Key Points
- The adult human esophagus is an 18- to 25-cm long muscular tube that has cervical, thoracic, and abdominal parts.
- The esophagus wall is composed of striated muscle in the upper part, smooth muscle in the lower part, and a mixture of the two in the middle.
- The myenteric plexus is well developed in the smooth muscle, but is also present in the striated muscle part of the esophagus.
- The function of the myenteric plexus in the striated esophagus is not well understood.
- Esophagus develops from foregut and by week 10 is lined by ciliated epithelial cells.
- Beginning at 4 months, the ciliated epithelium starts to be replaced by squamous epithelium. At either end of the esophagus the ciliated epithelium gives rise to esophageal glands.
- The upper esophagus is derived from branchial arches 4, 5, and 6, but the derivation of the lower esophagus is not known.
- The development of various elements of esophageal wall requires coordination of a variety of genes and mediators.
- Esophageal peristalsis appears in the first trimester, and gastroesophageal reflux can be documented in the second trimester.
Introduction
From mouth to stomach, the food conduit consists of the oral cavity, pharynx, and esophagus. The esophagus serves as a dynamic tube, pushing food toward the stomach, where digestion and absorption can take place. Mucus produced by the esophageal mucosa provides lubrication and eases the passage of food. Active peristaltic contractions propel residual material from the esophagus into the stomach. During vomiting and reflux, the esophagus also serves as a passageway for gastrointestinal (GI) contents traveling retrograde from the stomach or small intestine.
Embryology
The first stages of life are divided into the embryonic and fetal periods. The embryonic period extends from fertilization to week 9. The fetal period lasts from the end of the week 9 to birth. From days 0 to 14, the human embryo develops into a bilaminar disk of ectoderm and endoderm, with the endoderm forming the lining of the yolk sac. The endoderm is the scaffold for the future digestive tract. The ectoderm gives rise to epidermis and neural plates. Through the neurulation process, the neural plates evolve to neural tube and neural crest cells. The neural tube is the precursor for the spinal cord and brain. The neural crest cells, placed between the dorsal neural tube and the overlying epidermis, migrate out to form the peripheral nervous system by week 4. On day 15, the third embryonic layer, the mesoderm, appears and provides the substrate for the connective tissue, angioblasts, smooth muscle, and serosal layers of the gut. By day 21, the mesoderm is thickened and forms longitudinal masses called the paraxial mesoderm. By day 28, the paraxial mesoderm fragments progressively from cranial to caudal into cubes of tissue called somites. This process ends with the formation of 33 to 35 somites by day 31 of embryo development.1
Mesoderm proliferation and segmentation, which takes place between the endoderm and ectoderm, induces numerous transformations in the endoderm.2 At the same time, the human embryo elongates craniocaudally and folds laterally. The dorsal part of the yolk sac, composed of endoderm, is compressed by the lateral folding of the embryo and is incorporated as a rim during the fourth week. Thus the human embryo becomes a "body cylinder" dividing the yolk sac into intraembryonic and extraembryonic parts.3 The intraembryonic part is the origin of digestive tube and its accessory glands. The extraembryonic part regresses and disappears around week 12. At this point, the early digestive system divides into foregut, midgut, and hindgut.
Gut development takes place in four major patterned axes: anterior-posterior, dorsal-ventral, left-right, and craniocaudal. Each axis development is based on the epithelial-mesenchymal interactions mediated by specific molecular pathways.4 Thus, growth factors such as Wnt5a (expressed by mesoderm), endodermal proteins Six2/Sox2, as well as Hoxa-2, Hoxa-3, and Hoxb-4 control esophageal development in the anterior-posterior axis.5 These factors affect both the esophageal environment and the neural crest cells by making the environment more permissive for neural crest cells and by preparing the neural crest cells to migrate within the esophagus4, 5 (Figure 1).
Figure 1: Primordial gut.
a: Lateral view of a 4-week embryo showing the relationship of primordial gut to yolk sac. b: Drawing of median section of the embryo showing the early digestive system and its blood supply. The primordial gut is a long tube extending the length of the embryo. Its blood vessels are derived from the vessels that supplied the yolk sac. (Source: Moore KL, Persaud TVN. The Developing Human, 7th ed. Philadelphia: Elsevier, Inc., 2003:256).
During week 4, the foregut develops a small diverticulum on its ventral surface adjacent to the pharyngeal gut. This tracheobronchial diverticulum subsequently elongates and separates gradually from the dorsal foregut through the formation of the esophagotracheal septum to become the primitive respiratory tract.
The remaining part of the foregut rapidly elongates with the craniocaudal growth of the embryonic body. In the seventh and eighth weeks, the luminal epithelium proliferates and almost completely occludes the foregut with only residual channels persisting. Unlike other species, complete occlusion of the foregut has not been observed in human embryos.6 By week 10, new vacuoles appear in the luminal cells of the foregut and coalesce to form a single esophageal lumen with a superficial layer of ciliated epithelial cells.1
During the fourth month, a stratified squamous epithelium begins to replace the ciliated epithelium, a process that continues until birth. Residual islands of ciliated epithelium at the proximal and distal ends of the esophagus remain and give rise to esophageal glands.1 Thus the primitive foregut endoderm is the origin for both the future esophageal epithelium and submucosal glands. During week 6 of gestation, the circular muscle coat and ganglion cells of the myenteric plexus form. During week 7, blood vessels enter the submucosa.
