Key Points
- Gastroesophageal reflux disease is a widespread public health concern. It can lead to the development of Barrett's epithelium, which confers a higher risk of esophageal adenocarcinoma.
- Surgical therapy restores the mechanical barrier of the lower esophageal sphincter and prevents reflux of gastric contents into the esophagus.
- Current surgical techniques emphasize return of the esophagogastric junction (EGJ) to the abdominal cavity, full mobilization of the gastric fundus, snug crural closure, and a short tension-free fundoplication.
- Esophageal stricture and erosive esophagitis can be treated successfully with antireflux surgery (ARS).
- Inasmuch as the finding of Barrett's esophagus (BE) represents long-standing severe GERD, these patients should be referred for surgery based on symptom control and disease-related complications. There is insufficient evidence to support ARS in the prevention of esophageal adenocarcinoma.
- The best predictors of a good surgical outcome are typical symptoms, objectification of reflux events, and correlation of these events with symptoms.
- Indications for operation include symptoms not well controlled by medication, intolerance to medication, atypical symptoms not well controlled by medication, complicated GERD, and desire for freedom from medication.
- The Nissen fundoplication is the procedure of choice for the treatment of GERD in patients with or without esophageal dysmotility. Evidence suggests dysmotility is improved after fundoplication.
- Partial fundoplications should be reserved for patients with achalasia, and those whose esophageal manometry reveals amplitudes less than 30 mmHg.
- The most common cause of mechanical fundoplication failure is transhiatal herniation into the chest.
- In experienced hands, the laparoscopic Nissen fundoplication has over a 90% satisfaction rate at 5 years. Recently available 10-year data indicate satisfaction rates remain high at 88%.
- Redo surgery for identifiable mechanical failure after fundoplication can result in a good outcome in the majority of patients and can often be performed laparoscopically.
Introduction
With nearly half of Americans experiencing heartburn symptoms at least monthly, gastroesophageal reflux disease (GERD) is a serious health concern in the Western world. In Europe, epidemiologic studies put the prevalence of GERD between 9% and 42%.1 In Asia, the prevalence of GERD is lower than in the United States. However, rates of GERD now approach those seen in Western countries. In Japan, the incidence of esophagitis is reported to be between 14% and 16%.2 Gastroesophageal reflux disease increases the risk of esophageal stricture, Barrett's esophagus (BE), and esophageal cancer, and has a negative impact on work productivity and quality of life.3, 4, 5 The modern era of GERD therapy have brought advances in diagnosis and treatment, and subsequently a better understanding of the pathophysiology of GERD. Improved medical therapies in the form of type 2 histamine receptor antagonists and proton pump inhibitors (PPIs) have brought both symptomatic relief and effective resolution of esophageal mucosal damage, which may help to ameliorate some of the long-term effects of GERD. Medical therapy demands lifelong use in the majority of patients with GERD and fails to prevent the reflux of bile or gastric contents. Antireflux surgery (ARS) can provide a permanent anatomic and physiologic cure that provides resolution of symptoms and helps prevent the adverse consequences of ongoing esophageal exposure to gastric contents.
History of Antireflux Surgery
It was not until the early 1900s when radiologic studies became ubiquitous that hiatal hernia was recognized as a pathologic entity. Early attempts at repair centered on hernia reduction and hiatal closure without fundoplication. Because the underlying defect of the lower esophageal sphincter (LES) was not repaired, surgery failed to control symptoms, and the procedure was not widely embraced.
In 1951, Philip Allison and Norman Barrett established the causal relationship among hiatal hernia, gastroesophageal reflux, and erosive esophagitis. Allison described the crural sling and clasp musculature as the anatomic correlate to the LES and the primary mechanism that prevents pathologic reflux. His method to restore the antireflux mechanism included reduction of the herniated cardia with suture fixation to the abdominal surface of the diaphragm followed by loose closure of the hiatus. Unfortunately, his attempts at surgical repair fell short and he had a long-term recurrence rate of 49% at 20 years.
In 1939, Rudolf Nissen improvised a fundoplication to protect an esophagogastric anastomosis in a young man with a penetrating esophageal ulcer. During follow-up, Nissen noted that the patient's reflux symptoms were eradicated. Some years later, and disappointed with contemporary hiatal hernia repairs, Nissen performed a fundoplication on a man with an incarcerated paraesophageal hernia. The clinical outcome was excellent. He published the first description of this procedure in 1956, thereby ushering in the modern era of antireflux surgery.6
Principles of Surgical Repair
Three mechanisms prevent excessive esophageal exposure to refluxate. First, the propulsive action of the esophagus clears ingested material and physiologic reflux; this peristaltic activity serves to limit the contact time of these substances with the esophageal mucosa. Second, the LES is a region of high pressure located at the esophagogastric junction and is the primary mechanism that prevents pathologic reflux. The tonic opposition of the collar sling musculature (greater curvature) and the clasp fibers (lesser curvature) at the level of the cardia creates this region of high pressure (Figure 1). This area is commonly referred to as the gastroesophageal flap valve based on the endoscopic appearance during retroflexion. Finally, proper gastric emptying is required to eliminate the source of refluxate and prevent elevated gastric pressure with subsequent retrograde "decompression" into the esophagus.
Figure 1: Opposing sling and clasp muscle fibers. The longitudinal muscle layer of the stomach has been cut away to show the opposing sling and clasp muscle fibers.
These fibers sit in tonic opposition until a swallow triggers receptive relaxation. It is thought that progressive stretching of these fibers leads to valve incompetence and subsequent gastroesophageal reflux disease (GERD). Antireflux procedures restore the anatomic barrier by re-creating the one-way valve of the lower esophageal sphincter (LES). (Source: Jobe BA, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol 2004;99(2):241 with permission from Blackwell Publishing.)
All antireflux procedures can be divided into two broad groups: total (360 degrees) and partial (less than 360 degrees) fundoplications. Now regarded as the primary surgical option for the treatment of GERD, the Nissen fundoplication is a well-established total antireflux procedure proven both durable and safe over a period of 20 years. Since its introduction in 1991, the number of laparoscopic Nissen fundoplications (LNFs) has increased annually.7, 8 The Nissen fundoplication has undergone many modifications aimed at minimizing postoperative side effects and improving the longevity of the repair.
