiratory apparatus is ideally measured in the absence of airflow by determining the compliance (C) of the respiratory apparatus, where:
C =  V/
V/ P
P
and  V represents the change in volume of the lungs that results from increasing the air pressure by
V represents the change in volume of the lungs that results from increasing the air pressure by  P. The total compliance of the respiratory apparatus (CT) includes two components: the compliance of the lungs (CL) and the compliance of the chest wall (CW) where:
P. The total compliance of the respiratory apparatus (CT) includes two components: the compliance of the lungs (CL) and the compliance of the chest wall (CW) where:
CL =  V / (Ppleura - Palveoli)
V / (Ppleura - Palveoli)
CW =  V / (Pairway - Ppleura)
V / (Pairway - Ppleura)

Ppleura is estimated from the pressure measured with a fluid-filled esophageal catheter. Because compliance of the lungs and chest decreases as the lungs are expanded, measurements of compliance must be made at a set lung volume, for example, between the volume of the lungs at FRC and FRC plus 0.5 L. Compliance measurements are commonly performed on patients who are receiving mechanical ventilation through an endotracheal tube. Ideally, the patient is fully paralyzed, to avoid active respiratory movements, and pressures within the lungs are allowed to stabilize. However, it is frequently impractical to arrest respiration for more than a brief period (e.g., 1 second) and a measurement of "dynamic" compliance may be made during ventilation. Airway pressures during mechanical expansion of the lungs tend to exceed those measured at the same volume when there is no airflow, because of the resistance of the bronchi to air entering the lungs.
Restrictive respiratory disease can be due to reduction in the compliance of either the lung or chest wall. Loss of surfactant (e.g., in acute respiratory distress syndrome) can also make the lungs less compliant. Furthermore, space-occupying lesions in the lung or pleura can reduce compliance. On the other hand, loss of elastic tissue in emphysema can cause inappropriate increases in the compliance of the lungs.
Although changes in airway resistance and lung compliance can be estimated with more sophisticated procedures, detection of abnormal pulmonary function is usually based on measurements of lung volumes and maximal flows. Both the volumes of the lungs and flow rates are compared to normal values that are estimated on the basis of the height, age, sex, and race of the patient. As indicated in Figure 4, the volume of air in the lungs at maximum inhalation is referred to as the total lung capacity (TLC) of the lungs. The TLC can be divided into the fraction that can be voluntarily exhaled (the vital capacity, VC) and the fraction that is not under the control of the subject (the residual volume, RV). The components of the vital capacity can be determined by simple spirometric tests. Because it is impossible to expel the RV of air in the lungs at end-expiratory volume, more sophisticated tests are needed to measure the RV, TLC, and FRC. This can be accomplished by washing N2 from the lungs with 100% oxygen, equilibrating the lungs with He, or using plethysmography (body box), which is based on Boyle's law.
The volume of air in the lungs at rest (the resting midposition) represents the FRC, which includes the RV and the expiratory reserve volume (ERV). The ERV represents the volume of air that can be exhaled with forced exhalation, primarily as a result of contraction of the abdominal muscles. The ERV is characteristically reduced with weakness of the abdominal wall musculature or abdominal distention due to obesity, ascites, or pregnancy because they push the diaphragms upward, and the patients are unable to further increase intraabdominal pressures. Reductions in lung or chest wall compliance and diaphragmatic weakness are associated with reductions in VC. Much of the dyspnea experienced by patients with restrictive respiratory diseases is attributable to increased work required to adequately inflate their lungs, and these patients tend to breathe with shallow breaths at an increased respiratory rates.
Airway obstruction can be due to bronchoconstriction (e.g., asthma), excess mucus in the airways (e.g., chronic bronchitis), or loss of lung elasticity (e.g., emphysema). During exhalation, the diameter of the intrathoracic airways decreases, resulting in an increase in airway resistance, which further compromises the ability of patients with obstructive diseases to empty the air from the lungs. Trapping of air within the lungs of patients with obstruction results in an increase in the TLC of the lungs, but this is largely because of an increase in the RV, which cannot be exhaled by the patients. This results in an increase in the RV/TLC ratio and a reduction in the VC. As emphasized above, exhalation is normally related to the elastic recoil of the air spaces. Once this elasticity is lost in patients with emphysema, they must depend on abdominal muscles to force air out of the lungs. This results in airway compression and a reduction in expiratory flows.
