Key Points
- Surgical treatment of achalasia has evolved over the last several years. Transthoracic or transabdominal Heller anterior myotomy provide excellent relief of dysphagia. However, it is associated with high rates of morbidity.
- Minimally invasive laparoscopic approach for Heller myotomy was introduced in 1990. It is superior to other surgical approaches as regards clinical improvement, morbidity, and mortality.
- A long myotomy extending 3 cm onto the stomach yields better control of dysphagia.
- Addition of a 270-degree posterior Toupet may help minimize the postoperative gastroesophageal reflux.
- Various steps in the surgical technique have been described in the text.
- Relief of dysphagia is reported in 90% of these cases, but postoperative heartburn and gastroesophageal reflux disease are common complications.
- Emerging surgical approaches include robot-assisted minimally invasive Heller myotomy and fundoplication.
Background
Achalasia is a rare primary esophageal motor disorder characterized by ineffective relaxation of the lower esophageal sphincter (LES) and concomitant loss of esophageal peristalsis. Patients present with progressive dysphagia and varying degrees of regurgitation, aspiration, chest pain, and weight loss. Misdiagnosis as gastroesophageal reflux disease (GERD) is a common reason for delay in treatment in patients with achalasia. Although achalasia can occur at any age, most patients present between the ages of 20 and 50 years. The reported incidence in the United States is 0.5 to 1 per 100,000. Normal esophageal motility and LES function cannot be restored. Therefore, treatment is directed at decreasing the LES pressure or disrupting the muscle fibers of the LES to allow passage of ingested material. Although several therapies of varying efficacy are available, laparoscopic surgical myotomy provides the most durable long-term results with success rates between 90% and 95% for dysphagia in large studies. The use of minimally invasive techniques over the last decade has reduced the morbidity of esophageal myotomy, making it the treatment of choice for most patients.
Etiology
Though achalasia was first described over 300 years ago, the etiology is still unknown. Histologic examination of affected esophagi demonstrates myenteric inflammation with loss of ganglion cells and fibrosis of the myenteric plexus.1 This process is likely autoimmune regulated as T-cell lymphocytes predominate in the inflammatory infiltrate.2 Additional research has shown that achalasia patients have decreased nitric oxide synthase in the myenteric plexus, resulting in reduced nitric oxide production.3 Because nitric oxide is a key factor in gastrointestinal smooth muscle relaxation, it at least partly explains the dysfunction of the LES.
Symptoms
The hallmark symptom of achalasia is progressive dysphagia, often first to solids and then to liquids. Because the dysphagia usually worsens very gradually, it is often quite severe upon presentation. Most patients adapt their eating behavior long before the diagnosis is made. Because stress and cold liquids may exacerbate the dysphagia, both liquids and solids may be poorly tolerated simultaneously at presentation. Chest pain is also a common symptom, and patients often undergo extensive cardiac evaluation prior to diagnosis. Previously, 10% to 39% of patients suffered bronchopulmonary complications from repeated regurgitation and aspiration.4 Now many of these complications are avoided by earlier diagnosis and treatment. Heartburn occurs in about one fourth of the patients, although it is usually caused by fermentation of unevacuated food in the esophagus rather than gastroesophageal reflux.5, 6
Patients often have a long history of symptoms, some greater than 30 years. Weight loss is common, although usually patients alter their diets enough to maintain body mass until the disease becomes intolerable and they seek therapy. Acute onset of symptoms or rapid weight loss should be a red flag for clinicians to rule out malignancy. It is crucial to rule out pseudoachalasia (presence of a distal esophageal tumor causing dysphagia) in older patients (>55 years), and in those with a shorter duration of symptoms (<6 months), or more profound, rapid weight loss (>15 lb). Any patient with a questionable diagnosis should be carefully evaluated, even pursuing endoscopic ultrasound or computed tomography (CT) scan to rule out pseudoachalasia.5, 7, 8
Diagnosis
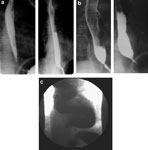
The preferred initial diagnostic test for most patients who present with progressive dysphagia is a contrast esophagram. This inexpensive and readily available study often reveals the classic findings of a dilated esophagus, impaired peristalsis, and the pathognomonic smooth tapering at the gastroesophageal junction (GEJ) commonly termed "bird's beak" esophagus. If diagnosed early, the esophagus may be of normal caliber (Figure 1a), though most patients present with some element of dilation (Figure 1b). Commonly an air-fluid level forms as esophageal emptying is delayed, or the barium tablet or marshmallow "hangs up" just above the GEJ and may require several minutes to pass. Food particles are often seen despite patients' fasting for several hours prior to the study, indicating a significant delay in esophageal emptying. In long-standing achalasia, the esophagus can become dilated and tortuous and has been termed sigmoid-shaped or megaesophagus (Figure 1c).