The smooth muscle of the lower esophagus and the lower esophageal sphincter (LES) are derived from the mesenchyme of the somites surrounding the foregut. The striated muscle forming the muscularis propria of the upper part of the esophagus and the upper esophageal sphincter is derived from mesenchyme of the branchial arches 4, 5, and 6. This origin explains the upper esophageal sphincter innervation by the vagal nerve (the branchial arch 5 nerve) and by the recurrent laryngeal nerve (a branch of the vagus nerve, the branchial arch 6 nerve). The embryologic origin of the gastroesophageal junction is still controversial, but gastric rotation together with augmentation of the fundus of the stomach are believed to determine its formation.7
The middle third of esophagus consists of a mixture of smooth and skeletal muscle. The origin of this mixture is controversial, with somites and endoderm influencing each other by molecular mechanisms.4 It was suggested that esophageal striated muscle arises from the smooth muscle by a process of transdifferentiation, however, it appears that the two muscle types may arise from two distinct differentiation pathways. When definitive endoderm was co-cultured with somitic mesoderm, it stimulated more smooth muscle development than skeletal muscle from the mesenchymal somitic cells.8
The smooth muscle differentiation begins after the neural crest cells colonize the gut and maturates on the rostrocaudal axis.9 Whether the circular muscle layer precedes or appears at the same time as the longitudinal muscle layer is still controversial, but both layers have been reported to mature into a rostrocaudal axis by week 9.9, 10
At the beginning of week 4, the neural crest cells enter the foregut and migrate rostrocaudally to reach the terminal hindgut by week 7 and give rise to the myenteric plexus.10 By week 6, the neural crest cells migrate centripetally through the circular muscle layer, giving rise to submucosal plexus.
Interstitial cells of Cajal (ICC) emerge from gut mesenchyme around week 9. By week 14, the ICCs form a network surrounding the myenteric plexus.9, 10 The ICCs are gut-pacemakers crucial to the generation of slow wave contractions and to neural transmission within the gut. The ICCs form after the differentiation of smooth muscle layers. Whether ICC differentiation requires neural crest cells has not been clearly established yet, and some recent studies identified ICC in the absence of neural crest cells.9, 11, 12
The development of concentric layers of smooth muscle, ICCs, and neural crest cells (as precursors of the enteric nervous system) is a coordinated process, controlled by numerous genes and signaling molecules including transcription factors (e.g., Phox2b, Sox10, Pax3, Mash1), components of the RET (RET proto-oncogene ) and ET(Endothelin)-3/EDNRB (endothelin receptor type B ) signaling pathways, secreted proteins (Hedgehog, BMPs(bone morphogenetic proteins)), neurotrophic factors (e.g., neurotrophin-3), and extracellular matrix (ECM) molecules (e.g., laminin).9, 13, 14, 15, 16 Perturbations in this coordinated process could result in clinical morbidities such as Hirschsprung disease, where the hindgut (usually colon) is devoid of enteric neurons and glial cells.17
The myenteric plexus has cholinesterase activity by week 9.5 and ganglion cells are differentiated by week 13. Several investigators have suggested that the esophagus is capable of peristalsis in the first trimester.18 Three different esophageal motility patterns have been described in the second trimester: simultaneous opening of the esophageal lumen from the oropharynx to the lower esophageal sphincter, propulsive peristaltic contractions, and reflux from stomach into the esophagus.19 Although peristaltic movements have been observed in ultrasound images during the second trimester, at birth the propagation of the peristalsis along the esophagus and at the LES is immature, resulting in frequent regurgitation of food during the newborn period. The pressure at the LES approaches that of the adult at 3 to 6 weeks of age.6
Adult Anatomy
Gross Anatomy
The esophagus is a flattened muscular tube of 18 to 26 cm from the upper sphincter to the lower sphincter. Between swallows the esophagus is collapsed but the lumen can distend to approximately 2 cm in the anterior-posterior dimension and up to 3 cm laterally to accommodate a swallowed bolus.20
The esophagus connects the pharynx to the stomach. Beginning in the neck, at the pharyngoesophageal junction (C5-6 vertebral interspace at the inferior border of the cricoid cartilage), the esophagus descends anteriorly to the vertebral column through the superior and posterior mediastinum. After traversing the diaphragm at the diaphragmatic hiatus (T10 vertebral level) the esophagus extends through the gastroesophageal junction to end at the orifice of the cardia of the stomach (T11 vertebral level).
Topographically, there are three distinct regions: cervical, thoracic, and abdominal. The cervical esophagus extends from the pharyngoesophageal junction to the suprasternal notch and is about 4 to 5 cm long. At this level, the esophagus is bordered anteriorly by the trachea, posteriorly by the vertebral column, and laterally by the carotid sheaths and the thyroid gland.
The thoracic esophagus extends from the suprasternal notch to the diaphragmatic hiatus, passing posterior to the trachea, the tracheal bifurcation, and the left main stem bronchus. The esophagus lies posterior and to the right of the aortic arch at the T4 vertebral level. From the level of T8 until the diaphragmatic hiatus the esophagus lies anteriorly to the aorta.
