Key Points
- Esophageal adenocarcinoma (EAC) arises from Barrett's esophagus (BE), which is the most serious complication of gastroesophageal reflux disease (GERD)—a common form of esophageal motility disorder.
- Esophageal adenocarcinoma is a highly lethal cancer, and its incidence has increased rapidly in the United States and Western Europe.
- Barrett's esophagus (BE) is a well-recognized premalignant condition, and patients with BE have a 30- to 50-fold greater risk of developing EAC.
- Risk factors for EAC include age, male gender, GERD symptoms, white race, obesity, family history, and use of lower esophageal sphincter–relaxing medications.
- Potential factors with negative association include dietary patterns, and use of aspirin or nonsteroidal antiinflammatory drugs.
- No single biomarker has yet emerged that is superior to histologic dysplasia in predicting progression to cancer.
- New developments in the endoscopic detection of early neoplastic lesions include chromoendoscopy and magnification endoscopy, narrow band imaging, optical coherence tomography, fluorescence spectroscopy, and brush cytology.
- Clinical staging can be accomplished by endoscopy, chest and abdominal computed tomography, endoscopic ultrasound, and laparoscopy.
- Treatment options include endoscopic mucosal resection, surgical resection, and ablative therapies for early cancer.
Introduction
Esophageal malignancies are the sixth leading cause of cancer death in the world and represent about 1% of the cancers diagnosed in the United States.1 The incidence of esophageal adenocarcinoma has increased rapidly in the United States and in large parts of Europe. It is estimated that there were 14,250 new cases of esophageal cancers in 2004 in the United States, with 13,300 cancer-related deaths.2 Esophageal adenocarcinoma (EAC) comprised a little more than half of esophageal cancers, or about 8000 incident cases.3 Survival rates have improved during recent years in some countries, but the 5-year survival is still only about 10% in most Western populations.4 In 2000, the last year of complete data available from the national cancer Surveillance, Epidemiology, and End Results database, the 5-year relative survival rates from esophageal cancer were the fifth lowest at 15.4%. Esophageal adenocarcinoma is a highly lethal cancer and its incidence has increased rapidly in the United States and Western Europe over the last three decades.5
Barrett's esophagus (BE) is a well-recognized premalignant condition for the development of EAC and results from chronic gastroesophageal reflux. It is characterized by a metaplastic transformation of the typically squamous epithelium native of the esophagus, to a columnar type highlighted by the presence of goblet cells appreciated on histologic evaluation.6 The condition entails a 30- to 50-fold greater risk of developing EAC. Patients with BE have an incidence of development of adenocarcinoma that approaches 0.5% annually.7 Some authors have reported a better survival rate among patients with cancer originating from BE compared with esophageal malignancies without this metaplasia. This might be explained by more prevalent symptoms of gastroesophageal reflux in patients with BE, leading to earlier endoscopy and confirmation of the diagnosis at an earlier tumor stage. Also, the introduction of endoscopic surveillance programs among patients with BE might improve long-term survival in this defined group of patients,8 although this remains to be proven.
History of Disease
Barrett's esophagus was not definitively described until 1950 by the English surgeon Norman Barrett. The association of columnar lined esophagus with EAC was first described in 1952 by Morson and Belcher.9 The first large series linking GERD, BE and EAC was reported by Naef et al.10 in 1975. Trier11 characterized the histologic lesion of specialized intestinal metaplasia (SIM) in 1970. It was subsequently appreciated that it is the SIM in BE that is associated with the development of EAC. Esophageal adenocarcinoma was considered a rare diagnosis even into the 1980s; in the past 30 years, however, the incidence of EAC has risen in the United States and other Western countries. From 1926 to 1976, most esophageal cancers were squamous with four surgical series reporting only 0.8% to 3.7% EAC. However, between 1979 and 1992, 54% to 68% of cancers were adenocarcinomas. Multiple population-based studies have also confirmed that the incidence of EAC increased threefold from 1976 to 1988.12, 13
Epidemiology
The incidence of EAC in the United States has increased approximately 300% to 500% in the last 40 years.14 Currently, 60% of all esophageal cancers are adenocarcinomas. In the white male population of the United States the reported annual increase has been close to 10%, which exceeds that of any other malignancy in that population.13 Incidence rates for women and blacks have also increased, although not as rapidly as for white men. The incidence of EAC has also been increasing in most Western industrialized countries. Highest incidence rates in the year 2000 were in Great Britain (5.0–8.7/100,000), Australia (4.8/100,000), and the Netherlands (4.4/100,000), followed by the United States (3.7/100,000). Reliable data from other countries are limited. Squamous cell cancer is still the predominant histology of esophageal cancer in Asia, and the incidence of EAC does not appear to be increasing significantly in Taiwan or Japan.15 Although, some misclassification or reclassification of tumors has likely occurred, detection bias and misclassification bias are inadequate to account for the rising incidence of EAC. A true increase in EAC incidence appears to have occurred.
Multiple investigators have assessed risk factors for EAC. The most consistently identified risk factors are increasing age, male gender, gastroesophageal reflux disease (GERD) symptoms, BE, white race, obesity, family history, use of lower esophageal sphincter–relaxing medications, and tobacco use. Potential factors with a negative association include selected dietary patterns and nutrients, Helicobacter pylori infection, and previous use of aspirin or nonsteroidal antiinflammatory drugs (NSAIDs) (Table 1). The use of alcohol or acid-suppressive medications does not appear to be independently associated with risk for EAC.