Asthma can be detected clinically by wheezing. A forced expiratory maneuver is used to determine the degree of airway obstruction. Obstruction reduces the volume of air that can be forcibly exhaled in 1 second (FEV1) compared to predicted values. Because reductions of FEV1 can also reflect decreases in lung volume, the total amount of air that the patient can exhale (forced vital capacity, FVC) must also be determined and the ratio of FEV1/FVC calculated. A decrease in FEV1/FVC must be documented before it can be concluded that an obstructive disorder is present. Asthmatic patients characteristically have reversible airway obstruction related to intermittent bronchospasm. Because they may be in remission at the time of pulmonary function tests, it may be necessary to induce bronchoconstriction by exposing the patients to low concentrations of an irritating solution (e.g., methacholine) or to exercise. Distinction between asthma and chronic obstructive lung disease is based on the relatively reversible nature of airway obstruction in asthma.
Dynamic compression of the airways in patients with emphysema can be visualized by plotting the flow of air from the lungs against the volume of air remaining in the lungs. As indicated in Figures 5 and 6, dynamic airway obstruction results in a fall in the expiratory flow as the lung volume falls, and the descending limb of the expiratory flow volume limb becomes curvilinear. In contrast, the flow volume curve in patients with restrictive lung disease retains a normal shape but is very much diminished in size. The flow-volume curve is also helpful in identifying the presence of extrathoracic obstruction (e.g., croup, epiglottitis, tracheal stenosis). The trachea tends to collapse during inspiration below the site of obstruction during inspiration because pressures in the extrathoracic trachea fall below atmospheric pressure in the surrounding air.
Figure 5: Flow-volume loops.
a: Normal. b: Intrathoracic obstruction. c: Restriction. d: Variable extrathoracic obstruction. e: Fixed airway obstruction.
Figure 6: Effects of intrathoracic and extrathoracic obstruction on the caliber of airways.
Intrathoracic obstruction is most severe during expiration and is relieved during inspiration. Extrathoracic obstruction is increased during inspiration because of the effect of atmospheric pressure to compress the trachea below the site of obstruction.
Interpretation of pulmonary function tests is based on comparing values measured in patients with values obtained in normal populations of individuals of the same age, height, sex, and race. These tests are designed to assess the nature and severity of abnormalities in lung function and can be augmented by cardiopulmonary stress tests to assess exercise capacity.
Gas Exchange
The principal role of the respiratory system is to permit efficient exchange of respiratory gases (O2 and CO2) with the environment.
Carbon Dioxide
The production of CO2 from food provides the principal source of energy in oxygen-breathing animals, and large volumes must be exhaled with each breath to avoid accumulation in the body. CO2 acts as a weak acid according to the equations:

Isoforms of the enzyme carbonic anhydrase in the red blood cells and on the endothelial surface accelerate the effective hydration of CO2, probably by the mechanism indicated in the second of these reactions. Accumulation of excess CO2 must inevitably result in a "respiratory acidosis." The arterial CO2 tension (PaCO2) is normally kept within a very narrow range (38 to 42 mmHg). The terms hyperventilation and hypoventilation are used to indicate increases and decreases in PaCO2. In contrast, hyperpnea and hypopnea designate the minute volume of ventilation (usually expressed in terms of the total amount of gas exhaled per minute, 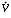 E). Care must be taken to distinguish between these parameters. For example, at low or moderate rates of exercise, patients characteristically are hyperpneic (
E). Care must be taken to distinguish between these parameters. For example, at low or moderate rates of exercise, patients characteristically are hyperpneic (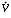 E increases) but they do not hyperventilate (PaCO2 remains unchanged). Tachypnea and bradypnea designate the rate of respiration and may not reflect either
E increases) but they do not hyperventilate (PaCO2 remains unchanged). Tachypnea and bradypnea designate the rate of respiration and may not reflect either 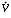 E or PaCO2 in the absence of measurements of the tidal volume (VT) and PaCO2.