The abdominal esophagus extends from the diaphragmatic hiatus to the orifice of the cardia of the stomach. Forming a truncated cone, about 1 cm long, the base of the esophagus transitions smoothly into the cardiac orifice of the stomach. The abdominal esophagus lies in the esophageal groove on the posterior surface of the left lobe of the liver.
Two high-pressure zones prevent the backflow of food: the upper and lower esophageal sphincter. These functional zones are located at the upper and lower ends of the esophagus but there is not a clear anatomic demarcation of the limits of the sphincters.
Structurally, the esophageal wall is composed of four layers: innermost mucosa, submucosa, muscularis propria, and adventitia. Unlike the remainder of the GI tract, the esophagus has no serosa.
On endoscopy, the esophageal lumen appears as a smooth, pale pink tube with visible submucosal blood vessels. The transition from esophageal to gastric mucosa is known as the Z-line and consists of an irregular circumferential line between two areas of different colored mucosa. The gastric mucosa is darker than the pale pink esophageal mucosa. Peristaltic waves can be seen during endoscopic examination.
Blood Supply
The rich arterial supply of the esophagus is segmental (Figure 2). The branches of the inferior thyroid artery provide arterial blood supply to the upper esophageal sphincter and cervical esophagus. The paired aortic esophageal arteries or terminal branches of bronchial arteries supply the thoracic esophagus. The left gastric artery and a branch of the left phrenic artery supply the LES and the most distal segment of the esophagus. The arteries supplying the esophagus end in an extensive, dense network in the submucosa. The copious blood supply and network of potentially anastomotic vessels may explain the rarity of the esophageal infarction.
Figure 2: Arterial blood supply of the esophagus
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The venous supply is also segmental. (Figure 3). From the dense submucosal plexus the venous blood drains into the superior vena cava. The veins of the proximal and distal esophagus drain into the azygous system. Collaterals of the left gastric vein, a branch of the portal vein, receive venous drainage from the mid-esophagus. The submucosal connections between the portal and systemic venous systems in the distal esophagus form esophageal varices in portal hypertension. These submucosal varices are sources of major GI hemorrhage in conditions such as cirrhosis.
Figure 3: Venous drainage of the esophagus
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
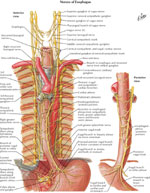
Innervation
The esophagus, like the rest of the viscera, receives dual sensory innervation, traditionally referred to as parasympathetic and sympathetic, but more properly based on the actual nerves, vagal, and spinal21 (Figure 4).
Figure 4: Parasympathetic and sympathetic innervation of the esophagus
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The vagal afferent neurons compose 80% of the vagal trunk and have cell bodies in the nodose ganglia and project to the nucleus solitarius.22 Vagal afferents merging from the esophageal smooth muscle layer are sensitive to mechanical distention, whereas polymodal (responding to multiple modalities of stimuli) vagal afferents with receptive fields in the mucosa are sensitive to various osmo-, chemo-, thermo-, and mechanical intraluminal stimuli.22 In general, vagal afferents do not play a direct role in visceral pain transmission, but through mechanoceptors vagal afferents transduce pressure into painful sensations.21
The spinal afferents have their cell bodies in the dorsal root ganglia and terminate in the spinal column and in the nucleus gracilis and cuneatus in the brainstem. From there, they project, through the thalamus, to primary sensory and insular cortical areas.23 The spinal afferents merging from nerve endings in the muscle layer and serosa act as nociceptors for perception of discomfort and pain and are mechanosensitive.24 The spinal afferents merging from intraepithelial nerve endings are involved in mediating acid-induced pain during topical exposure to intraluminal acid.25 Many of the spinal afferents contain calcitonin gene-related peptide and substance P, which are neurotransmitters that are important in mediating visceral nociception.21, 22
The motor innervation of the esophagus is predominantly via the vagus nerve. The cell bodies of the vagal efferent fibers innervating the upper esophageal sphincter and the proximal striated muscle esophagus arise in the nucleus ambiguus, whereas fibers destined for the distal smooth-muscle segment and the LES originate in the dorsal motor nucleus of the vagus nerve.
The esophagus receives parasympathetic and sympathetic innervation that regulates glandular secretion, blood vessel caliber, and the activity of striated and smooth muscle. The parasympathetic nerve supply comes from the nucleus ambiguus and dorsal motor nucleus of the vagus nerve and provides motor innervation to the esophageal muscular coat and secretomotor innervation to the glands. The sympathetic nerve supply comes from the cervical and the thoracic sympathetic chain (spinal segments T1–T10) and regulates blood vessel constriction, esophageal sphincters contractions, relaxation of the muscular wall, and increases in glandular and peristaltic activity.