Figure 1: Esophagrams of a patient with early achalasia pre- and posttreatment.
a: Initial esophagram of patient with early achalasia and no esophageal dilation. b: Patient after 2 years of nonoperative treatment. Note significant esophageal dilation and air-fluid level compared to pretreatment. c: End-stage achalasia with sigmoid or megaesophagus.
Manometry is essential in making the diagnosis with the vast majority of patients exhibiting the classic findings of incomplete LES relaxation and aperistalsis of the esophageal body. In a minority of patients, manometry tracings show simultaneous contractions, often of normal amplitude, which some have coined "vigorous achalasia." A common misconception is that the LES must also be hypertensive. Although the LES pressure can occasionally be elevated, most patients have normal LES pressures (<45 mmHg) with incomplete LES relaxation with deglutition.
Endoscopy is necessary to exclude pseudoachalasia and to evaluate for atypical anatomy such as epiphrenic or traction diverticula. Characteristic endoscopic findings include a dilated esophagus with failure of the LES to open with insufflation and some mild resistance to passage of the scope through the GEJ commonly described as a "pop." Retained food and debris in the esophagus are common. If suspicion of pseudoachalasia persists, an endoscopic ultrasound with biopsy or CT scan should be included in the evaluation.
Surgical Treatment
History
Ernst Heller9 first described the two-cardiomyotomy technique (one anterior and one posterior) along the GEJ for achalasia in 1914. It is currently modified such that only an anterior myotomy is performed. Heller myotomy provides excellent results and relief of dysphagia in 90% to 95% of patients.5, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Traditionally, surgery was accomplished via a transthoracic or transabdominal approach. Each was associated with the morbidity of a major open procedure, often with expected hospital stays of 7 to 10 days expected.
For this reason, 10 to 15 years ago most patients were treated by less invasive therapies such as pneumatic dilatation despite superior long-term results from surgical myotomy. In the early 1990s, as minimally invasive techniques developed, surgery became more acceptable. The thoracoscopic approach was first described in 1991. This minimally invasive approach decreased postoperative pain and shortened hospital stays without compromising the relief of dysphagia.25, 26
In the mid-1990s multiple factors led to a change from the thoracoscopic to the laparoscopic approach. First, with the increase in the number of laparoscopic fundoplications being performed, surgeons became more adept at laparoscopically operating on and around the esophageal hiatus. Second, it became clear that extending the myotomy well onto the stomach was critical for consistent and durable relief of dysphagia.21 Third, the incidence of postoperative reflux was high with the thoracoscopic approach even with limited gastric myotomy, thus the need for a fundoplication became apparent. This is much easier to perform laparoscopically rather than from the chest. Additionally, laparoscopy avoids the need for single lung ventilation with a double-lumen endotracheal tube and tube thoracostomy. The laparoscopic approach has since been proven superior to both open and thoracoscopic procedures with respect to complications, morbidity, mortality, length of hospital stay, relief of dysphagia, prevention of postoperative reflux, and operative times.18, 21, 27, 28, 29
University of Washington Experience
At the University of Washington, we converted from a thoracoscopic to a laparoscopic Heller myotomy in 1994 for two distinct reasons. First, we recognized that a limited gastric myotomy (0.5–1.0 cm) failed to protect the patient from gastroesophageal reflux (GER). In fact, when pH monitoring was performed, 80% of patients had pathologic reflux.21 Second, 17% of patients in our studies returned with recurrent dysphagia, and half of them responded to extension of the gastric myotomy between 1.5 and 2.0 cm via the laparoscopic approach. Between 1994 and 1998, 52 patients underwent laparoscopic myotomy with this longer gastric myotomy, yielding excellent improvement in dysphagia in over 90% of the patients. Still, occasional patients had inadequate relief or recurrence of dysphagia, some of whom improved with further extension of the gastric myotomy. Therefore, we began extending the gastric myotomy