The current technique emphasizes return of the esophagogastric junction into the abdominal cavity by transhiatal esophageal mobilization, division of the short gastric vessels to achieve complete mobilization of the gastric fundus, snug crural closure, and a short, tension-free fundoplication designed to re-create the distal high-pressure zone. By performing a fundoplication, the distal esophagus is essentially "submerged" into the proximal stomach, which serves to re-create the acute angle of His and distal high-pressure zone. As intragastric pressure and volume increase, the enveloped distal esophagus is compressed, thereby preventing reflux. When viewed with a retroflexed endoscope, a nipple valve the length of the fundoplication becomes evident (Figure 2).9, 10, 11
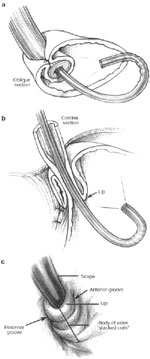
Figure 2: a. An endoscopic view of a 360-degree fundoplication.
b. The fundic wrap viewed from a retroflexed endoscope appears as a nipple valve. c. The body of the valve takes on a "stacked coils" appearance and should run perpendicular to the endoscope. (Source: Jobe BA, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol 2004;99(2):241 with permission from Blackwell Publishing.)
Antireflux Surgery and the Complications of Gastroesophageal Reflux Disease
Esophagitis and Peptic Stricture
In those with erosive esophagitis, PPIs will enable complete mucosal healing in 90% of patients. However, if the medication is stopped, esophagitis will return within 1 year in 80% of patients.12 This highlights the limitation of medical therapy—it does not address the mechanical etiology of GERD. Multichannel intraluminal impedance studies have recently demonstrated that acid-suppression therapy does not affect the total number of reflux episodes; rather, the refluxate is rendered less acidic and is not detected with standard pH monitoring. Other than acid, undefined characteristics of refluxed gastric fluid may be contributing to ongoing esophageal mucosal damage. This may explain the failure of medical therapy in some patients with reflux disease.
With the widespread use of effective medical therapy, peptic stricture is now relatively uncommon. In a large Veterans Administration study of typical GERD patients, approximately 13% developed stricture.13 Overall, the reported incidence ranges between 10% and 25%. Therapy for stricture begins with PPIs, which have been shown to effectively treat esophagitis, reduce the need for dilations, and alleviate dysphagia.14 The PPIs successfully treat 90% of patients; however, 80% experience a recurrence if medical therapy is withdrawn. A number of patients require dilation or multiple dilations to treat recalcitrant dysphagia, especially if they have long-standing disease. These patients may benefit from surgical intervention. In a study of 74 patients with peptic stricture refractory to medical therapy, Klingler et al.15 showed that laparoscopic antireflux surgery (LARS) diminished the need for further dilations fivefold and significantly reduced dysphagia scores. Dysphagia scores dropped from a mean of 6.8 to 3.7 (p <.0001). Ninety-one percent of patients reported satisfaction with the procedure. Twelve percent of patients required ongoing medication for residual symptoms or dyspepsia. Laparoscopic antireflux surgery is as effective as medical therapy in treating stricture and reduces dysphagia and the need for dilation.
Barrett's Esophagus
Barrett's esophagus (BE) occurs most frequently in those with long-standing GERD symptoms. It is intestinal metaplasia of the esophageal lining in response to the chronic and injurious effects of reflux. The presence of BE represents a 40 to 60 times increase in the risk of a patient developing esophageal adenocarcinoma when compared to a person without BE. Whether BE represents an indication for surgery in and of itself has been intensely debated. Many surgeons feel that LARS is the best way to prevent ongoing exposure to all forms of reflux and thus reduce the risk of dysplasia and cancer. Although several retrospective studies have demonstrated regression of BE or low-grade dysplasia after antireflux surgery,16, 17 there is no conclusive evidence that surgery prevents or decreases the risk of developing adenocarcinoma. Inasmuch as the finding of BE represents long-standing severe GERD, these patients should be referred for surgery based on symptom control and disease-related complications.
Asthma and Atypical Symptoms
Our understanding of GERD has expanded to include its effects not just in the esophagus but also in the upper aerodigestive tract. Laryngopharyngeal reflux can manifest as hoarseness, cough, wheeze, aspiration pneumonia, pulmonary fibrosis, and laryngeal dysfunction. Initially, treatment consists of high-dose PPIs. However, atypical symptoms fail to resolve with medical therapy in more than 40% of patients. Surgery is a viable option in patients with laryngopharyngeal symptoms that have proximal reflux events based on pH monitoring. The overall symptomatic response rate with laparoscopic antireflux surgery in patients with laryngopharyngeal reflux symptoms and asthma is less than for patients with only typical GERD symptoms.
Preoperative Evaluation and Objective Testing
Proper patient selection is the key to successful surgical treatment of GERD. Beginning with a history and physical exam, symptoms are classified as either typical or atypical. Typical GERD symptoms include heartburn, regurgitation, belching, and dysphagia. Atypical symptoms include hoarseness, chest pain, laryngospasm, globus sensation, cough, and wheezing. Frequency and timing of reflux symptoms, relationship to meals, symptom exacerbation with supine or upright position, and difficulty swallowing are noted. As a diagnostic tool, symptoms alone cannot provide or exclude a diagnosis of GERD. Symptoms have a limited positive predictive value in the initial presentation of GERD18 and in those patients with postoperative symptoms.19
Routine preoperative objective evaluation includes 24-hour pH monitoring, manometry, esophagogastroduodenoscopy (EGD), and barium swallow. Gastric emptying studies may reveal gastroparesis in those with symptoms of nausea and vomiting or those suspected of having a vagal nerve injury after a primary antireflux operation. The goal of preoperative testing is to verify that symptoms are the result of GERD, and assess for any complicating factors such as short esophagus, stricture, BE, or cancer that may change the operative intervention. The best predictor of a good surgical outcome is objectification of reflux events and correlation of these events with symptoms.