Age, Sex, and Race Distribution
The age distribution is similar to most gastrointestinal cancers, with an increased risk with advancing age. The median age at diagnosis is about 60 years. Barrett's esophagus associated EAC is more common in men than women with a ratio of 7:1.13 It is more common in Caucasians; however, an increase also has been observed in Hispanics in the United States.16 The role of socioeconomic variables and occupation as an explanation for these differences is unclear.17, 18
Heredity
Although, familial clustering of both BE and EAC occurs,19, 20 the influence of genetic factors in the etiology of EAC seems to be of limited importance among cases occurring in the community at large. The recent increase in incidence of EAC is not related to hereditary factors because a change of gene pool in 20 to 30 years appears unlikely.
Barrett's Esophagus
The strongest risk factor for EAC is BE. The excess risk has been estimated to be 30- to 60-fold relative to the risk of the general population and the majority of cases of EAC arise from BE.21, 22, 23 The main predictor of malignant potential of BE has been the histologic classification of dysplasia on endoscopic biopsy. The degree of dysplasia has been shown to correlate with the risk of developing cancer, and high-grade dysplasia (HGD) is associated with the greatest risk.24 Additionally, surgical series show concurrent undetected cancer in the resection specimen in up to 50% of patients with HGD.25, 26, 27 The progression through grades of dysplasia is neither orderly nor inexorable. Subjects may progress from nondysplastic BE straight to HGD or cancer without an intervening detectable low-grade dysplasia (LGD) phase.28 Alternatively, subjects with HGD may undergo apparent regression of the disease and spend months or even years with no detectable dysplasia. However, the data on disease progression are compromised by our random-sampling endoscopic biopsy techniques, and hence it is difficult to know what percentage of apparent disease regression is real. Studies also suggest that patients with long segment BE ( 3 cm) have the highest propensity for malignant transformation compared with short segment BE (<3 cm). The latter group of patients is still at an increased risk compared with patients without BE.29, 30, 31 At this time, we are unable to predict, by endoscopic appearance alone, which patients with short segment BE or long segment BE will progress to cancer.
3 cm) have the highest propensity for malignant transformation compared with short segment BE (<3 cm). The latter group of patients is still at an increased risk compared with patients without BE.29, 30, 31 At this time, we are unable to predict, by endoscopic appearance alone, which patients with short segment BE or long segment BE will progress to cancer.
Gastroesophageal Reflux Symptoms
The role of gastroesophageal reflux symptoms in the development of EAC has been extensively investigated. In a Swedish population-based, nationwide, case-control study, patients with any history of reflux had an odds ratio (OR) of 7.7 [95% confidence interval (CI), 5.3–11.4] compared with those patients without reflux symptoms. This OR increased to 43.5 (95% CI, 18.3–103.5) for EAC in those with the most frequent, severe, and long-standing symptoms.32 The widely disseminated information from this study and others33, 34, 35 suggest a strong and probably causal relationship between gastroesophageal reflux symptoms and EAC.
Tobacco and Alcohol
Case-control studies have reported a moderately increased risk (OR, 1.5–3.4) of EAC among tobacco smokers.17, 36, 37, 38 This strength appears to be weak compared with squamous cell carcinoma of the esophagus. There is evidence to suggest that there is little decrease in OR on stopping smoking such that the OR for current and ex-smokers was 2.0 times greater in the cancer cases even 30 years after cessation of smoking.17
On the other hand, based on the available data, alcohol is not associated with an increased risk of EAC.17, 39, 40
Obesity
The increasing trend in EAC closely resembles the epidemic increase in obesity, and a high body mass index (BMI) has been found to be a risk factor for EAC in several epidemiologic case-control studies.36, 39, 41 In a population-based, case-control study from Sweden, a strong dose-dependent relationship existed between BMI and EAC (adjusted OR, 7.6; CI, 3.8–15.2) among patients with the highest BMI quartile compared with those in the lowest quartile.41 The mechanism behind this association remains to be identified.
Diet
The best established risk is low intake of fruits and vegetables.42, 43 The antioxidants in these dietary items seem to have a protective effect.42 Low intake of fiber appears to increase the risk according to two large population-based case-control studies.42, 44 Other potential dietary risk factors include high intake of dietary fat, dietary cholesterol, and animal protein.42 There has been increasing speculation about the role of nitrosamines as well as for the possible protective role of selenium.45, 46
Medications
Data suggest that continuous and long-standing use of medications that can relax the lower esophageal sphincter, and thereby aggravate gastroesophageal reflux, increases the risk of developing of EAC.47, 48 The implicated drugs include nitroglycerins, aminophylline,  -receptor agonists, anticholinergics, and benzodiazepines; the strongest association is seen with anticholinergics.
-receptor agonists, anticholinergics, and benzodiazepines; the strongest association is seen with anticholinergics.
The role of acid suppressive agents, aspirin, NSAIDs, and cyclooxygenase-2 (COX-2) inhibitors is discussed below (see Chemoprevention and Other Novel Therapies).
Helicobacter pylori
Evidence of an inverse association between Helicobacter pylori infection and risk of EAC is accumulating.49, 50, 51 Some investigators have suggested that H. pylori infection and cagA strains in particular may "protect" against the development of BE and progression to EAC. The postulated mechanism is through its ability to introduce atrophic gastritis, and possibly also by increasing intragastric ammonia production.52 The potentially negative association of H. pylori infection in relation to EAC needs to be further defined.