a full 3 cm in 1998. We also began performing a Toupet, rather than a Dor fundoplication. We felt an anterior fundoplication more difficult with a 3-cm cardiomyotomy and suspected the 270-degree posterior Toupet would provide better control of reflux. A comparison of the two approaches confirmed that an extended myotomy with Toupet fundoplication (EM/Toupet) more effectively obliterated the LES than the shorter myotomy with Dor fundoplication (SM/Dor). The evidence for this was a greater reduction in the residual LES pressure (9.5 vs. 15.8 mmHg respectively), as well as better, more durable relief of dysphagia. Dysphagia was both less frequent (once a month vs. once a week on average) and less severe (3.2 vs. 5.3 on a 10-point visual analog scale) in the EM/Toupet group. Most importantly, in the subsequent 7 years, no patient has required surgical intervention for recurrent dysphagia. Furthermore, this complete obliteration of the LES did not result in more reflux, as the mean distal esophageal acid exposure was equivalent in the two groups (EM/Toupet 6.0% vs. SM/Dor 5.9%).30
Patient Positioning and Preparation
Patients are placed in a modified lithotomy position with a beanbag beneath them and both arms tucked at the sides. The beanbag overhangs the edge of the operative table to allow formation of a saddle around the patient's perineum. This technique secures the patient's position while protecting against soft tissue damage or nerve compression. The operative surgeon stands between the patient's legs with the assistant at the patient's left side. Monitors should be located ergonomically above the patient's head.
Port Placement
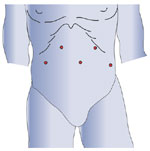
Abdominal access is initially obtained at the costal margin in the left upper quadrant. Pneumoperitoneum is established with a Veress needle, and an optical trocar (Visiport, United States Surgical Corp., Norwalk, CT) is inserted. Four working ports are then placed under direct visualization, as shown in Figure 2. The camera port is placed approximately 10 to 12 cm inferior to the left upper quadrant port and 4 cm left of midline. Remaining ports include a 5-mm trocar in the right upper quadrant for the surgeon's left hand, a 10-mm trocar in the left lower quadrant for the assistant's right hand, and a 10-mm trocar in the right lateral quadrant for a liver retractor. Our preference is an expandable paddle liver retractor that can be secured to the operative table with a Bookwalter retractor post and flexible arm (Codman, Raynham, MA). Alternatively, a 5-mm subxiphoid incision can be made and a Nathanson liver retractor (Cook, Bloomington, IN) utilized. After port placement, the patient is placed in steep reverse Trendelenburg positioning. A 10-mm, 30-degree angled laparoscope provides superior visualization.
Figure 2: Laparoscopic port sites for Heller myotomy.
Alternatively, the right lateral port can be placed in the subxiphoid position if using a Nathanson liver retractor.
Operative Steps
Our initial approach involves dividing the left phrenoesophageal and phrenogastric ligaments, allowing exposure of the left crus. Next, we mobilize the gastric fundus to create a tension-free fundoplication (Video 1). An ultrasound dissector (Autosonix, United States Surgical Corp., Norwalk, CT) is used to divide the short gastric vessels beginning at the inferior pole of the spleen and continuing superiorly to the previously exposed left crus. After the left phrenoesophageal ligament is divided, the gastrohepatic ligament is incised. The right and anterior phrenoesophageal ligament and peritoneum overlying the anterior abdominal esophagus are divided, being cognizant of the underlying anterior vagus nerve. When a posterior Toupet fundoplication is planned, a posterior esophageal window is created. During this step the posterior vagus nerve should be visualized and protected. If an anterior fundoplication is performed, only the anterior esophagus requires full exposure.
Adequate mediastinal esophageal mobilization is crucial for a long esophageal myotomy and tension-free fundoplication (Video 2). A Penrose drain may be placed around the GEJ and used to retract the esophagus caudally and laterally during hiatal and mediastinal mobilization. To clear a path for the myotomy across the GEJ, we resect the cardioesophageal fat pad to the left of the anterior vagus nerve while simultaneously mobilizing the vagus from the esophagus. This allows a straight plane to perform the myotomy.