Twenty-four-hour pH Monitoring
Twenty-four-hour esophageal pH monitoring is the most sensitive and specific test for GERD. This test should be performed to establish the diagnosis of GERD, particularly in any patient with a nondiagnostic EGD or atypical symptoms of GERD. An abnormal finding would be a drop below a pH of 4 for more than 4% of a 24-hour period. A DeMeester score is calculated to grade GERD severity. The test is performed with the patient off all antisecretory medications. Twenty-four-hour pH studies also provide insight into the pattern of reflux; some patients may exhibit positional reflux (upright, supine, mixed).
Possibly supplanting the cumbersome catheter based pH monitoring system is the BRAVO probe (Medtronic, Shoreview, MN), a pH-sensing capsule endoscopically secured in the distal esophagus. It boasts minimal discomfort and it detaches spontaneously, so there is no need for retrieval. The pH sensor transmits a signal to a portable recording device, which is then downloaded. Designed to free patients from the inhibition imposed by a nasal catheter, the BRAVO system has been widely accepted by patients and physicians in the diagnosis of GERD. It is equal to or better at detecting acid when compared with a catheter-based system. Additionally, the BRAVO probe is able to extend the period of ambulatory monitoring, which may improve the diagnostic accuracy.20
Barium Swallow
The barium swallow is useful in the diagnosis of structural abnormalities such as stricture, nonreducible hiatal hernia, paraesophageal hernia, and diverticula. The esophogram demonstrates extrinsic compression and mucosal abnormalities, and offers a real-time view of the action of the esophagogastric junction. However, the ability of the esophogram to detect GERD is limited, with a sensitivity of 34%.21 Important characteristics of hiatal hernia to note during esophogram are reducibility and size. A nonreducing hernia may predispose to failure of ARS. A larger (>5 cm) hiatal hernia indicates long-standing disease with associated mediastinal scarring and possible shortened esophagus. The challenge is to reduce the large hernia (which may require extensive mediastinal dissection) and provide enough intraabdominal esophageal length so as not to put tension on the fundoplication. Tension with subsequent mediastinal herniation is the most common form of mechanical failure after surgery.
Esophagogastroduodenoscopy (EGD)
All patients considered for ARS must undergo EGD, which can establish a diagnosis of GERD in the case of severe, erosive esophagitis. In addition, retroflexed endoscopic view of the gastroesophageal junction can provide an assessment of the competency of the gastroesophageal flap valve and identify a hiatal hernia. Furthermore, EGD is necessary to rule out alternate causes of symptoms, such as malignancy, and identify BE.22 Esophagogastroduodenoscopy also offers the opportunity to identify and dilate strictures. Although esophagitis on endoscopy has traditionally been used to confirm a diagnosis of GERD, studies suggest that all patients regardless of their endoscopic exam should undergo pH testing. In a study by Khajanchee et al.,23 patients with esophagitis with a normal DeMeester scores had a significantly less favorable outcome than those with abnormal scores [odds ratio (OR) 9.02, p <.01). It is important to perform barium swallow before EGD in patients who complain of dysphagia. This may alert the endoscopist to diverticula, hiatal hernia, webs, or other structural abnormalities that may be treated during the endoscopy or explain the patients symptoms.
Manometry
Manometry is performed in all patients undergoing evaluation for an antireflux procedure. Manometry confirms aboral peristalsis, assess adequacy of esophageal peristaltic pressure, and locates and measures the upper and lower esophageal sphincter and their resting pressures. A mechanically defective LES is characterized by three parameters: total length shorter than 2 cm, abdominal length shorter than 1 cm, and a resting pressure less than 6 mmHg. A hypotensive LES is the most common finding in patients with GERD. If one component of the triad is defective, there is a 69% to 76% prevalence of GERD. Having all three parameters be defective guarantees a dysfunctional LES. Manometry identifies motility disorders such as achalasia or diffuse esophageal spasm that would complicate or otherwise preclude an antireflux procedure. If the esophageal contraction amplitude is not >30 mmHg, the pressure needed to pass a food bolus past a Nissen fundoplication, then a partial type fundoplication should be considered. A partial fundoplication may diminish the chance of developing dysphagia postoperatively.
Multichannel Intraluminal Impedance-pH
Recent research utilizing a technology called combined multichannel intraluminal impedance and pH-metry (MII-pH) has demonstrated that many reflux events go undetected by pH testing alone. This technology allows the detection of nonacid and weakly acid (a drop in pH of one unit with a nadir above pH 4) reflux in addition to traditional acid reflux events. In addition, this technology enables one to determine the duration and proximal extent of reflux episodes. Utilizing a thin catheter similar to a pH probe, six sequential electrode pairs measure the impedance to electrical current between them. As these electrode pairs are bridged with refluxate, the impedance decreases, indicating an event. With respect to antireflux surgery, impedance testing may present the opportunity to identify nonacid reflux events as a cause of symptoms that are refractory to medical therapy. It is theorized that these patients would benefit from LARS much like patients with typical acid reflux. Although prospective studies are lacking, MII-pH holds promise in enhancing the understanding of atypical symptoms, laryngopharyngeal symptoms, and persistent symptoms in patients on maximal medical therapy.
Other Factors
In addition to objective measures, one should assess the patient's ability to tolerate an operation physically and emotionally. The surgeon must evaluate patient compliance and understanding of the procedure and possible side effects. Setting expectations is a major component of the preoperative workup. Preexisting psychiatric diagnoses have been linked with failed antireflux surgery24. Velanovich et al. showed that 95% of patients without psychiatric diagnosis were satisfied with ARS, whereas only 11% of psychiatric patients found the surgery to be helpful25. Patients who have stress aerophagia or who have eating disorders such as bulimia should be approached cautiously (if at all).