Pathogenesis
The pathogenesis of cancer in BE is multifactorial. Esophageal carcinogenesis is a multistep process recognized phenotypically as the histologic sequence of metaplasia, LGD, HGD, and adenocarcinoma. In the past decade, we have gained significant insight into the pathophysiology and molecular pathways associated with carcinogenesis. Reflux of gastroduodenal contents can promote chronic mucosal injury, resulting in inflammation, which is known to promote carcinogenesis. Also, the geographic distribution of EAC indicates that dietary habits, obesity, upper gastrointestinal infections, and exposure to carcinogens might interact and be responsible for the development of carcinogenesis in BE.
Role of Gastric Acid and Bile Reflux
Patients with complicated BE (dysplasia and cancer) reflux significantly more gastric acid and bile into their esophagus than patients with uncomplicated BE (nondysplastic BE) and GERD patients without BE.53 The arachidonic acid pathway, which controls inflammation, can be activated by gastric acid or bile salts and may contribute to carcinogenesis in BE. Low pH, as well as bile salts, can induce expression of COX-2, which is a key enzyme of the arachidonic acid pathway, in both an ex vivo culture model of human BE and in EAC cell lines.54, 55 Cyclooxygenase-2 expression increases concomitantly with neoplastic progression in BE, supporting the association of arachidonic acid pathway and EAC development.56 It is proposed that protein kinase C, extracellular signal-related kinase (ERK), and p38 mitogen-activated protein kinase (MAPK) mediate bile and acid-induced COX-2 expression.54, 55 Cyclooxygenase-2 can catalyze the conversion of arachidonic acid into various prostaglandins, including prostaglandin E2 (PGE2), and PGE2 induces the proliferation of BE cells, which provides an opportunity for these cells to accumulate replicated errors.57 Prostaglandin E2 also inhibits tumor surveillance by inhibiting natural killer cell activity. Thus chronic induction of PGE2 could facilitate the accumulation of abnormal cells with genomic instability and therefore promote carcinogenesis.58 Chronic injury related to acid and bile salts can also induce the production of reactive oxygen species, deplete antioxidants, and increase the expression of oxidative-stress related genes.59 At present, the clinical evidence for the role of acid and bile reflux in the progression of neoplasia in BE remains controversial.
Role of Hypergastrinemia
A large number of patients with BE are on acid-suppressive therapy that increase postprandial and fasting serum gastrin levels, which may have a role in promotion of carcinogenesis.60, 61 Gastrin can bind to the cholecystokinin (CCK2) receptor, the stimulation of which can induce expression of epidermal growth factor (EGF) and trefoil peptide. This can eventually induce COX-2 expression, which appears to be important for carcinogenesis in BE. Barrett's mucosa expresses more CCK2 receptors, and it has been shown that gastrin exposure increases proliferation in an esophageal cancer cell-culture model. Further downstream effects of gastrin-related CCK2 receptor stimulation can inactivate proapoptotic factors and therefore potentiate carcinogenesis. The importance of these observations in the clinical management of patients with BE remains unclear.
Role of Obesity and Diet
Obesity has emerged as an important risk factor for EAC, as has the modest decrease in dietary intake of fresh fruits and vegetables. Obesity increases circulating levels of many steroid hormones as well as insulin-like growth factor-1 (IGF-1). Increased expression of IGF-1 receptor (IGF-1R) is associated with progression of neoplasia in BE.62 Binding of increased levels of circulating IGF-1 to IGF-1R could transducer signals through several intracellular pathways, stimulating the malignant transformation of various epithelial and mesenchymal cells, and also protecting these cells against apoptosis.63 Possible links among obesity, dietary factors, and EAC need to be explored further.
Role of Molecular Aberrations
Apart from the cellular and architectural morphologic changes, a wide range of molecular abnormalities, both phenotypic and genotypic, occur during the process of malignant degeneration in BE.64, 65 Phenotypic changes encompass, among others, increased proliferation (marker: Ki-67),66 increased expression of growth factors [EGF, c-erbB2, and transforming growth factor- (TGF-
(TGF- )],67 increased expression of inflammatory factors [COX-2, tumor necrosis factor-
)],67 increased expression of inflammatory factors [COX-2, tumor necrosis factor- (TNF-
(TNF- )],68, 69 and disturbed cell adhesion (reduced expression of E-cadherin).64, 67 In addition, multiple genetic abnormalities have been reported: apart from gross chromosomal abnormalities, specific mutations-in the tumor-suppression genes p53 and p16 have been described. Finally, changes in the cell cycle of the involved epithelium, with an increased number of cells in the S phase (corresponding to DNA synthesis) and the G2 phase (premitosis), have been described.70 Currently, the changes suggested to be of potential help in the surveillance of patients include changes in DNA content (DNA aneuploidy), increased proliferation, and alterations of p53. However, no biomarker has yet emerged that is superior to histologic identification of dysplasia and cancer.
)],68, 69 and disturbed cell adhesion (reduced expression of E-cadherin).64, 67 In addition, multiple genetic abnormalities have been reported: apart from gross chromosomal abnormalities, specific mutations-in the tumor-suppression genes p53 and p16 have been described. Finally, changes in the cell cycle of the involved epithelium, with an increased number of cells in the S phase (corresponding to DNA synthesis) and the G2 phase (premitosis), have been described.70 Currently, the changes suggested to be of potential help in the surveillance of patients include changes in DNA content (DNA aneuploidy), increased proliferation, and alterations of p53. However, no biomarker has yet emerged that is superior to histologic identification of dysplasia and cancer.