The thin nerve fibers and numerous ganglia of the intramural myenteric and the submucosal plexi provide the intrinsic innervation of the esophagus. The ganglia that lie between the longitudinal and the circular layers of the tunica muscularis form the myenteric or Auerbach's plexus, whereas those that lie in the submucosa form the submucous or Meissner's plexus. Auerbach's plexus regulates contraction of the outer muscle layers, whereas Meissner's plexus regulates secretion and the peristaltic contractions of the muscularis mucosae. A network of fibers interconnects these two plexi.28 The ganglia of the myenteric plexus are more numerous in the smooth muscled esophagus than in the striated muscle esophagus.29 In the smooth muscled esophagus, the neurons of the myenteric plexus are relay neurons between the vagus and the smooth muscle. In the striated muscle the role of the neurons of the myenteric plexus is largely unknown.30
Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been used to map the central nervous system projections from the esophagus. Esophageal stimulation at the subliminal and liminal levels is sensed peripherally and transmitted to the brain for further processing and modulation. Esophageal sensory innervation is carried by the vagus nerve to the nodose ganglion and projects through the brainstem, through the thalamus, to terminate in the cortex.26, 27 Regions that are activated by esophageal stimulation include secondary sensory and motor cortex, parieto-occipital cortex, anterior and posterior cingulated cortex, prefrontal cortical cortex, and the insula.31
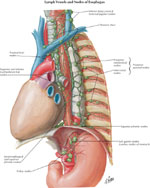
Lymphatics
Lymphatic drainage in the esophagus consists of two systems: the lymph channels and lymph nodules. (Figure 5).
Figure 5: Lymphatic drainage
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The lymph channels begin in the esophageal tissue space as a network of endothelial channels (20–30  m) or as blind endothelial sacculations (40–60
m) or as blind endothelial sacculations (40–60  m).32 The location of the lymphatic capillary origin is not known precisely. Some authors propose that precapillary spaces exist in the lamina mucosa, but others contend that there is an absence of true lymphatic capillaries in the upper and middle levels of the lamina mucosa.6 Electron microscopic studies show anastomotic lymph capillaries in the lower mucosal levels and small lymphatic vessel in the submucosa.
m).32 The location of the lymphatic capillary origin is not known precisely. Some authors propose that precapillary spaces exist in the lamina mucosa, but others contend that there is an absence of true lymphatic capillaries in the upper and middle levels of the lamina mucosa.6 Electron microscopic studies show anastomotic lymph capillaries in the lower mucosal levels and small lymphatic vessel in the submucosa.
Lymph capillaries drain into collecting lymph channels (100–200  m) that continue through the esophageal muscular coat and are distributed parallel to the long axis of the esophagus. Paired semilunar valves within the collecting channels determine the direction of flow. The collecting lymph channels merge into small trunks that open into the regional lymph nodes.
m) that continue through the esophageal muscular coat and are distributed parallel to the long axis of the esophagus. Paired semilunar valves within the collecting channels determine the direction of flow. The collecting lymph channels merge into small trunks that open into the regional lymph nodes.
As with esophageal innervation, the lymphatic drainage of the esophagus differs in the striated and smooth muscle regions. The lymphatics from the proximal third of the esophagus drain into the deep cervical lymph nodes, and subsequently into the thoracic duct. The lymphatics from the middle third of esophagus drain into the superior and posterior mediastinal nodes. Lymphatics of the distal third of the esophageal follow the left gastric artery to the gastric and celiac lymph nodes. There are considerable interconnections among these three drainage regions primarily owing to the dual embryologic origin of lymphatic pathways from branchiogenic and body mesenchyme.7, 32 The bidirectional lymph flow in this region is responsible for the spread of malignancy from the lower esophagus to the upper esophagus.
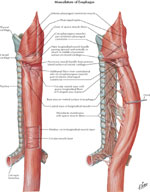
Musculature of the Esophagus
The muscular coat consists of an external layer of longitudinal fibers and an internal layer of circular fibers (Figure 6). The longitudinal fibers are arranged proximally in three fasciculi. The ventral fasciculus is attached to the vertical ridge on the posterior surface of the lamina of the cricoid cartilage by the tendocricoesophageus. The two lateral fasciculi are continuous with the muscular fibers of the pharynx. The longitudinal fibers descend in the esophagus and combine to form a uniform layer that covers the outer surface of the esophagus.
Figure 6: Musculature of the esophagus
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The circular muscle layer provides the sequential peristaltic contraction that propels food toward the stomach. The circular fibers are continuous with the inferior constrictor muscle of the hypopharynx; they run transverse at the cranial and caudal regions of the esophagus, but oblique in the body of the esophagus. The internal muscular layer is thicker than the external muscular layer. Below the diaphragm, the internal circular muscle layer thickens and the fibers become semicircular and interconnected, constituting the intrinsic component of the LES.
Accessory bands of muscle connect the esophagus and the left pleura to the root of the left bronchus and the posterior of the pericardium. The muscular fibers in the cranial part of the esophagus are red and consist chiefly of striated muscle; the intermediate part is mixed; and the lower part, with rare exceptions, contains only smooth muscle.
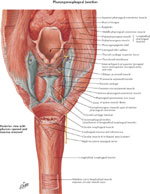
Upper Esophageal Sphincter
The upper esophageal sphincter (UES) is a high-pressure zone situated between the pharynx and the cervical esophagus (Figure 7). The UES is a musculocartilaginous structure composed of the posterior surface of the thyroid and cricoid cartilage, the hyoid bone, and three muscles: cricopharyngeus, thyropharyngeus, and cranial cervical esophagus. Each muscle plays a different role in UES function.33 These three muscles spread upward, posteriorly, where they insert into the esophageal submucosa after crossing the muscle bundles of the opposite side. The thyropharyngeus muscle is obliquely oriented, whereas the cricopharyngeus muscle is transversely oriented. Between these two muscles, there is a zone of sparse musculature—the Killian's triangle, from which Zenker's diverticulum might emerge.