Minimally Invasive Antireflux Surgery
The popularization of laparoscopic fundoplication has been fueled by both patient demand and growing familiarity among surgeons with advanced laparoscopic techniques. In general, LNF is associated with a shorter hospital stay, decreased postoperative pain, earlier return to solid food, and better cosmesis than the open approach.26 In addition, a laparoscopic approach may provide better visualization of the hiatus and allow extended dissection into the mediastinum. Randomized clinical trials comparing open and laparoscopic Nissen fundoplication have found no difference in long-term symptom relief, esophageal acid exposure, esophageal sphincter pressure, postoperative dysphagia, and overall satisfaction26, 27, 28 (Tables 1 and 2). Although laparoscopic Nissen fundoplication has largely overtaken its open counterpart, it is an operation that requires advanced laparoscopic skills and has a significant learning curve. In the early phase of the learning curve, generally considered the first 25 to 30 cases, intraoperative complications such as esophageal perforation and bleeding are higher, conversion rates to laparotomy are higher, and operative time is longer.29 Although the perioperative complications are higher, the long-term quality of life score as well as objective functional outcomes are similar for operations performed in the early and late phases of the learning curve.30
Regardless of the procedure chosen, the reconstruction must provide a functional barrier to reflux while producing the fewest side effects possible. Additionally, three essential goals must be met: (1) adequate intraabdominal esophageal length to allow a tension-free fundoplication, (2) mobilization of the fundus to facilitate a torsion and tension-free fundoplication, and (3) closure of any associated hiatal defect. Partial-type fundoplications are now reserved for those undergoing myotomy for achalasia and those with severe hypomotility on manometry. The "tailored approach," which calls for partial wraps in those with esophageal motility disorders has lost favor, being supplanted by the favorable results obtained with total fundoplications even in those with esophageal motility disorders. However, practices differ in many parts of the world, with similar outcomes using different techniques. Regardless of technique, emphasis should be placed on safety, reducing side effects, and performing the most durable repair to prevent gastric refluxate from entering the esophagus.
Indications for Operation
The key to success with antireflux surgery resides in proper patient selection. Many patients with moderate to severe GERD are candidates for LARS especially with the advent of minimally invasive techniques that promise low morbidity, shorter hospital stays, and greater than 90% success rates in experienced hands. Although many patients find relief of their symptoms with PPIs, as many as 10% of medically treated patients still suffer breakthrough symptoms of GERD and up to 50% of patients require lifelong medication. In severe erosive esophagitis, PPIs heal 90% of patients, but the condition recurs within 1 year in 80% after drug withdrawal. Along with residual symptoms, intolerance to medication and the development of GERD-related complications represent reasons to pursue LARS. Medication failure is perhaps the most common reason to refer for surgical management. Additionally, many patients weigh the long-term cost and lifestyle adjustments as factors when deciding whether to pursue surgery. The patients most likely to benefit from LARS are those who have abnormal 24-hour pH testing scores, typical symptoms, and a good response to medical therapy.31
Contraindications to Surgery
Antireflux surgery is contraindicated in patients who cannot tolerate general anesthesia or, in the case of the laparoscopic approach, a pneumoperitoneum. An uncorrectable coagulopathy and severe cardiopulmonary disease both preclude surgery. In patients with previous foregut surgery or in those who have had prior upper abdominal surgery, the laparoscopic approach can be attempted initially. Most repairs are completed laparoscopically; however, these patients do have a higher risk of conversion to laparotomy. Adhesions and large fatty left liver lobe are the primary reasons for conversion to open procedure.
Operative Techniques
Total Fundoplications
Laparoscopic Nissen Fundoplication (360-Degree Fundoplication)
After general anesthesia has been induced, the patient is positioned in the low lithotomy position. The surgeon operates from between the patient's legs. An assistant helps retract the liver from the right. Placing the patient in steep reverse Trendelenburg position helps to retract the abdominal contents away from the esophageal hiatus (Figure 3).
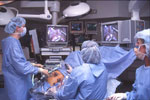
Figure 3: A typical operating room setup for performing laparoscopic antireflux surgery (LARS).
Setup consists of placing the patient in the lithotomy position with the surgeon operating from between the patient's legs. This allows easy access to the foregut and hiatus. The assistant stands to the left of the patient. (Source: Courtesy of B.A. Jobe, M.D.)
After dividing the gastrohepatic omentum, the phrenoesophageal membrane is opened, gaining access to the posterior mediastinum. With sharp dissection, the right and left diaphragmatic crura are defined, and the esophagogastric junction is returned to its proper intraabdominal position with circumferential esophageal mobilization. If there is a hiatal hernia, it is reduced with the mediastinal dissection. The diaphragmatic crura are then approximated with nonabsorbable suture posterior to the esophagus. The tightness of the closure is calibrated such that there is a snug fit around the esophagus, but a laparoscopic instrument can easily pass through the hiatus.
A harmonic scalpel allows efficient division of the short gastric vessels. This enables complete mobilization of the gastric fundus in anticipation of a tension-free fundoplication. Finally, the fundus is passed posterior to the esophagus, wrapped 360 degrees, and sutured anteriorly using nonabsorbable sutures (Figure 4). Use of the "shoeshine" maneuver prior to completing the fundoplication ensures that the stomach is not twisted and that the proper portion of the stomach is employed in the repair; the surgeon grasps both ends of the fundus and pulls it back and forth behind the esophagus to ensure adequate mobility and no tension (Video 1). Most surgeons calibrate the fundoplication over an esophageal dilator in order to prevent a tight a closure with subsequent postoperative dysphagia; however, some debate the advantage of wrap calibration in light of the added risk of perforation upon passage of the dilator (Figure 5). At completion, the fundoplication should be 2 cm in length (Video 2).
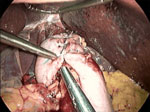
Figure 4: Nissen fundoplication.
This intraoperative view is looking superiorly toward the hiatus and shows a complete Nissen fundoplication wrapping posteriorly around the distal esophagus. The grasper to the left (patient's right) points to three sutures holding the two ends of the fundus securely over the distal esophagus. The liver is seen being retracted superiorly by a laparoscopic liver retractor, an essential piece of equipment for exposing the hiatus. The grasper in the middle shows the course of the esophagus as it enters the abdomen. The crura are not seen in this picture. (Source: Courtesy of B.A. Jobe, M.D.)