Screening and Surveillance for Esophageal Adenocarcinoma
Screening
Recent guidelines from the American College of Gastroenterology recommend that patients with chronic gastroesophageal reflux symptoms, especially white men older than 50 years, are those most likely to have BE and should undergo upper endoscopy.6 There has been ongoing debate about screening in the literature, and at the current time there is no direct evidence that has validated the use of screening for EAC in the United States.71, 72, 73 Screening methods for the detection of BE and cancer have included standard endoscopy, unsedated endoscopy with ultrathin endoscopes, catheter-based cytology, balloon cytology, and more recently wireless capsule endoscopy.374, 75, 76, 77 A study aimed at assessing the cost-effectiveness of endoscopic screening in patients with GERD to rule out HGD in BE showed that compared with no screening, screening endoscopy cost $24,700 per life-year saved. It was concluded that under favorable conditions, general screening by endoscopy of all patients with reflux symptoms to prevent death from EAC may represent a cost-effective strategy; however, clearly such conditions may be difficult to meet.78 Although preliminary studies indicate some promise with these technologies in terms of screening for EAC, there have not been any definitive trials to allow recommendation of these techniques.
Surveillance
Surveillance is structured follow-up testing of BE to detect progressive dysplastic changes in the mucosa that herald the development of carcinoma. The goal of surveillance is to diagnose early stages of cancer in patients with known BE and to intervene so as to prevent progression to invasive cancer. Despite the difficulties with surveillance, the detection of HGD or early cancer has been shown to have the potential to improve survival in patients with BE.73, 79, 80 Cancers confined to the esophagus are associated with a 5-year survival rate of 70% compared with a survival rate of 20% for patients with more invasive cancers.81 Nodal involvement is far less likely to occur in patients found to have cancer on surveillance endoscopies compared with those patients who are not undergoing surveillance.73, 81 This leads to significant improved survival in surveyed patients compared to those patients who underwent surgery for symptomatic disease, as shown by several retrospective studies.
A systematic endoscopic biopsy protocol, generally accepted to be four-quadrant biopsy specimens taken every 1 to 2 cm of Barrett's mucosa starting at the gastroesophageal junction using standard or jumbo biopsy forceps, can provide tissue for histologic diagnosis of dysplasia. Increasing grade and extent of dysplasia are associated with increasing risk of cancer. Also, presence of mucosal abnormalities noted on endoscopy and the extent of HGD noted on histology predict the development of EAC. The presence of mucosal nodularity has been associated with a 2.5-fold increased risk of cancer development in patients with HGD (p = .01). Focal HGD, defined as involvement of 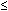 5 crypts in one biopsy specimen from the entire set of biopsy specimens, has been found to be associated with significantly less risk of cancer development than is diffuse HGD (p = .02).24
5 crypts in one biopsy specimen from the entire set of biopsy specimens, has been found to be associated with significantly less risk of cancer development than is diffuse HGD (p = .02).24
Current guidelines and reviews suggest that surveillance of nondysplastic Barrett's mucosa established by two endoscopies should be done at 3- to 5-year intervals. Surveillance of patients with LGD should be done at 1-year intervals. High-grade dysplasia should be confirmed in a second endoscopy and reviewed by an expert pathologist. If confirmed, the patient can be offered surveillance at 3-month intervals for 2 years and then every 6 months. Any mucosal abnormality should be carefully investigated and removed by endoscopic mucosal resection (EMR) for diagnosis. Surgical resection and mucosal ablation with techniques such as photodynamic therapy (PDT) can be offered for patients with confirmed HGD.82
There are several challenges in the surveillance of Barrett's patients. The efficacy and effectiveness of such intervention has not been shown in prospective randomized controlled trials. There is a high intra- and interobserver variability in the histologic grading of biopsies, especially for the diagnosis of LGD. The incidence of cancer, currently estimated at 0.5% per year, reduces the cost-effectiveness of any surveillance strategy; the vast majority of Barrett's patients will never develop EAC but currently still are candidates for an expensive and labor-intensive surveillance program.
At a recent American Gastroenterological Association BE workshop, a critical review of the literature in BE pertaining to the diagnosis, screening, surveillance, and treatment of BE was conducted. With regard to surveillance, the panel of experts agreed that endoscopic surveillance detects curable neoplasia in patients with BE and that endoscopy with multiple systemic biopsies is needed for detection of dysplasia or adenocarcinoma for the surveillance of BE. Half of the panelists rejected the statement that endoscopic surveillance in patients with BE has been shown to prolong survival, and the panel did not feel that endoscopic surveillance of all BE patients, in general, is cost-effective.7
Clinical Features
Early stages of cancer may cause no symptoms. Unfortunately, once patients are symptomatic, they generally have advanced and incurable disease.83 Patients with EAC, much like those with esophageal squamous cell carcinoma, can have dysphagia, odynophagia, weight loss, abdominal pain, or occult gastrointestinal bleeding. Weight loss is an independent indicator of poor prognosis if there is a loss of more than 10% of body mass. Dyspnea, cough, hoarseness, and pain (retrosternal, back, or right upper abdominal) occur less often but may reflect the presence of extensive, unresectable disease.84 Some patients may report melena or chest pain. Cough aggravated by swallowing raises the possibility of an esophagopulmonary fistula, a devastating complication associated with a high 30-day mortality rate.85 It is believed that the high frequency of advanced disease at symptomatic presentation is because the esophagus has a rich lymphovascular supply and does not have a serosa (enabling tumors to expand outward before luminal stenosis results). These tumors can also be recognized during endoscopic surveillance in patients with known BE.