E or PaCO2 in the absence of measurements of the tidal volume (VT) and PaCO2.
Alterations of PaCO2 can be either primary or secondary. This is illustrated by comparing the increase in PaCO2 of central respiratory depression, a primary disorder that causes acidosis, with the secondary ("compensatory") rise in PaCO2 that occurs in patients with metabolic alkalosis. Secondary disorders of PaCO2 restore arterial toward more normal values. Primary elevations in PCO2 can also be due to mechanical disorders of ventilation. This is more frequently observed in patients with obstructive lung disease, but also occurs in more advanced restrictive disease.
Clinical manifestations of hypercapnia are very nonspecific. Patients may become somnolent, complain of headache, or lapse into coma, and on occasion, papilledema may be observed due to dilation of intracerebral vessels. Detection of increased PaCO2 is usually based on measurements of arterial blood gases. In the absence of arterial blood data, elevations in venous bicarbonate concentration may suggest secondary renal compensation for respiratory alkalosis. However, elevations in serum HCO3- may also be due to metabolic alkalosis. End-tidal PCO2 (PETCO2) measured in air at the end of exhalation is sometimes used to detect elevations in arterial PCO2 (PaCO2). These measurements are made either digitally or graphically on the basis of the exhaled profile of PCO2 (capnometry and capnography). High levels of PETCO2 indicate that PaCO2 must also be elevated. Unfortunately, PETCO2 can significantly underestimate PaCO2 if significant dead space is present in the lungs.
Dead Space
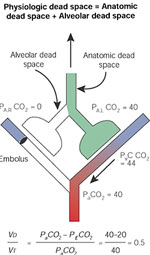
The concept of dead space is illustrated in Figure 7. The "anatomic dead space" designates the volume of the conductive airways (nonrespiratory bronchi, trachea, larynx, nose, and mouth). There is no gas exchange in this portion of the lungs, and PCO2 levels in this compartment are similar to that in the atmosphere (0.03%). Most of this gas is exhaled at the beginning of exhalation (Figure 8), and its contribution is minimized in end tidal samples of exhaled air. The volume of the anatomic space is approximately equal to 150 mL in a normal adult, and it can be reduced by one half with a tracheostomy.
Figure 7: Dead space.
Distinction between anatomic dead space, which is in series with the alveolar compartment, and the alveolar dead space, which is in parallel with the perfused alveoli.
Figure 8: Measurement of dead space.
The total (physiologic) dead space reduces the PCO2 of the mixed expired gas (PECO2) and can be calculated by measuring the arterial and mixed expired gas. Partial pressures of CO2 at the sites indicated by the eyes are indicated on the right.
The alveolar dead space is represented in Figure 7 by lung units that are not perfused and therefore contain room air. Gas exhaled from this portion of the lungs contains no CO2 and dilutes the PETCO2 (Figure 8). The sum of anatomic and alveolar dead space is sometimes referred to as "physiologic" dead space, an unfortunate term because the volume may be increased by pathologic conditions. It is less confusing to designate the sum of anatomic and alveolar space as "total" dead space:
VDtotal = VDanatomic + VDalveolar
The Bohr equation can be used to estimated the ratio of total dead space to tidal volume:

where VD and VT indicate the dead space and tidal volumes, and PE-CO2 represents the PCO2 in the mixed expired gas. It will be noted that either reducing VD or increasing VT can decrease this ratio. Increases in VD can impair excretion of CO2 and contribute to hypercapnia. Elevations in VD/VT can be associated with pulmonary vascular embolization or a wide variety of lung disorders associated with lung units that are not well perfused.
Oxygen
Elevations in PaCO2 indicate decreased ventilation. In contrast, although a decrease in arterial PO2 (PaO2) can be caused by hypoventilation, it can also be caused by exposure to high altitudes and by various forms of "venous admixture." The differential diagnosis of hypoxia is consequently much more complicated than that of hypercapnia. The source of this difference is related to the fact that most of the oxygen in the blood is carried by hemoglobin, which normally becomes fully saturated when blood traversed the pulmonary capillaries (Figure 9).
Figure 9: Oxyhemoglobin curve.