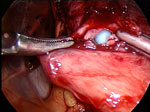
Figure 5: Intraoperative view of an esophageal perforation.
The blue bougie has perforated the esophagus. Perforations are immediately repaired and followed closely postoperatively. A postoperative barium swallow may be necessary to exclude a leak. There is also usually a period in which the patient remains NPO (nothing by mouth) to allow the perforation to heal. (Source: Courtesy of John Hunter, M.D.)
Video 1: Shoeshine A and B Cinepak.

Use of the "shoeshine" maneuver prior to completing the fundoplication ensures that the stomach is not twisted and that the proper portion of the stomach is employed in the repair. The surgeon grasps both ends of the fundus and pulls it back and forth behind the esophagus to ensure adequate mobility and no tension.
Video 2: Final Nissen 2 Cinepak New.
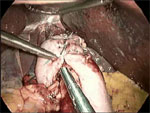
Most surgeons calibrate the fundoplication over an esophageal dilator to prevent a tight a closure with subsequent postoperative dysphagia; however, some debate the advantage of wrap calibration in light of the added risk of perforation upon passage of the dilator. At completion, the fundoplication should be 2 cm in length.
There is some evidence that the wrap itself is less likely to be the cause of persistent dysphagia (>6 weeks); rather, the hiatal closure appears to play a critical role in the development of this infrequent complication. Based on radiologic and EGD studies, Granderath et al.32 categorized 50 patients referred for post-ARS dysphagia. Fifteen of 18 patients identified as having crural stenosis with an intact wrap were effectively treated with pneumatic dilation, lending credence to the hypothesis that too tight of a crural closure (or accumulated scar tissue) leads to constriction of the esophagus with resulting dysphagia. Three patients in this group required reoperation. In all, the authors found a tight hiatal closure effectively narrowing the esophagus. Those patients with transthoracic migration of the wrap (n = 27) underwent reoperation. All were found to have complete or partial wrap migration into the mediastinum with evidence of constriction at the hiatus. In almost 60% of these patients, the crural closure was intact but inadequate in preventing wrap migration. Only five patients of the 50 had problems with the wrap itself (discovered at reoperation) that accounted for their dysphagia. These findings point to the importance of proper hiatal closure in preventing persistent or late-onset postoperative dysphagia.32
Hill Posterior Gastropexy
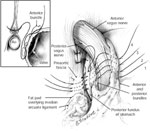
The Hill repair is not a fundoplication per se, but a recalibration of the antireflux barrier. The Hill repair aims to secure the esophagogastric junction into the abdominal cavity, recalibrate the LES, and re-create the acute angle of His. This is accomplished by esophageal mobilization, return of the esophagogastric junction to the abdominal cavity without tension, crural closure, and suture fixation of the right and left phrenoesophageal bundles to the preaortic fascia. The tightness of the sutures is calibrated using intraoperative manometry (Figure 6).
Figure 6: The Hill repair.
The anterior and posterior phrenoesophageal bundles ("the esophagogastric junction") are sutured to the preaortic fascia after esophageal mobilization and hiatal closure. Tightening of the sutures is performed in conjunction with intraoperative manometry to calibrate the fundoplication tightness. (Source: Courtesy of B.A. Jobe, M.D.)
Collis Gastroplasty for Short Esophagus
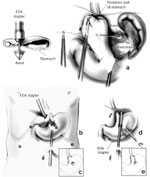
In about 10% of patients undergoing LARS, there will not be adequate intraabdominal esophageal length. Because the most common cause of failure after antireflux surgery is related to transdiaphragmatic herniation, at least 2.5 cm of tension-free intraabdominal esophagus must be present in order to perform a proper Nissen fundoplication. In most patients, maximal esophageal mobilization reaching up to the aortic arch will enable adequate length to be achieved. However, despite these efforts, some patients require an esophageal lengthening procedure such as a Collis gastroplasty. This procedure creates a tubularized portion of stomach that acts as a continuation of the esophagus (Figure 7). The fundoplication is subsequently performed around the neoesophagus.
Figure 7: Collis gastroplasty.
The Collis gastroplasty is an esophageal lengthening procedure that addresses a shortened esophagus. First, an anvil is passed alongside an esophageal dilator through the fundus (a). This guides an EEA stapler used to create a sealed "buttonhole" (b,c). Through this transgastric circular window, a linear cutting stapler is then placed and fired parallel to the esophageal dilator (d,e) essentially converting a cuff of proximal stomach into the distal esophagus. The wrap is then performed around the neoesophagus. EEA, end-to-end anastomotic stapling device; GIA, gastrointestinal anastomotic stapling device. (Source: Horvath KD, et al. The short esophagus: pathophysiology, incidence, presentation, and treatment in the era of laparoscopic antireflux surgery. Ann Surg 2000;232(5):630–640., with permission from Lippincott, Williams & Wilkins.)
The short esophagus may be suggested in the preoperative workup; however, the final diagnosis can only be made intraoperatively. Four findings have been established to correlate with the presence of short esophagus: (1) large nonreducing hiatal hernia, (2) esophageal stricture, (3) Barrett's esophagus, and (4) an LES at 35 cm or less from the incisors by manometry.
Although there is excellent symptomatic relief of GERD symptoms after combined Collis gastroplasty and Nissen fundoplication, long-term results demonstrate that the neoesophagus (tubularized portion of stomach) continues to secrete acid proximal to an intact fundoplication and can result in mucosal damage.33, 34 In a follow-up study of patients treated with Collis gastroplasty, recurrent erosive esophagitis owing to pathologic acid exposure was found in an alarming 80% of patients. Despite the high incidence of ongoing mucosal damage, 65% of patients with recurrent disease reported significant symptomatic improvement compared to preoperative scores.34 Accordingly, patients should undergo follow-up with objective testing and be placed on PPIs if there is an abnormal level of acid exposure regardless of the presence or absence of symptoms. It must be understood that many of these patients have advanced GERD with a severely damaged esophagus and severe, medically refractory volume reflux; in this setting, the efficacy of Collis gastroplasty and fundoplication in ameliorating chronic symptoms is outstanding.