Excellent visualization and exposure is essential to performing a safe and adequate myotomy. A lighted 52-French bougie is placed into the body of the stomach, serving both to illuminate the esophagus and muscle layers as well as to provide a stable platform to perform the myotomy. The myotomy is begun approximately 3 cm below the GEJ and an L-shaped hook electrocautery device is used to divide the muscle fibers. During the myotomy, electrocautery should be avoided unless absolutely necessary. Individual muscle fibers are divided by hooking them and applying gentle upward traction (Video 3). Bleeding from the muscle or submucosa is controlled with pressure and time. These steps are important to avoid delayed perforation from unrecognized thermal mucosal injury. Progressive division of the longitudinal and then circular muscle layer is performed as the myotomy is carried superiorly, 6 to 8 cm above the GEJ. Once the circular muscles are divided, a mucosal plane is reached with smooth, white, bulging mucosa. Thus, the entire myotomy spans approximately 9 to 11 cm (3 cm below to 6-8 cm above the GEJ). The most difficult dissection involves the 3-cm myotomy on the stomach where the plane of dissection becomes blurred with intervening sling muscular fibers and the underlying gastric mucosa is thinner, increasing the risk of perforation. Mucosal perforations are repaired with a fine (4-0 or 5-0) absorbable monofilament suture and rarely require further intervention. In cases with a perforation, performing an anterior (Dor) fundoplication is prudent to buttress mucosal repairs and prevent leakage or fistula formation. Endoscopy can be utilized to evaluate the completeness of the myotomy and check for a missed perforation.
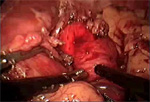
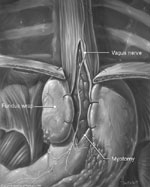
After satisfactory cardioesophageal myotomy, we perform a Toupet fundoplication (Video 4). The posterior fundus of the stomach is brought around the esophagus and secured to the right crus and the right cut edge of the myotomy. In a similar (in fact mirror image) fashion the anterior fundus is sutured to the left crus and left edge of the myotomy (Figure 3). Rarely, patients with large hiatal hernias require crurapexy closure of the hiatus.
Figure 3: Heller myotomy and Toupet posterior fundoplication.
(Source: University of Washington, 2002, with permission.)
Postoperative Management
Typically, patients receive liquids the evening of their operation. They are then advanced to a soft diet and discharged the following day. It is important to treat nausea aggressively with antiemetics. Patients are advised to avoid strenuous activity and heavy lifting for 4 to 6 weeks. A majority of patients resume normal activities within 1 to 2 weeks, and a regular diet in 2 to 6 weeks.
Postoperative Outcomes
Long-term outcomes data from the open surgical era suggests successful relief of dysphagia in 65% to 70% of patients.31 However, the majority of these patients underwent open thoracotomy with minimal extension of the myotomy onto the stomach. This lack of sufficient myotomy onto the cardia has been proven the cause of most recurrent dysphagia after thoracoscopic or open myotomy via the chest. Additionally, many of these late failures are the result of severe, intractable reflux affecting quality of life and requiring surgical intervention. Since the advent of the abdominal approach, and because laparoscopic techniques greatly improve visualization and allow longer transabdominal myotomies, success rates have improved dramatically. Several authors now support performing the myotomy with intraoperative endoscopic or manometric guidance to ensure complete obliteration of the LES. Specifically the sling fibers of Willis (the oblique component of the LES) must be surgically divided to provide adequate passage of food. Transthoracic approaches also make performing an antireflux procedure more difficult. These problems have been substantially corrected with laparoscopic techniques.27 Long-term outcomes for dysphagia in patients undergoing a laparoscopic Heller myotomy and antireflux procedure range between 80% and 95% in large studies.21, 32, 33, 34 Results for control of reflux may be inferior to results for dysphagia.35
Surgery for Symptom Recurrence
Laparoscopic techniques are the approach of choice in patients with previous a thoracoscopic myotomy, thus avoiding the previous operative site and allowing extension of the myotomy and an antireflux procedure. Successful amelioration of dysphagia in achalasia requires complete division of the LES. As mentioned earlier, approaches via the chest did not allow proper extension of the myotomy onto the cardia, resulting in recurrence of dysphagia. These operations can be particularly difficult, requiring advanced laparoscopic skills and an understanding of the thoracic and abdominal approaches to avoid perforations and other complications. Other forms of treatment such as pneumatic dilation may offer relief of recurrent symptoms, especially if the patient has already had a myotomy. Botulinum toxin should be reserved only for very poor operative candidates, as it makes further surgery more difficult36 and commonly lasts only between 6 and 12 months.34