The sigmoid relationship between PO2 and oxygen content and saturation reflects the properties of hemoglobin to bind oxygen.
Desaturation of hemoglobin can be clinically detected by the presence of cyanosis. "Peripheral" cyanosis is caused by slow passage of blood through cutaneous capillaries in patients who are cool or in shock. Peripheral desaturation is caused by increased extraction of O2 from the cutaneous vessels and arterial saturation is normal. "Central" cyanosis is due to desaturation of the arterial blood. Cyanosis is difficult to appreciate if saturation is above 90% or the patient is anemic. Cyanosis may also be observed in the presence of methemoglobin, which is the ferric form of hemoglobin that cannot participate in oxygen exchange and is very dark in color. Carbon monoxide readily combines with hemoglobin, resulting in a cherry red color.
Saturation of the blood represents the percentage of hemoglobin that is combined with oxygen and was previously measured by removing and measuring the amount of oxygen in a sample of blood before and after exposing it to 100% oxygen. It is now measured from the color of various forms of hemoglobin in the blood with a co-oximeter. The co-oximeter measures the absorption of light at four wavelengths to calculate the concentrations of oxyhemoglobin, unsaturated hemoglobin, methemoglobin, and carboxyhemoglobin (Figure 9). The introduction of the pulse oximeter represented an important advance because it permits noninvasive estimates of saturation based on the color of the finger. To distinguish the color of arterial blood from that of the skin and venous blood, the signal is analyzed in terms of the increase in colors of the blood during systole. These devices provide approximate data and cannot separate the contribution of carboxyhemoglobin to the oxyhemoglobin estimate, or methemoglobin from the reduced hemoglobin signal.
As noted in Figure 9, saturation of the blood is related to PO2 by a sigmoidal curve that can be shifted by a variety of factors. Acidosis, hypercapnia, anemia, and high levels of the metabolite 2,3-diphosphoglycerate shift the curve to the right, whereas alkalosis, hypocapnia, and low concentrations of 2,3-diphosphoglycerate shift the curve to the left. A shift to the left is generally considered deleterious, because it indicates excess affinity of hemoglobin for oxygen, resulting in tissue hypoxia. A shift to the right has an opposite effect. Hemoglobin has an affinity for carbon monoxide that is over 200 times greater than for oxygen, and low concentrations of CO can effectively displace oxygen from hemoglobin. In addition, carbon monoxide acts to shift the oxyhemoglobin curve of the remaining oxygen on hemoglobin to the left, further impairing delivery of oxygen to the tissues. The effects of anemia and carbon monoxide exposure on oxyhemoglobin are best plotted as oxygen content against PaO2 (Figure 9).
Venous Admixture
Venous admixture represents the most common cause of arterial hypoxia. Venous admixture can reflect one of three problems:
Anatomic Shunts
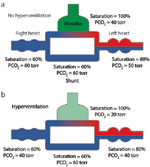
As indicated in Figure 10, an anatomic shunt can deliver unsaturated venous blood to the arterial circulation. The arrival of hypoxemic blood to the carotid bodies stimulates ventilation and reduces PaCO2. However, increasing ventilation has little effect on oxygen saturation in blood issuing from the ventilated vessels, which is already nearly saturated. Once venous blood enters the systemic arteries, there is virtually nothing that can be done to improve arterial saturation, and hyperventilation provides little clinical benefit. These shunts can be intrapulmonary (arteriovenous fistulas, e.g., in Osler-Weber-Rendu disease) or extrapulmonary (e.g., patent foramen ovale, atrial or ventricular septal defects, and patent ductus arteriosus). However, extrapulmonary shunts are initially associated with flow of arterialized blood from the systemic to the pulmonary circulation, where hydrostatic pressures are normally low. Such shunts increase the flow of blood through the pulmonary circulation and eventually increase the resistance of the pulmonary circulation but do not cause desaturation. Venous blood enters the arterial circulation when pressures in the pulmonary circulation reach those in the corresponding systemic structures (Eisenmenger's syndrome). The degree of shunt can be calculated from the shunt equation:
Figure 10: Effect of shunting.