Partial Fundoplications
Toupet Fundoplication (270-Degree Fundoplication)
The Toupet fundoplication is a 270-degree posterior fundoplication that is most commonly employed after Heller myotomy in patients with achalasia. After the crura is closed, the fundus is passed posterior to the esophagus similar to the approach used for the laparoscopic Nissen fundoplication. The limbs of the fundoplication are then sutured together to the anterior esophagus, taking care to avoid the anterior vagal trunk (Figure 8). Mounting evidence in the surgical literature weighs heavily against the continued use of partial fundoplication as a primary therapy for medically refractory GERD.35, 36, 37, 38
Figure 8: Toupet repair.
In the Toupet repair, the fundus is wrapped 270 degrees around the distal esophagus. Securing the fundoplication entails suturing the fundus on either side of the esophagus. Identification of the anterior vagal branch helps prevent incorporation into a suture. Suturing the lateral aspects of the wrap to the crural edges stabilizes the repair. (Source: Peters, JH, DeMeester, T (eds). Minimally Invasive Surgery of the Foregut. St Louis, MO: Quality Medical Publishing; 1994, with permission)
Studies of laparoscopic Toupet fundoplication have demonstrated comparable short-term results to Nissen fundoplication; however, in long-term follow-up, the Toupet repair is associated with a high symptomatic failure rate.37 In addition, Toupet fundoplication has been demonstrated to be inadequate in the treatment of the most severe forms of GERD.36 A study by Heider et al.39 showed that, compared to partial fundoplication, most patients with dysmotility have improved esophageal peristalsis after undergoing an LNF. This suggests that GERD-related esophageal injury plays a role in causing dysmotility, and that abolishing pathologic reflux corrects the motility disorder.
Postoperative Care
Possibly the most attractive feature of laparoscopic surgery is the prompt recovery time. The hospital stay is generally 1 to 4 days. Some centers perform LARS as a day surgery in selected patients. Following an initial trial with clear liquids, patients begin a pureed diet on postoperative day 1. Liberal use of preemptive antiemetics helps to control postoperative nausea and vomiting, a common cause of early wrap disruption or herniation. If there is concern that an occult perforation occurred during surgery, then a barium swallow is performed before a diet is started. Typically, patients are maintained on a soft diet for 4 to 6 weeks and then transitioned to solid foods as tolerated. Bread and meat should be avoided for the first 6 weeks, as these items are notoriously troublesome to swallow. Frequently, there is some dysphagia in the early postoperative period; however, this should resolve within 6 to 8 weeks.
Complications of Nissen Fundoplication
Intraoperative and Early Complications
Intraoperative complications include esophageal perforation, pneumothorax, splenic injury, bleeding, and missed visceral injury. Esophageal and gastric perforations occur in approximately 1.5% of cases; if detected, they are repaired primarily, and drains are placed to minimize the risk of peritonitis or mediastinitis. Pneumothorax is usually self-limited because CO2 is rapidly reabsorbed from the pleural space. Chest tube placement is rarely indicated. Splenic injury can take the form of infarction or bleeding. Superior pole infarction can occur with ligation of the short gastric arteries and does not require intervention. Splenic bleeding may require conversion to a laparotomy and urgent splenectomy. The rate of splenectomy should be less than 1% in experienced hands. Cautery injury can result in delayed intestinal perforation and peritonitis. Meticulous dissection and gentle retraction can help prevent injury. It is paramount that the instruments be in view during the laparoscopic procedure to avoid an occult injury. An abdominal survey before closure can help identify any signs of bleeding or visceral injury. Overall, the complication rate associated with Nissen fundoplication is 13%.40
Late Complications
Although Nissen fundoplication has greater than 90% success in eliminating reflux symptoms, over time a proportion of patients develop new or recurrent foregut symptoms. Some dysphagia, gas bloating, and mild residual esophagitis are common in the early postoperative period, but these symptoms generally resolve within 3 months; severe or persistent symptoms may indicate failure and the need for further investigation.
Of patients undergoing LARS, 2% to 6% eventually require reoperation.41, 42, 43, 44 Reported mechanical causes of failure vary significantly among studies, but transthoracic herniation occurs in 10% to 60% of failures and "slipped" fundoplications are responsible in approximately 15% to 30% of patients. Tight fundoplication, missed motility disorders, and paraesophageal hernias are other modes of LARS failure41, 45, 46, 47, 48, 49, 50 (Table 3).
One of the reasons for pursuing LARS is to offer patients, especially the young, freedom from daily medication dependence. However, data suggest that many patients are using antisecretory medications after ARS. Some construe this as surgical failure. Closer examination of prescribing patterns reveals that many patients are receiving antisecretory medication for symptoms unrelated to the presence of recurrent GERD. Lord et al.19 showed that only 24% of 86 symptomatic, medically treated patients post-ARS had abnormal distal esophageal acid exposure. This study indicates that many patients with foregut symptoms after ARS are taking antisecretory medication based on symptoms alone and not on objective evidence to support their use. In this study, the authors found a very poor positive predictive value of symptoms, including moderate to severe heartburn and regurgitation, and the presence of abnormal acid exposure. Latent, preexisting foregut disorders may be unmasked by the eradication of reflux symptoms by ARS; therefore, failure of the wrap should not be assumed when addressing post-ARS symptoms. Symptoms after ARS should be investigated to rule out esophageal motility disorders, gastroparesis, delayed gastric emptying, irritable bowel syndrome, gastritis, and nonulcer dyspepsia, and to ensure the integrity of the fundoplication. Resuming medication after ARS should be based on objective evidence of GERD as measured by 24-hour pH monitoring.