The physical examination is usually unremarkable. Lymphadenopathy, particularly in the left supraclavicular fossa (Virchow's node), hepatomegaly, and pleural effusion are all indicators of metastatic disease.84
Diagnosis
The diagnosis of EAC rests on histologic documentation, and this is accomplished with flexible upper endoscopy, recognition of the tumor, and biopsy. The features, location, and size of the tumor can be more accurately assessed by endoscopy than by radiographic studies. Barium swallow usually has a limited role as a diagnostic test for these tumors and typically shows a stricture or ulceration of the esophagus. A barium esophagram may be very helpful in the analysis of malignant stenoses that may be too narrow to be traversed by the endoscope and to confirm the presence of esophagopulmonary fistulas.86 In patients with advanced cancers, esophageal dilatation may be required to allow for a standard [outer diameter (OD), 9.8 mm] endoscope to traverse the obstructed lumen. Alternatively, an ultrathin (OD, 5.3–6 mm) endoscope may pass through the stenosis and allow completion of examination in 75% of cases.87 Biopsy specimens are required for histologic confirmation. The diagnostic yield reaches 100% when six or more samples are obtained using a standard endoscopic biopsy forceps. As an adjunct, brush cytology can be helpful in sampling tight malignant strictures, which may not be easily accessible to conventional biopsy techniques.82
Detection of Early Esophageal Adenocarcinoma
There have been several new developments in the endoscopic detection of early neoplastic lesions in BE patients:
Chromoendoscopy and Magnification Endoscopy
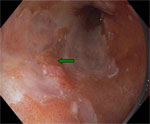
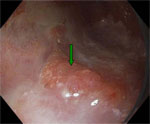
This technique involves the topical application of dyes during endoscopy in an effort to enhance the detection of mucosal pattern or lesions on basis of staining characteristics.88 Although some randomized studies have shown that methylene blue staining increases the detection of dysplasia and early cancer in BE,89 others have found it to be disappointing.90 High-resolution endoscopes with high-quality charge coupled device chips (>850K pixels) and variable focal distance are now commercially available. These endoscopes have a mechanically or electronically movable lens at the distal tip that enables the endoscopist to zoom in on an area of interest (Figures 1 and 2). Magnification endoscopy, which enhances mucosal detailing, is generally used in combination with chromoscopy.
Figure 1: Endoscopic image of Barrett's adenocarcinoma using high-resolution endoscopy.
The green arrow denotes a nodular mucosa that revealed early Barrett's adenocarcinoma on biopsy. The pink-colored areas are Barrett's metaplasia and the whitish areas are normal squamous epithelium.
Sharma et al.91 studied 80 patients who had columnar-lined distal esophagus using indigo carmine dye and 115  magnification endoscopy. Three types of mucosal patterns were noted: ridge/villous, circular, and an irregular/distorted pattern. The presence of the ridge/villous pattern for detecting intestinal metaplasia had high sensitivity, specificity, and positive predictive value (97%, 76%, and 92%, respectively). Low-grade dysplasia, seen in 18 patients, had the ridge/villous pattern. Six patients had an irregular/distorted pattern, and biopsies revealed HGD in all these patients. It is yet to be determined if this technique will decrease the need for endoscopic biopsy or significantly enhance the diagnostic yield over conventional techniques.
magnification endoscopy. Three types of mucosal patterns were noted: ridge/villous, circular, and an irregular/distorted pattern. The presence of the ridge/villous pattern for detecting intestinal metaplasia had high sensitivity, specificity, and positive predictive value (97%, 76%, and 92%, respectively). Low-grade dysplasia, seen in 18 patients, had the ridge/villous pattern. Six patients had an irregular/distorted pattern, and biopsies revealed HGD in all these patients. It is yet to be determined if this technique will decrease the need for endoscopic biopsy or significantly enhance the diagnostic yield over conventional techniques.
Narrow Band Imaging
The mucosal detail as seen with magnification endoscopy can be further increased by using narrow band imaging (NBI), and in addition the superficial vascular network can be assessed in detail as well. The NBI system has a standard high-resolution mode in which the white light image is composed of the sequential imaging through a red, green, and blue band-pass filter. In the NBI mode, the bandwidths of these three filters have been narrowed and the relative contribution of the blue filter has been increased, resulting in improved mucosal contrast and detail. This technique holds the promise of obtaining images comparable to magnification endoscopy with chromoscopy but without the use of dyes.92, 93
Optical Techniques
These techniques use light to enhance the detection of cancerous lesions. Included in this category are spectroscopic techniques and optical coherence tomography (OCT). Fluorescence endoscopy involves stimulation of certain molecules (fluorophores) by ultraviolet or blue light. Upon excitation, these fluorophores emit fluorescent light spread over a range of longer wavelengths from the green to the red spectrum. This is called autofluorescence and responsible endogenous fluorophores include collagen, reduced nicotinamide adenine dinucleotide (NADH), aromatic amino acids, and porphyrins. The value of light-induced fluorescence spectroscopy has been clinically evaluated with promising results in distinguishing nondysplastic areas from areas with HGD or cancer.94 However, it samples only a small mucosal area (1–3 mm), which makes this technique impractical for scanning larger areas. The technique, therefore, has been incorporated in an endoscopic system- fluorescence imaging. Initial studies suggested that fluorescence imaging increased the detection rates of HGD and cancer.95 However, in a recent randomized crossover study comparing this with standard endoscopy, fluorescence imaging did not improve the detection of HGD or early cancer.96 Optical coherence tomography is a technique that uses infrared light for excitation after which the reflected light is analyzed for its delay and intensity of reflection. Current endoscopic OCT devices are catheter based, allowing scanning of esophagus in a linear, transverse, or radial fashion. Using predetermined OCT criteria for SIM, the linear scanning OCT system was found to be 97% sensitive and 92% specific for identifying specialized intestinal metaplasia. A quantitative analysis of the OCT signal seemed to identify HGD with high sensitivity (100%) and specificity (85%).97 Spectroscopy and OCT are techniques in evolution, and further refinements are anticipated that may improve its diagnostic accuracy for the detection of preneoplastic lesions.