Anatomic shunting results in transfer of venous blood into the arterial circulation. a:. Normal ventilation of lung (PCO2, 40 mmHg). B:. Hyperventilation of lung (PCO2, 20 mmHg). Increased ventilation in response to hypoxemia results in a fall in the PCO2 in the ventilated regions of the lungs but cannot appreciably increase the saturation in the ventilated regions. Consequently, hyperventilation (B) can lower the PCO2 of the arterial blood to normal or below normal levels, but it has little effect on the arterial saturation. "Torr" represents partial pressure in millimeters of mercury.
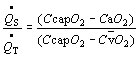
where CcapO2, CaO2, and C O2 designate capillary, arterial, and mixed venous oxygen content. Precise determination of the shunt requires sampling of mixed venous blood from the pulmonary artery. Capillary O2 content is estimated with the alveolar gas equation (see below), but simpler estimates can be made. The persistence of arterial hypoxia with exposure to 100% indicates the presence of an anatomic shunt. A small amount of venous blood normally enters the arterial circulation from bronchial veins draining into the pulmonary veins, and the thebesian veins, some of which drain from the myocardium into the left chambers of the heart. Localization of an anatomic shunt requires further workup, frequently involving radiologic, echocardiac, and nuclear medical techniques.
O2 designate capillary, arterial, and mixed venous oxygen content. Precise determination of the shunt requires sampling of mixed venous blood from the pulmonary artery. Capillary O2 content is estimated with the alveolar gas equation (see below), but simpler estimates can be made. The persistence of arterial hypoxia with exposure to 100% indicates the presence of an anatomic shunt. A small amount of venous blood normally enters the arterial circulation from bronchial veins draining into the pulmonary veins, and the thebesian veins, some of which drain from the myocardium into the left chambers of the heart. Localization of an anatomic shunt requires further workup, frequently involving radiologic, echocardiac, and nuclear medical techniques.
Regions of Decreased Ventilation to Perfusion Ratio (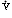 /
/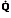 )
)
If one region of the lungs is poorly ventilated, blood emerging from the local pulmonary veins will be hypoxemic, and desaturated blood will reach the systemic arteries, regardless of the rate at which other alveoli are ventilated (Figure 11). Regions of decreased 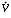 /
/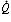 appear to be responsible for much of the hypoxia associated with both obstructive and restrictive lung diseases.
appear to be responsible for much of the hypoxia associated with both obstructive and restrictive lung diseases.
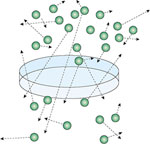
Figure 11: The effect of mismatched ventilation 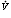 and perfusion
and perfusion 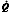 on arterial oxygenation.
on arterial oxygenation.
Low 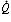 /
/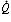 ratio in right lung results in decreased saturation that cannot be compensated for by increasing ventilation to the left lung.
ratio in right lung results in decreased saturation that cannot be compensated for by increasing ventilation to the left lung.
Impairment of Diffusion
Although convection is responsible for most the movement of fresh air into and out of the airways of the lungs, the subsequent movement of gases in the alveoli and between the alveoli and pulmonary capillary blood depends on diffusion. Diffusion of O2 and CO2 in the lungs is mediated by the random movement of gas molecules from regions where concentrations are high to those where concentrations are low (Figure 12). This accounts for movement of oxygen from alveoli to blood and from blood to alveoli. The rate of diffusion across a membrane is set by the differences in concentration on either side of the membrane, membrane area, and membrane permeability. To calculate the diffusion coefficient of the air-blood interface for a test gas, information must be available concerning the concentration of the gas in both the alveoli and capillary blood. Normally oxygen equilibrates with blood entering the alveoli well before the blood emerges from these capillaries, and it is therefore difficult to estimate the average oxygen concentration of blood in the capillaries. Because hemoglobin has a much higher affinity for CO than for O2, concentrations of CO in the plasma remain negligible; they can be assumed equal to zero. The diffusion of CO can consequently be calculated from the rate at which CO is removed from the inhaled air and estimates of alveolar CO. Alveolar CO is usually estimated by incorporating an inert gas (e.g., He or Ne) with the CO in the inhaled gas. Patients are asked to inhale a mixture of inert gas and CO and hold it within the lungs. The diffusion capacity for CO (DLCO) can then be calculated by comparing concentrations of the CO with those of the inert gas in the exhaled air.