Contraindications
The inability to undergo surgery or general anesthesia precludes Heller myotomy. Previous hiatal or esophageal surgery may be relative barriers to laparoscopic surgery. Some feel that a megaesophagus or grade IV dilation (>8 cm) is a contraindication to a myotomy because of poor relief of dysphagia and instead recommend esophagectomy. With minimally invasive techniques, there is little to lose in attempting a myotomy while reserving an esophagectomy for treatment failures. With this approach, most patients with severely dilated or even sigmoid-shaped esophagi have substantial improvement in their dysphagia and subsequently avoid esophagectomy and its significant morbidity and mortality.21
Controversies
Length of the Myotomy
The exact length and dimensions of the esophageal myotomy have been a source of much debate. Multiple authors have shown a correlation between dysphagia relief and gastric-myotomy length. We first noted this in our thoracoscopic group of patients where the myotomy extended only 0.5 cm onto the gastric wall. The rate of persistent dysphagia was 27% due to incomplete myotomy.21 A critical flaw to the thoracoscopic approach is its inherent inability to perform an extended distal myotomy onto the stomach. Therefore, we switched our technique to a laparoscopic approach incorporating a myotomy carried out 1 to 1.5 cm on the gastric wall. This additional length of myotomy distally, decreased the postoperative dysphagia rate to 11%.21
In 1998, our group hypothesized that postoperative dysphagia could be further decreased with an extended myotomy (EM) carried 3 cm distal to the gastroesophageal junction. We compared outcomes in the EM patients with those undergoing short myotomy (SM) 1 to 1.5 cm onto the gastric wall. Our data clearly showed that EM resulted in significantly less postoperative dysphagia severity and frequency when compared to SM (p = .001 for both).30 With EM there was no difference in the frequency of postoperative heartburn, regurgitation, or chest pain when compared to SM (p = .36, p = .19, p = .71, respectively). Additionally, postoperative 24-hour pH studies demonstrated no difference in proximal or distal esophageal acid exposure between EM and SM (p = .55 and p = .96, respectively).
Antireflux Procedure
Whether or not to perform an antireflux procedure at the time of a Heller myotomy has previously been the most controversial surgical issue in the treatment of achalasia. This is no longer the case. With few exceptions, an antireflux procedure should be performed. Performing a cardiomyotomy obliterates the primary barrier to GER. Thus a partial fundoplication that does not inhibit bolus transit from the aperistaltic esophagus into the stomach but diminishes GER would seem intuitive.
It has been shown that patients with achalasia have decreased esophageal chemoreceptors and therefore do not sense acid reflux when compared to normal subjects.37 This is likely the cause of the low rates of postoperative GER reported by patients treated with Heller myotomy without an antireflux procedure. Although the GER may remain silent to the patient, continuous acid exposure of the esophagus can lead to aspiration, peptic strictures, Barrett's esophagus, and esophageal cancer.38 Proponents of cardiomyotomy without fundoplication cite their shorter operative times, diminished risk of causing dysphagia, and ease at which GER can be treated with modern acid suppression medication.
We and others demonstrated that an antireflux procedure could reduce the incidence and severity of GER without diminishing the effects on dysphagia.21, 39, 40, 41 Even one of the most vocal groups against addition of an antireflux procedure recently published a prospective randomized study specifically addressing whether an antireflux procedure is necessary.42 Their studies now support the use of antireflux procedures for all patients undergoing esophagomyotomy. They discovered the addition of an antireflux procedure to Heller myotomy clearly decreased the incidence of GER (nine-fold) without increasing the rate of dysphagia. Subjectively, patients undergoing Heller only and Heller plus Dor had similar decreases in the median dysphagia score, showing that the antireflux procedure did not in fact cause patient dissatisfaction or increased dysphagia as previously feared. There are now few surgeons who do not perform an antireflux procedure after myotomy.