Other emerging spectroscopic techniques, including elastic scattering spectroscopy, Raman spectroscopy, and multimodal optical spectroscopy have shown initial promise in differentiating Barrett's epithelia in feasibility studies.
Brush Cytology
With brush cytology the entire surface area of the Barrett's mucosa may be sampled using a brush that is advanced through the working channel of the endoscope. This may reduce the sampling error, detect relevant abnormalities as dysplastic cells are shed easier, and take considerable less time than obtaining four-quadrant random biopsies. The morphologic analysis of brush cytology specimen is difficult and in fact inferior to standard histology for surveillance of BE.98 This can perhaps be overcome by using fluorescent in situ hybridization (FISH), a technique in which small fluorescent DNA probes are used for detection of specific chromosomal abnormalities. These probes bind specifically to relevant areas of different chromosomes, which enable identification of amplification or loss of areas known to be important for the development of cancer. Thus, FISH may allow earlier detection of patients at risk for malignancy. In a recent study, a high sensitivity was demonstrated to detect relevant histologic abnormalities by using multicolor FISH on brush cytology specimens of BE patients with HGD or early cancer. A DNA probe set specific for the locus specific regions harboring the p53 and p16 genes and centromeric probes for the chromosomes 9, 17, and Y were used. The number of genetic abnormalities increased as dysplasia progressed and invasive disease occurred, whereas no FISH abnormalities were shown in control patients.99
Staging
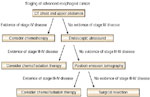
Clinical staging depends on the anatomic extent of the primary tumor, which can be ascertained by examination before treatment, as opposed to pathologic staging, which is based on surgical exploration and examination of the resected specimen. The staging of esophageal cancer is critical to guide further therapy for the patient. Staging helps define whether there is any hope of curative treatment and facilitates rational treatment choices. It also allows identification of patients with advanced disease unlikely to benefit from extensive evaluation and aggressive treatment. Unfortunately, none of the currently available staging modalities has been shown to be able to stage all aspects of the tumor, and as a consequence these patients tend to undergo multimodality staging. The survival of patients with EAC is stage dependent and defined by the T (tumor), N (node involvement), and M (metastasis) system (Table 2). The primary tumor (T) is defined by the depth of invasion into the wall of the esophagus and adjacent structures. Regional lymph nodes (N) and metastases (M) are characterized by their presence or absence. Esophageal cancer is not a disease with a uniformly bleak prognosis, and clinical stage is an accurate predictor of prognosis. Reported 5-year survival rates by T stage are T in situ, >85%; T1, 46%; T2, 30%; T3, 22%; and T4, 7%.100, 101 Furthermore, the most powerful independent prognostic factor in esophageal cancer is the ability to obtain an R0 resection (i.e., complete macroscopic and microscopic resection), and this depends primarily on preoperative stage. Nodal status is also a significant prognostic factor. Among patients with resectable disease, the 5-year survival rate is 40% for N0 patients compared with 17% with N1 patients.100, 101, 102 Clinical staging can be accomplished by endoscopy, chest and abdominal computed tomography (CT), endoscopic ultrasound (EUS) with fine-needle aspiration (FNA), and laparoscopy (Figures 3 and 4).
Figure 3: Algorithm for staging and treatment of early cancers.
(Source: Wang et al.,82 with permission from American Gastroenterological Association).
Figure 4: Algorithm for staging and treatment of advanced cancers.
(Source: Wang et al.,82 with permission from American Gastroenterological Association).