Figure 7: Upper esophageal sphincter and upper esophageal musculature
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The cricopharyngeus (CP) muscle is a striated muscle attached to the cricoid cartilage. It forms a C-shaped muscular band that produces maximum tension in the anteroposterior direction and less tension in lateral direction.34 Structurally, biochemically, and mechanically, the CP is different from the surrounding pharyngeal and esophageal muscles. It is composed of a mixture of fast- and slow-twitch fibers, with the slow fibers being predominant and having a diameter of 25 to 35  m. The CP is suspended between the cricoid processes, surrounds the narrowest part of pharynx, and extends caudally where it blends with the circular muscle of the cervical esophagus.
m. The CP is suspended between the cricoid processes, surrounds the narrowest part of pharynx, and extends caudally where it blends with the circular muscle of the cervical esophagus.
The cervical esophagus contains predominantly striated muscle fibers, but occasionally smooth fibers are found in the center of the muscle.33 The muscle fibers are arranged in two layers: the external layer containing longitudinal arranged fibers, and the internal layer containing circular or transversely arranged fibers. The external longitudinal layer of the cervical esophagus originates from the dorsal plane of the cricoid cartilage constituting a sparse muscle area: the Laimer's triangle. The external longitudinal layer courses down the length of the entire esophagus. At its distal end the longitudinal fibers become more oblique and end along the anterior and posterior gastric wall.35 The internal circular layer of muscle originates at the level of cricoid cartilage and in descending forms incomplete circles.35
Upper esophageal sphincter function is controlled by a variety of reflexes that involve afferent inputs to the motor neurons innervating the sphincter. These reflexes elicit either contraction or relaxation of the tonic activity of the UES. Inability of the sphincter to open or discoordination of timing between the opening of the UES with the pharyngeal push of ingested contents leads to difficulty in swallowing known as oropharyngeal dysphagia.33
Lower Esophageal Sphincter
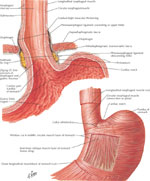
The lower esophageal sphincter is a high-pressure zone located where the esophagus merges with the stomach (Figure 8). The LES is a functional unit composed of an intrinsic and an extrinsic component. The intrinsic structure of LES consists of esophageal muscle fibers and is under neurohormonal influence. The extrinsic component consists of the diaphragm muscle, which functions as an adjunctive external sphincter that raises the pressure in the terminal esophagus related to the movements of respiration. (Figure 9). Malfunction in any of these two components is the cause of gastroesophageal reflux and its subsequent symptoms and mucosal changes.36
Figure 8: Gastroesophageal mucosal junction and muscular arrangement at the lower esophagus
(Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
Figure 9: Diaphragmatic crura and esophageal opening viewed from below (a) and as viewed from above (b).
The esophageal opening is created by a loop of right crux of the diaphragm. (Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
The intrinsic component of the LES is composed of circular layers of the esophagus, clasp-like semicircular smooth muscle fibers on the right side, and sling-like oblique gastric muscle fibers on the left side.37 The circular muscles of the LES are thicker than the adjacent esophagus. The clasp-like semicircular fibers have significant myogenic tone but are not very responsive to cholinergic stimulation, whereas the sling-like oblique gastric fibers have little resting tone but contract vigorously to cholinergic stimulation.37
The extrinsic component of the LES is composed of the crural diaphragm, which forms the esophageal hiatus, and represents a channel through which the esophagus enters into the abdomen. The crural diaphragm encircles the proximal 2 to 4 cm of the LES, and determines inspiratory spike-like increases in LES pressure as measured by esophageal manometry.38
The endoscopic localization of the LES is different from the manometric localization. The endoscopic localization of the LES is presumably determined by changes in the esophageal mucosa color owing to transition from nonstratified squamous esophageal epithelium to the gastric mucosa, changes known as the Z-line. A study correlating manometric and endoscopic localization of the LES (Z-line) found that the functional location of LES was 3 cm distal to the Z-line.39, 40
Three-dimensional (3D) manometric measures of the lower esophageal high-pressure zone showed a marked radial and longitudinal asymmetry, with higher pressures toward the left posterior direction. Radial pressures peak at the respiratory inversion point during esophageal manometry where inspiration converts from a positive pressure as measured by pressure sensors to a negative pressure as the pressure sensor enters the intrathoracic cavity. The high-pressure zone appears to coincide with asymmetric thickening of the muscular layer at the gastroesophageal junction, which corresponds to the gastric "sling" fibers and to the semicircular "clasp" fibers.41
The LES is innervated by both parasympathetic (vagus) and sympathetic (primarily splanchnic) nerves, with the vagal pathways being essential for reflex relaxation of LES.42 Vagal sensory afferents from the LES and distal esophagus end in nucleus tractus solitarius of the hindbrain. The motor innervation of the LES is topographically provided through preganglionic fibers from the dorsal motor nucleus of the vagus. The dorsal motor nucleus and the tractus solitarius nucleus form a dorsal vagal complex in the hindbrain that coordinates reflex control of the sphincter.42
Histology
Light Microscopy
The wall of the esophagus consists of four layers: mucosa, submucosa, muscularis propria, and adventitia. Unlike other areas of the GI tract, the esophagus does not have a distinct serosal covering. This allows esophageal tumors to spread more easily and makes them harder to treat surgically.43 The missing serosal layer also makes luminal disruptions more challenging to repair.