A "slipped" or misplaced fundoplication occurs when the proximal stomach (instead of the distal esophagus) is wrapped with the fundoplication. Endoscopically, there is a pouch of stomach proximal to the narrowing caused by fundoplication. The slippage is usually the result of transthoracic herniation and represents one of the most common forms of failure. This may be the result of tension on the diaphragmatic closure secondary to an unrecognized short esophagus (Figure 9). Twisting of the wrap is a technical error that can result in a poor outcome. Twisting results from employing the distal greater curvature (antrum) of the stomach as the fundoplication; this results in a two-compartment, twisted stomach and episodic emesis and abdominal pain. Endoscopically, twisted wraps appear as obliquely running folds with respect to the endoscope. These patients require reoperation.
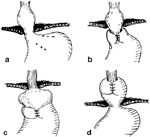
Figure 9: Mechanisms of fundoplication failure.
a: Total disruption of the fundoplication with herniation of the fundus into the mediastinum. b: Herniation of the fundus into the chest through the fundoplication—a slipped Nissen. This complication is most likely caused by an unrecognized short esophagus. c: The fundus has been wrapped around the body of the stomach—a technical error. d: Total herniation of the fundoplication into the chest.
A focused evaluation of postoperative symptoms begins with reviewing all prior records and objective testing. Again, repeat 24-hour pH testing, EGD, manometry, and esophagram are helpful studies to elucidate mechanisms of failure. By measuring the LES length, position, and resting pressure, manometry can determine the integrity of the fundoplication and gauge whether it is tight or located intrathoracically. Esophageal function is evaluated for undiagnosed achalasia, diffuse esophageal spasm, or other motility disturbances that might account for postoperative symptoms. Twenty-four-hour pH testing will confirm recurrent reflux but will not identify the anatomic mechanism of failure. Esophagram is a very valuable test in the workup of fundoplication failure. This exam identifies fundoplication herniation or contrast retention secondary to a tight fundoplication. Esophagogastroduodenoscopy is also very valuable in determining the anatomic causes of fundoplication failure.
Redo Surgery
In our practice, we consider patients for redo surgery if they present with persistent or recurrent foregut symptoms or if they develop symptoms not present prior to surgery. Surgical intervention is carried out in those who have an identifiable anatomic abnormality or a physiologic cause based on objective testing. Redo surgery can be technically challenging in even the most experienced hands. To provide a durable repair, the cause of the failure must be unequivocally identified so as to avoid a subsequent failure; this mandates that the entire fundoplication be dismantled prior to reconstruction. A laparoscopic technique is typically the initial approach, but a laparotomy is always an option in difficult cases. Rarely, a thoracic approach may be necessary. Esophageal perforation is the complication most feared when performing redo surgery secondary to adhesions and scarring.
Smith and colleagues51 reported a 2.8% failure rate requiring reoperation in a series of 1892 patients undergoing antireflux surgery over a 13-year period; of the failures, 73% required reoperation within the first 2 years of surgery. The most common mechanism of failure was transdiaphragmatic wrap herniation (61%). The laparoscopic approach was used in the majority of these patients and the conversion rate to laparotomy was 8%. Similarly, Dutta et al.52 reported a reoperation-related conversion rate of 7%.
Although not as good as for a primary operation, it is important to mention that satisfaction rates after reoperative antireflux surgery are quite respectable. Almost 90% of patients have an outstanding or acceptable outcome with redo fundoplication. In a retrospective review of 118 patients, one study demonstrated that symptomatic response rates approach those of primary surgery.53 Redo laparoscopic ARS is feasible with acceptable complication rates and good success rates. It can provide a clinically effective means in the management of recurrent GERD symptoms.
Long-Term Results
Safety, Morbidity, and Mortality
Laparoscopic ARS is safe, effective, and durable. When selecting patients for surgery, it is important to understand that ARS is an elective procedure, meant to alleviate symptoms and prevent long-term complications of GERD. As of yet, no randomized controlled trial has shown that LARS prevents the progression of Barrett's esophagus into dysplasia or adenocarcinoma. Some series demonstrate a trend toward decreased cancer risk, but these findings are weakened by retrospective design and sample size.54, 55, 56 Esophageal cancer is a rare entity, posing difficulties in its study.
Mortality rates associated with LARS are very low, ranging from 0.008% to 0.8%57 in large series. In fact, many large, single-institution retrospective studies reported zero mortality. Carlson and Frantzides49 examined 10,000 antireflux operations and demonstrated a morbidity rate of 2%. These data came from large, academic referral centers and private practice experience. Early wrap herniation, pneumothorax, gastric perforation, and hemorrhage were the most common serious complications, each occurring around 1% of the time. This study did not mention splenectomy. In another large retrospective study of over 5000 patients undergoing ARS, splenectomy occurred in 2.3%, esophageal laceration in 1.1%, and the mortality was 0.8%. The study period was 1992 to 1997, during the developmental phases of the laparoscopic approach to Nissen fundoplication. Adverse events were significantly more likely in the surgeon's first 15 cases. This speaks to the need for specialized training to guide the surgeon through the learning curve and ideally avoid perforations and other disastrous complications.58
Predictors of Outcome
Campos et al.31 identified three main predictors of good outcome in a study of 199 patients undergoing LARS. At a median follow-up of 15 months, 87% of patients reported good to excellent results. The most likely to benefit from LARS were those who had abnormal 24-hour pH testing scores, typical symptoms, and a good response to medical therapy. The strongest indicator of good surgical outcome was the 24-hour pH study [OR 5.4, 95% confidence interval (CI) = 1.9–15.3]. In studies with respiratory symptoms related to GERD, symptoms correlation with reflux events were strong indicators of good outcome.
Patient Satisfaction and Symptom Control
Most large studies for LARS report high patient satisfaction rates at least 5 years out. A study published by Anvari et al. showed an impressive follow up of 181 patients at 5 years post–laparoscopic Nissen fundoplication (Table 4).59 These patients agreed to undergo objective testing including manometry and 24-hour pH testing. Significant improvements in symptom scores, lower esophageal sphincter pressure (LESP), and normalization of esophageal pH were maintained at 5 years postoperative. Ten-year results are emerging from Belgium where the first laparoscopic Nissen fundoplication was performed by Dallemagne et al.58 Of the 86 patients evaluated at 5 years, 69 returned for follow-up. Results demonstrate that at 10 years, 89.5% of patients were symptom free. Only 9% were taking PPIs, and 7% had undergone revision surgery.