Endoscopy can accurately define the length of a tumor that can be traversed and is a helpful to which additional studies are needed next.82
Computed tomography scan is the most commonly used staging procedure, and the strength of this test is its ability to detect distant metastatic disease.103 It has a limited role in determining the T stage of esophageal tumors.101 Common sites for distant metastasis in patients with recently diagnosed esophageal cancer include abdominal lymph nodes (45%), liver (35%), lung (20%), bone (9%), adrenal (2%), brain (2%), pericardium (1%), pleura (1%), and spleen (1%).104 Initial staging with CT shows distant metastases or unresectable disease in up to 50% of patients at presentation. However, this finding may be inaccurate in up to 40% of cases when compared with surgical staging.105 Overall, CT scanning has a sensitivity of 37% to 66% in screening for distant metastases.101 The role of magnetic resonance imaging (MRI) in staging of EAC remains unclear. It has higher contrast sensitivity than CT (especially with regard to delineating margins between the esophagus and mediastinal fat) and is useful for detecting solid organ metastases larger than 5 to 10 mm; however, it has not been shown to have a distinct advantage over CT and is equally poor for T staging.106
Endoscopic ultrasound is the only readily available modality that provides high-resolution, real-time images of the gut wall layers (i.e., mucosa, submucosa, and muscularis), periesophageal structures, and regional nodes, and is thus ideally suited for staging EAC.107 Linear EUS scanners also allow safe EUS-guided FNA of lymph nodes. The diagnostic utility of EUS is in determining the depth of tumor invasion and presence of locoregional adenopathy. This technology is limited in terms of detecting distant metastasis.82 At 7 to 12 MHz ultrasound frequency, the esophageal wall usually can be imaged as a five-layer structure. Tumor depth of invasion (T) has been correlated to the level of wall-layer abnormality on EUS.108 A meta-analysis has demonstrated the accuracy of EUS for T staging to be 89%.109 The accuracy of EUS to ascertain tumor infiltration tends to increase with increasing stage, and staging accuracy is generally more than 90% for T3 disease.
There are limitations to the ability of EUS to predict T stage, such as distinguishing mucosal (T1m) from submucosal (T1sm) invasion, which is crucial to identify tumors amenable to local ablative treatment (PDT or EMR).110 However, new EUS probes that operate at higher frequency (20 and 30 MHz) can visualize the esophageal wall as a series of seven or nine layers. It has been demonstrated that these high-frequency ultrasound probes, often called "miniprobes," increased the accuracy of differentiating between T1 and T2 lesions from 76% (standard EUS) to 92% accuracy.108, 111
Esophageal adenocarcinoma can sometimes present as malignant stenosis, and initial studies have shown that the accuracy of EUS in determination of T stage for nontraversable tumors to be slightly lower than that of traversable cancer (77% vs. 84%).112 Miniprobes may have a role in more accurate T staging than low-frequency probes in these patients, and stenotic lesions may be traversed without dilation. Contributors to inaccurate staging include operator inexperience, microscopic invasion, and peritumoral inflammation. Endoscopic ultrasound is less accurate for nodal staging but is still the most accurate modality for staging locoregional lymph nodes. In a meta-analysis, the accuracy of conventional EUS for N staging was 79%.113 Criteria for malignant nodes include a diameter greater than 10 mm, uniform hypoechogenicity, round shape, and sharp borders.108
The use of EUS-FNA is now becoming more widespread, and has been shown to improve the accuracy of EUS for N staging by providing cytologic material to confirm malignant involvement of lymph nodes.114 Most significantly, EUS-FNA has improved the ability to investigate celiac axis lymph nodes, particularly important as the presence of malignant celiac nodes is a sign of distant metastatic spread. Although EUS has a limited role in the restaging of esophageal cancer after neoadjuvant therapy, measurement of tumor diameter seems to provide a reasonably accurate assessment of therapeutic response.108
Positron emission tomography (PET) is a novel nuclear study that is being used increasingly to stage gastrointestinal malignancies. It uses fluorine 18 (18F)-fluorodeoxyglucose (FDG) for the detection of nodal or distant metastasis in EAC.115 This substrate is taken up by metabolically active cells and then phosphorylated, after which it cannot be further metabolized. Positron emission tomography can detect degeneration of this material. However, inflammatory tissues are also fast glucose metabolizers, and metastases have often to be differentiated from inflamed tissue, leading to false-positive results. A number of case series have found that PET is not as sensitive as EUS or CT for locoregional disease.116, 117 This technology provides no definition of the esophageal wall and hence cannot define the T stage. However, it appears to be superior to CT for detecting distant metastases. The reported sensitivities and specificities for detecting distant metastases range from 69% to 88% and 90% to 93%, respectively.115, 118, 119 New advances in PET technology include fusion PET, which combines the CT image with the PET image to allow better tumor localization.
Other staging procedures that have been investigated include minimally invasive surgery or laparoscopic staging before esophagectomy. Case series have found that performance of laparoscopic- or thoracoscopic-guided biopsies of lymph nodes increases the detection of metastatic disease and can change management in 15% to 20% of patients. Thoracoscopy has a significant advantage in detecting thoracic lymph nodes, and when compared with laparoscopy appears to be superior to EUS, PET, and CT staging.82, 101
As per a recent American Gastroenterological Association technical review82 on the management of esophageal carcinoma, it is recommended that CT of the chest and upper abdomen be the first staging procedure, followed by EUS and FNA if there is no evidence of distant metastasis found on CT and the procedure is available. If surgical resection is still considered, PET can be used, if available, because of its increased sensitivity for distant metastasis. In patients with potentially early-stage disease (tumors <2 cm and nonobstructing), EUS with endoscopic mucosal resection should be considered as a staging procedure if available for histologic staging of the cancer.