The mucosa is thick and reddish cranially and more pale caudally. It is arranged in longitudinal folds that disappear upon distention. It consists of three sublayers:
- Mucous membrane: a nonkeratinized squamous epithelium. It covers the entire inner surface of the esophagus, except the LES, where both squamous and columnar epithelium may coexist. The mucous membrane is composed of
- stratum basale, including basophilic cells that can divide and replenish the superficial layers
- stratum intermedium
- stratum superficialis.
- Lamina propria: a thin layer of connective tissue
- Muscularis mucosa: a thin layer of longitudinally, irregularly arranged smooth muscle fibers. The muscularis mucosa extends through the entire esophagus and continues into the rest of the GI tract, being much thinner in the proximal part of the esophagus than in its distal part.44 At the pharyngeal end of the esophagus, the muscularis mucosa is represented by a few scattered smooth muscle fibers. Caudally, approaching the cardiac orifice, the muscularis mucosa forms a thick layer. The muscularis mucosa separates the lamina propria from the submucosa and retracts when it is sectioned during surgical procedures.
Submucosa
The submucosa contains connective tissue as well as lymphocytes, plasma cells, nerve cells (Meissner's plexus), vascular network (Heller plexus), and mucous glands. The esophageal glands are small racemose glands (which have acini arranged like grapes on a stem) of mucous type. Their secretion is important in esophageal clearance and tissue resistance to acid.45
Muscularis Propria
The muscularis propria is responsible for motor function. The upper 5% to 33% is composed exclusively of striated (skeletal) muscle, and the distal 33% is composed of smooth muscle. In between there is a mixture of both, called the transition zone. Functionally the transition zone can be observed with manometry as a region where there is no significant contraction amplitude during a peristaltic contraction that travels down the body of the esophagus.46
Adventitia
The adventitia is an external fibrous layer that covers the esophagus, connecting it with neighboring structures. It is composed of loose connective tissue and contains small vessels, lymphatic channels, and nerve fibers.
Developmental Anomalies
Tracheoesophageal Fistula and Atresia
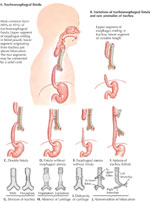
Tracheoesophageal fistula and esophageal atresia are the most frequent congenital esophageal abnormalities. Tracheoesophageal fistula results from defects in the separation of the respiratory tract from the foregut. Esophageal atresia results from failure of the primitive gut to recanalize during week 8. Five types of congenital esophageal atresia with or without tracheoesophageal fistula have been recognized32: (Figure 10).
Figure 10: Main types of tracheoesophageal fistulae
The figure shows both tracheoesophageal fistula (A-E) and tracheal abnormalities ( F-J). Note that A-E do not correspond with the classification of the type of tracheoesophageal fistula. A shows type C, B type B, C type D, D type E, and E type A tracheoesophageal fistula, respectively. (Source: Netter medical illustration with permission from Elsevier. All rights reserved.)
- Type A—pure esophageal atresia (7.6%)
- Type B—esophageal atresia with proximal tracheoesophageal fistula (0.8%)
- Type C—esophageal atresia with distal tracheoesophageal fistula (86.5%)
- Type D—esophageal atresia with proximal and distal tracheoesophageal fistula (0.7%)
- Type E—"H-type" tracheoesophageal fistula without esophageal atresia (4.4%)
The most common variant is type C, with an incidence of 86.5%. It presents as a blind esophageal pouch with a fistula between the trachea and the distal esophagus. The fistula often enters the trachea close to the carina.
The second most common anomaly is type A, pure esophageal atresia without tracheoesophageal fistula.
Esophageal atresia with tracheoesophageal fistula occurs in one in 3000 to one in 5000 births. In 93% cases of esophageal atresia there are associated malformations (VACTERL) and in 7% esophageal atresia is found as a solely malformation.47 The VACTERL association describes the following more commonly associated combination of defects: vertebral, anorectal, cardiac, tracheal, esophageal, renal, and limb. When studies over the last 30 years had been conducted, there were no changes in the incidence of tracheoesophageal fistula and esophageal atresia, but decrease in the subsequent mortality had been found.48
Clinically, esophageal atresia should be suspected when polyhydramnios is present in the mother. Polyhydramnios develops as a consequence of the inability of the fetus to swallow and thus absorb amniotic fluid. On physical examination of the newborn, a scaphoid abdomen and the regurgitation of saliva are indicators of GI obstruction. If rapid onset of choking, coughing, and regurgitation are present at the first feeding, suspicion of esophageal atresia is raised. When esophageal atresia is suspected, a nasogastric tube insertion should be attempted. Failure to pass a nasogastric tube into the stomach, together with chest radiography showing air/contrast collection in the upper esophageal segment, confirms the diagnosis of esophageal atresia. If atresia is present, an inserted nasogastric tube will typically stop at 10 to 12 cm.