Fundoplication Technique
Owing to the aperistaltic esophagus in achalasia patients, a complete 360-degree wrap is avoided as it would potentially re-create the obstruction relieved by the myotomy. A majority of surgeons perform either an anterior (Dor) or a posterior (Toupet) 270-degree fundoplication. The advantage of the Dor fundoplication is its technical ease due to avoidance of extensive gastric mobilization, and its ability to buttress small mucosal perforations created during myotomy. A Toupet fundoplication inherently splays apart the myotomy edges, theoretically preventing fibrosis between them and recurrent dysphagia. A small nonrandomized study showed patients undergoing Toupet fundoplication having less postoperative reflux when compared to patients undergoing Dor fundoplication.41 It is the authors' choice to perform a Toupet fundoplication for the above advantages, though until a proper randomized control trial comparing Dor to Toupet fundoplication is performed, this controversy will continue.
Sigmoid-Shaped or Megaesophagus
Megaesophagus represents end-stage achalasia, as the combination of an aperistaltic esophagus and failure of LES relaxation leads to progressive esophageal dilatation and lengthening (Figure 1c).43 Previously, patients with megaesophagus were thought best treated with esophagectomy rather than myotomy,44, 45, 46 with the rationale being that the dilated and often tortuous aperistaltic esophagi do not empty sufficiently to improve dysphagia, even when the LES is disrupted. With the drastic decrease in morbidity with minimally invasive approaches, many surgeons have elected to try a myotomy first. Two studies have demonstrated good postoperative results in patients with megaesophagus treated with Heller myotomy. The obvious advantage of Heller myotomy is avoidance of the morbidity and mortality associated with esophagectomy. Patti et al.47 performed laparoscopic Heller myotomy on patients with dilated esophagus (>6 cm) with straight axis configuration and sigmoid shaped configuration. They reported no increased difficulty in performing the surgery and no increase in complications, with 92% of the patients reporting excellent or good relief of dysphagia. Likewise, Mineo and Pompeo48 studied 14 patients with sigmoid esophagus treated with Heller myotomy. With a median follow-up of 85 months, excellent or good results were reported by 72%, and no patient required an esophagectomy. Postoperative dysphagia and regurgitation scores decreased significantly (p = .002 and p = .001, respectively) and were equivalent to postoperative scores from a nondilated esophagus group undergoing Heller myotomy. Health-related quality of life was evaluated with the Short-Form 36 (SF-36) questionnaire, showing statistically improved general health, social function, and mental health. Interestingly, esophageal width was found to narrow with time, on average 10 mm in 24 months.
Complications
Early Complications
Operative perforations occur in 1% to 5% in most series; however, the great majority are repaired with little or no morbidity or deleterious effects on successful relief of dysphagia. This is in comparison to dilation, where esophageal rupture has significant potential morbidity and mortality. Pneumothorax, bleeding, intraabdominal abscess, and wound infection can occur in approximately 3% of cases. Pneumothorax rarely requires treatment, as the carbon dioxide used for insufflation is readily absorbed. Other complications, such as vagal injuries, unrecognized mucosal perforations, and splenic injuries, are reported, but rare.
Late Complications
Late complications are related to recurrent dysphagia. Incomplete myotomy, perihiatal scarring, peptic stricture, or obstructing tumors can all cause recurrent symptoms.
Emerging Therapy
Robot-Assisted Surgery
Robot-assisted minimally invasive surgery has recently emerged as a therapy for achalasia. Antireflux procedures have been performed utilizing robot assistance and in some cases without the need for an additional surgeon.50, 51 Advantages include increased range of motion of the instruments (7 degrees of freedom at the "wrist"), improved tissue manipulation, lack of tremor, improved 3-D visibility with depth perception, and ease of fine manipulations in smaller spaces.50, 51, 52 Esophageal myotomy lends itself to robotic surgery because most of the approach is in a single direction such that the robotic arms can be placed widely apart, allowing full range of motion50 without intraoperative movement of the robotic apparatus. We have begun performing many laparoscopic esophageal myotomies with the da Vinci Surgical Robot (Intuitive Surgical, Sunnyvale, CA) and find the procedure well suited to robotic assistance.
Conclusion
The treatment of achalasia has undergone substantial changes over the last 20 years. Less effective therapies such as pneumatic dilation and botulinum toxin (Botox) injections are being supplanted by surgical intervention. The minimally invasive approach removes the majority of surgical morbidity and successfully relieves dysphagia in over 90% of patients. Laparoscopic esophageal myotomy and Toupet fundoplication should be considered the primary treatment for achalasia for most patients.