Figure 12: Membrane diffusion.
Diffusion of gases is mediated by the random movements of molecules, which tend to proceed from regions with higher concentrations to regions of lower concentration. Diffusion is directly proportionate to the concentration difference between the alveoli and capillaries, and the permeability and surface areas of the barrier separating these compartments, and is inversely related to the thickness of the barrier. The diffusing capacity measured with CO (DLCO) is influenced by the hemoglobin concentration of the blood, the distribution of the gas to the alveoli, and the capillary surface area, but is relatively insensitive to the thickness and permeability of the alveolar-capillary membranes. The amount of gas that diffuses through the barrier is directly proportionate to the partial pressure gradient of the gas ( p), surface area of the membrane (A), the exposure time (
p), surface area of the membrane (A), the exposure time ( t), and the permeability of the membrane (P), and is inversely proportionate to the thickness of the membrane (
t), and the permeability of the membrane (P), and is inversely proportionate to the thickness of the membrane ( x).
x).
Because most of the CO removed from the alveoli is associated with hemoglobin, anything that reduces the amount of blood in the lungs must reduce the DLCO. This includes diseases that reduce the volume of capillaries in the lungs (pulmonary vascular disease, emphysema, and pulmonary fibrosis) and anemia. Because oxygen consumption is linked to metabolism, the cardiac output must be maintained despite the loss of pulmonary vasculature, and the velocity of blood in the remaining capillaries must consequently increase. If the time of transit of red cells through the capillaries becomes sufficiently short, there may be inadequate time for oxygen to reach and combine with hemoglobin. This helps explains why patients frequently become more hypoxemic when exercising than at rest, and measurements of saturation should be made at both rest and with exercise. DLCO can be increased in patients with overperfused lungs (e.g., atrial septal defect) and asthmatics.
Effects of Hypoventilation and Venous Admixture on PaO2
Hypoventilation increases PCO2 and reduces PO2 in the arterial blood. The effect of increases in PaCO2 on alveolar PO2 (PAO2) can be estimated with the simplified alveolar gas equation:
PAO2 = PIO2 - PaCO2 / R
R designates the ratio of CO2 production to O2 consumption determined at the mouth and is referred to as the respiratory exchange ratio (R). PIO2 represents partial pressure of the inspired oxygen (about 150 mmHg). In the absence of venous admixture, the PAO2 will closely approximate PaO2. Under these circumstances, an increase in PaCO2 from 40 to 80 mmHg with an R of 0.8 results in a fall of PaO2 from 110 mmHg to 60 mmHg.
Detection of venous admixture is based on finding that PaO2 is significantly below the value calculated for PAO2. This difference, PAO2-PaO2, should be less than 10 mmHg in young persons and less than 20 mmHg in persons over 60 years old who are breathing room air, and less than 100 mmHg in those breathing 100% O2. PAO2-PaO2 can only be used to estimate venous admixture if the patient is in a steady state. For example, breath holding will cause an abrupt decrease in PaO2 and a much slower rise in PaCO2, which can result in an overestimate of venous admixture.
Conclusion
The lungs represent an organ that originated at what might be considered the wrong site, the upper gastrointestinal tract. The structure and function of the lungs represent a remarkable compromise and adaptation that allow it to serve a number of very different needs: respiration, filtration of blood flowing to the systemic circulation, and metabolism of metabolically active peptides in the circulation. Although extremely attenuated and specialized to enhance gas exchange between air and gas over an enormous surface in a very small volume, it is able to withstand environmental attacks by innumerable particles, droplets, acids, bases, gases, and organisms over the life span of the organism.
The list of suggested readings in Table 1 provides further information on the topics covered in this review.







![Figure 11 : The effect of mismatched ventilation |[Vdot]| and perfusion |[Qdot]| on arterial oxygenation. Unfortunately we are unable to provide accessible alternative text for this. If you require assistance to access this image, or to obtain a text description, please contact npg@nature.com](/gimo/contents/pt1/thumbs/gimo73-f11.jpg)