The atretic upper esophagus ends in a blind pouch, and the trachea communicates with the distal esophagus. Air enters the GI tract via the tracheoesophageal fistula and the newborn presents clinically with a gas-filled abdomen and frequent aspiration pneumonias due to gastric reflux into the respiratory tract through the fistula. Confirmation of the type of esophageal atresia is obtained by esophagography with or without bronchoscopy.
Treatment of esophageal atresia and tracheoesophageal fistula is surgical, and the procedure depends on the type of esophageal atresia and the distance between the two esophageal segments. Thus, if the distance between the esophageal segments is short, end-to-end anastomosis is the preferred surgical procedure. If the distance between segments is long, lengthening of the upper esophageal segments in some cases can be achieved using bougienage or intraoperative myotomy.47 A colonic segment may be inserted between the esophageal segments when the lengthening of the upper esophageal segments is not possible or adequate.
The results of surgical correction are generally excellent when the esophageal malformation is an isolated anomaly. Overall outcome is determined by the associated genetic malformation, age of infant, and birth weight.47 Postoperative complications develop more often in very premature and premature infants, with term infants having a higher survival rate and better prognostic than preterm infants.49
Long-term outcome after esophageal atresia repair indicates that the most significant problems are GI and respiratory symptoms. Reported incidence of these types of complications in adults and children varies from 30% to 60%, with respiratory infections being more severe in childhood and eventually improving through adolescence.50 The most frequent GI complications are gastroesophageal reflux disease (GERD) (48%) and dysphagia (43%).50
Gastroesophageal reflux disease symptoms may be alleviated by pharmacologic treatment or with time, owing to the patient's accommodation with the medical condition, but there is a higher risk of developing chronic esophagitis and Barrett's metaplasia compared to the normal population.51 This increased risk is owing to impaired esophageal luminal acid clearance. Nissen fundoplication is the preferred surgical treatment for GERD, postesophageal atresia repair, with a 15% to 30% failure rate and requirement for reoperation.52 Dysphagia, owing to esophageal dysmotility, has been found to be the most troublesome GI symptom, with negative impact on the quality of life in adults who have been treated for esophageal atresia as infants.53
The aesthetic aspects of the surgical treatment are now becoming more important in adults with esophageal atresia owing to good functional results of applied treatments.53
Congenital Esophageal Stenosis
Congenital esophageal stenosis represents narrowing of the esophageal lumen. It can be located at any level of the esophagus, but is more frequent in the distal third. It appears either as a web (membranous diaphragm) or a long segment of esophagus with a threadlike lumen (fibromuscular stenosis). Usually, the esophageal stenosis results from incomplete esophageal recanalization during the eighth week of human embryologic development, but it also may result from failure of esophageal blood vessels to develop in the affected area.32 The presence of respiratory tissue in some esophageal stenosis cases (as hyaline cartilage, or respiratory mucus gland) suggests an incomplete separation of the respiratory bud as the etiology in some cases.
The incidence of esophageal stenosis is low, occurring in 1 in every 25,000 live births.54 In a recent study, no significant changes in population characteristics of patients with congenital esophageal stenosis were observed over the last three decades, but mortality and postoperative complication rates decreased and associated cardiac anomalies became by far the most important risk factor for mortality.48
Congenital esophagus stenosis has been classified histologically as follows:
- Group I: tracheobronchial rests (cartilage, respiratory mucus glands, ciliated epithelium)
- Group II: membranous diaphragm
- Group III: fibromuscular stenosis
Patients may present with aspiration and recurrent pneumonia in early infancy. Dysphagia and regurgitation of solid food are symptoms that appear later in childhood, when more solid foods are added to the child's diet. If the esophageal stenosis is not severe, its diagnosis may be postponed until adulthood, when a history of long-standing solid foods dysphagia could be documented.
Once suspicion of congenital esophageal stenosis is raised, an upper endoscopy with biopsy and pH monitoring of the esophagus help with diagnosis and eliminate the possibility of a stricture secondary to gastroesophageal reflux. Barium swallow typically demonstrates narrowing of the esophagus lumen.
The first approach to treatment is with dilation, which may include bougienage or pneumatic dilation under fluoroscopic guidance. Dilation may be diagnostic and therapeutic. Although pneumatic dilation expands and stretches a fibromuscular stenosis, a persistent "waist" in the balloon may indicate a cartilaginous ring and the necessity for surgical resection. The efficacy of dilatation seems to be limited and may even result in severe complications such as chest pain, mucosal tears, or esophageal rupture. When pneumatic dilation fails, surgical treatment may be required for removal of the abnormal segment.55
Laser lyses of webs or stenosis stenting have been described and may be attempted in selected cases.49
When respiratory tissue is present on esophageal stenosis biopsy, surgical removal of the involved segment is necessary owing to high risk of malignant transformation.55
Congenital Esophageal Duplication and Duplication Cyst
Foregut duplications include esophageal cysts (tubular duplications) and bronchogenic cysts. They originate from failure of the primitive foregut to become completely vacuolated during the embryonic life. These cyst- or tube-like structures develop independently and rarely are in continuity with the esophagus. They can be associated with other congenital malformations, such as tracheoesophageal fistulas, spinal abnormalities, esophageal atresia distal to duplication, and other segmental GI duplications (small bowel is more frequent).56
Duplications of the GI tract have three common characteristics:
- They are contiguous with some segment of the GI tract.