Antireflux Surgery vs. Medical Therapy
In most patients, the long-term management of GERD can be achieved with medical or surgical therapy. Several randomized controlled trails have compared surgical to medical therapy. Unfortunately, each investigation is limited by sample size and variables that make interpreting and comparing the results challenging. For example, the largest of the studies was performed in veterans who, as a group, are quite different from the general population. From this, it is difficult to determine whether the results are generalizable.
The first randomized controlled trial investigating medical versus surgical therapy is the Veterans Administration GERD Study Group trial, which provided the longest follow-up data. This trial demonstrated the superiority of surgery over histamine receptor blockade and lifestyle modifications in the treatment of typical GERD symptoms and esophagitis in 247 veterans.60 The 10-year follow-up of this study demonstrated that 62% of surgical patients were on antisecretory medication. Rates of esophagitis and abnormal esophageal acid exposure were equivalent between the two groups.13 To some, the results of this follow-up study were misconstrued to imply that surgical therapy for GERD is ineffective. It is important to remember that this was an intention to treat analysis, and several of the patients who were originally randomized to surgery, never received this therapy and thus remained on antisecretory therapy. A symptom-based questionnaire was delivered to subjects both on and off medical therapy; compared to the medically treated patients, those who underwent antireflux surgery had only a slight increase in GERD-related symptoms off medication. Furthermore, they had significantly lower symptom scores off medication than their medically treated counterparts. The implication is that surgery is effective in maintaining symptom control, and that many patients are placed on PPI therapy for non–GERD-related reasons.
Lundell et al.61 examined medical versus surgical therapy in a well-designed randomized controlled trial. At 5-year follow-up (n = 310), surgery was demonstrated to be superior to medical treatment under a strict set of symptomatic and endoscopic "failure" criteria. However, if the dosage of PPI was increased to accommodate for breakthrough symptoms in the medically treated subjects, the two therapies were found to be equivalent.
A recent randomized controlled trial that examined medical versus surgical treatment comes from Mahon et al.62 in the United Kingdom. Three-month follow-up showed significantly less acid exposure to the distal esophagus by pH testing in the surgical arm. At 12 months, surgical patient's gastrointestinal and general well-being scores were significantly improved over the PPI group. The dosage of PPIs was titrated to abolish all reflux symptoms. Long-term results of this study will be eagerly awaited.
In all, there have been few randomized controlled trials that use consistent criteria to evaluate medical and surgical therapy. However, certain conclusions can be drawn. Surgery in the properly selected patient can achieve excellent and durable symptom control and can ameliorate long-term effects of esophageal acid exposure. For a multitude of reasons, LARS does not necessarily free patients from medication dependence.
Surgical Results in Patients with Atypical Symptoms
Respiratory and atypical symptoms that stem from GERD have been shown in several studies to be improved by LARS over medical therapy. One recent study by Ciovica et al.63 showed a significant improvement in 126 patients with preoperatively documented respiratory symptoms and GERD treated with PPIs. The patients then underwent LARS. Cough, sore throat, hoarseness, and laryngeal symptoms improved postoperatively and remained improved at 12 months. In another study with 21 GERD patients with respiratory symptoms, relief was obtained in 66% of patients and 19% showed improvement of respiratory symptoms. The medically treated control group only showed a 14% improvement in respiratory symptoms at 6-month follow up. Regurgitation symptoms were well controlled in the LARS group but unaffected in the medically treated group. This offers good supporting evidence that reflux reaches the upper airways and triggers respiratory symptoms.64
Farrell et al.65 also found improvement in atypical symptoms in response to LARS, although much less impressive than improvement in typical GERD symptoms. Ninety-three percent of patients showed improvement and 48% complete resolution in atypical symptoms in 56 patients who underwent LARS. Hunter's group66 found in a retrospective study of 39 patients with GERD-triggered asthma that systemic steroid use significantly decreased after LARS. Allen and Anvari67 examined the effect of LARS on cough in patients with GERD. Cough was significantly improved at 5-year follow-up.
In contrast to PPI therapy, LARS provides a mechanical barrier to reflux preventing refluxate, whether acid, nonacid, or alkaline, from reaching the larynx and airway. Theoretically, reducing vagally mediated bronchospasm from reflux-related distal esophageal injury is another mechanism by which LARS alleviates symptoms. Patients with atypical symptoms can be diagnostically and therapeutically challenging to the clinician; however, these studies indicate that LARS may be superior to medical therapy at alleviating atypical and respiratory symptoms.
Cost-Effectiveness
Several conflicting studies have surfaced recently that address the cost-effectiveness of LARS versus medical therapy with PPIs. For example, a Veterans Administration Cooperative study by Arguedas et al.68 demonstrated that long-term management with PPIs was superior to LARS in terms of quality adjusted life years (QALY) and cost over 10 years. They projected that medical therapy would total $8,798 versus $10,475 for operation at 10 years, and QALY would be 4.59 versus 4.55 in the surgical group. In contrast, a recent British study by Cookson et al.69 has shown that laparoscopic Nissen fundoplication is cost-effective after 8 years compared with maintenance therapy with PPIs in patients with severe GERD. A previous study by Heikkinen et al.28 from Finland showed that total cost was actually lower for Nissen fundoplication mostly owing to earlier return to work.
Conclusion
From Rudolph Nissen's first fundoplication to the current endeavors in endoscopic therapy, the treatment of GERD continues to undergo refinements on many fronts. Antireflux surgery has established itself as a safe, durable, and effective therapy for typical GERD. Long-term outcome studies have consistently demonstrated LARS to provide reconstruction of the antireflux barrier that translates into effective symptom control and prevention of GERD-related complications. The results achieved with the Nissen fundoplication should serve as the standard for surgical and endoscopic therapies directed at the treatment of GERD.