- They are lined by alimentary epithelium.
- They have smooth muscle in theirs walls.44
The congenital duplication cysts represent 0.5% to 2.5% of esophageal benign tumors.57, 58 Originating from foregut, they could be attached to the esophagus or to the tracheobronchial system. They are lined by squamous columnar, cuboid, or ciliated epithelium, surrounded by two layers of smooth muscle.59 They are usually located in the right posterior mediastinum. Usually they are accidentally discovered on chest computed tomography (CT) scans and are asymptomatic. Cysts may become symptomatic owing to complications such as respiratory system compression (causing stridor, cough, or tachypnea), digestive system compression (causing chest pain or dysphagia), cardiac compression (causing cardiac arrhythmias), infarction, rupture, or, rarely, neoplastic dysplasia.60, 61
Congenital esophageal duplications may be separated from the esophagus or may share a common wall. Duplications may also contain gastric mucosa. Duplications of the esophagus can be associated with vertebral anomalies and intraspinal cysts and often are associated with intraabdominal intestinal duplications.60
The diagnosis of esophageal duplication can be made by CT chest exam or MRI body exam. A chest x-ray may demonstrate a soft tissue mass with a mediastinal shift. Barium swallow can detect tubular esophageal duplication, but miss the esophageal cyst that does not communicate with the esophageal lumen.61 Ultrasound can help distinguish a solid from a cystic mass, and barium contrast study can demonstrate extrinsic compression of the esophagus. A CT scan delineates anatomy of the mass prior to surgical resection, and a nuclear (technetium) scan may help identify ectopic gastric mucosa.
Definitive treatment involves complete surgical resection of the duplication, even for asymptomatic cysts.
Congenital Esophageal Rings
The congenital esophageal ring is a concentric extension of the normal esophageal tissue, usually consisting of different anatomic layers including mucosa, submucosa, and sometimes muscles. The location is variable, but most are found in the distal esophagus. There are three types of esophageal rings: types A, B. and C.62
Esophageal rings may originate from incomplete vacuolization of the esophageal columnar epithelium during early embryonic life; however, they are also associated with immunologic63 or inflammatory conditions (such as scleroderma, chronic graft-versus-host disease),64, 65 and gastroesophageal reflux.66
The type A esophageal ring is a muscular ring located roughly 2 cm proximal to the squamocolumnar junction and it represents a proliferation of the proximal border of the LES. It consisting of three layers: mucosa, submucosa, and muscularis propria. It is the least frequent type and usually asymptomatic.67
The type B or Schatzki's ring is a mucosal ring located at the squamocolumnar junction. Because it is difficult to exactly localize the squamocolumnar junction and the LES, the exact anatomic relationship between the Schatzki's ring and squamocolumnar junction remains controversial. Typically it is associated with the proximal margin of a hiatal hernia. It consists of two layers, mucosa and submucosa, having squamous epithelium on its upper surface and columnar epithelium on its lower surface.68
The type B ring is the most common esophageal ring and is found in 6% to 14% of subjects undergoing an upper GI series. No differences in the prevalence of rings based on gender has been noticed, but they appear to be more common in subjects over 40 years old.62 Characteristically, Schatzki's ring causes intermittent dysphagia for solid food, a common complication being meat impaction (steakhouse syndrome). Esophageal rings usually exist as a single lesion but can be multiple. Owing to its mucosal nature, Schatzki's ring has been proposed to be caused by GERD, but no clinical association had been found.62
The type C esophageal ring is an indentation caused by the diaphragmatic crura, sometimes seen on radiographic studies. It is never symptomatic. Routine upper GI series, including barium radiography and upper esophagogastric endoscopy are diagnostic for this type of ring. The prognosis is good, and patients with symptoms are advised to change their dietary habits and to cut and chew all food carefully. If no improvement in the symptoms is noticed, serial progressive dilations are recommended.62
Congenital Esophageal Webs
The congenital esophageal web is defined as a thin, usually eccentric, transverse membrane. It consists of two layers, mucosa and submucosa, and is formed from connective tissue covered by normal squamous epithelium. Its location is variable, but most frequently is found in the cervical esophagus, where it is frequently associated with heterotopic gastric mucosa. Most cases are asymptomatic. Intermittent dysphagia for solid food is the usually complaint. Esophageal web occurs more frequently in females.62
As for congenital esophageal rings, it is thought that webs result from incomplete vacuolization of the esophageal columnar epithelium during early embryonic life. They were also associated with iron-deficiency conditions, blistering skin diseases (such as epidermolysis bullosa),69 heterotopic gastric mucosa in the upper esophagus,70 and gastroesophageal reflux.66
Radiographic techniques are the most sensitive diagnostic methods and esophageal webs may be confused with esophageal stenosis. Treatment consists of bougienage, and rarely transendoscopic incision or surgical resection is necessary.