Treatment
Treatment of Early Cancers
Early esophageal cancers, those confined to the mucosa or upper submucosa of the esophagus, are termed T1, N0, M0 by the American Joint Commission on Cancer terminology. There are no randomized treatment trials for these cancers because they are rare, accounting for <5% of esophageal cancers diagnosed in most series.82 The traditional approach for these early cancers is surgical resection. Limited reports have results with a 100% rate of total excision without any operative mortality.120, 121, 122 However, this is likely to be influenced by reporting bias. The reported mortality rate for esophagectomy performed on patients with HGD is at least between 2% and 6%.123, 124
The major concern with surgical therapy is the 40% incidence of morbidity associated with the procedure.82 Localized esophageal cancer is most commonly resected with the use of either a right transthoracic or a transhiatal approach. The right transthoracic approach combines a laparotomy and right-sided thoracotomy, leading to an esophagogastric anastomosis either in the upper chest (the Ivor-Lewis technique) or in the neck (the three-field technique). The transhiatal approach uses a laparotomy with blunt dissection of the thoracic esophagus and places the anastomosis in the neck.84 Although neither retrospective trials nor prospective trials have demonstrated any significant differences in survival or operative mortality between these two types of surgery, the results of one trial suggest that the transhiatal approach has a lower rate of perioperative complications (mainly fewer pulmonary complications and a lower incidence of chylous leakage).125 Possible procedure-related complications include anastomotic strictures, leaks, chronic aspiration, infection, and chylothorax. Minimally invasive esophagectomy has been evaluated for resection of esophageal cancer with the use of laparoscopy for gastric mobilization and resection.126 The gastroesophageal anastomosis is accomplished with a thoracoscope or manually. Although the procedure has not been shown to improve patient outcomes in terms of morbidity or mortality, it has been attractive to patients with superficial cancer or HGD who desire an improved cosmetic result. Lymph node metastasis, histologic grade, and extension of the tumor through the wall of the esophagus or within periesophageal tissues are the most important predictive factors for survival after surgery.
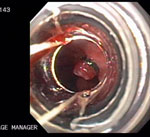
Because of the morbidity and mortality associated with esophagectomy, there has been increased interest in endoluminal therapy for superficial esophageal cancers and polypoid high-grade dysplasia. Several different EMR techniques have been developed. Piecemeal resection using a saline lift technique imitates the technique used for sessile colonic adenomas.127 This technique can be used in association with snare removal of the lesion. Other lesions may be amenable to resection with a banding technique similar to that used for esophageal varices; a snare is used to remove the pseudopolyp produced after banding so that the specimen can be sent to pathology to ensure complete removal.127, 128, 129 Finally, novel EMR caps, with a beveled edge and a snare that fits along a groove at the top of the cap, have been described.130 These caps may be positioned over the lesion of interest, after which suction is applied, and the lesion is pulled into the cap. The snare is tightened over the base of the lesion, which is then truncated. This method is especially useful for larger lesions, which will not easily fit in a variceal ligation cap (Figures 5 to 8).
Early EACs have been endoscopically treated in the setting of BE. These have been described in large case series using EMR alone or in combination with PDT.128, 129 Lesions most amenable to EMR are those that are polypoid, elevated, and <2 cm in size, and have a low-grade cancer.128 Endoscopic mucosal resection was used in 115 patients (83% with early cancers) and resulted in a 3-year overall survival rate of 88%. Complications occurred in 9% of patients, including bleeding and stricture formation. However, because BE without HGD or cancer was left untreated, 30% of the patients developed additional neoplastic lesions.131 For this reason, it is generally advised that the remainder of BE be treated after resection of cancer. Photodynamic therapy has been used for this purpose, with 94% initial success rates at 1 year.129
Endoscopic therapy using PDT, laser therapy, and argon plasma coagulation (APC) combined with effective acid-suppression therapy has also been reported.132, 133, 134, 135 These treatments have had limited success in completely eliminating BE and cancer, with response rates of 25% to 94%. Laser therapy with neodymium:yttrium-aluminum-garnet (Nd:YAG), which operates at a wavelength of 1063 nm, penetrates quite deeply and has been favored for tumor ablation. Lasers that operate in the 540-nm wavelength, such as the argon or potassium-titanyl-phosphate (KTP-YAG) lasers, have a much more limited depth of penetration. Thermal therapies, such as APC applied at high-power setting, have also been used to treat early cancers. Photodynamic therapy is influenced by the type of photosensitizer used for therapy. Aminolevulinic acid is not approved in the United States at this time, but it has found favor in Europe because it is not associated with the prolonged cutaneous photosensitivity that is found with the sodium porfimer that is used in the United States. Owing to its very limited depth of penetration, aminolevulinic acid has only been found to be useful for cancer therapy if the thickness of the cancer is <2 mm.
These endoscopic techniques hold a great deal of promise for elderly patients with significant morbidities, poor surgical candidates, and patients that decline surgical resection.
Treatment of Advanced Cancers
The prognosis for EAC remains dismal, with an overall 5-year survival of approximately 20%.14, 122 This poor result is attributed in part to the advanced stage of the cancer when it is usually diagnosed, and more than 50% of those with this cancer present with stage III or IV disease.122
Primary surgical therapy for cancers limited to the esophagus, stage I or IIa disease, has had good results without the need for or morbidity of chemotherapy. Surgical therapy for more advanced cancer has been performed as either a limited resection or en-bloc resection of lymph nodes, which can be performed via the transhiatal or transthoracic (Ivor-Lewis) approach.82 It is clear from the surgical literature that the experience of the surgeon and the volume of the center providing the treatment are crucial predictors of mortality and morbidity of the treatment. Low-volume centers have average 30-day mortality rates of 18.7% for esophagectomy, whereas high-volume surgeons have corresponding rates of 9.2%.136 These differences may be attributed to the greater experience of the surgical operators and to the specialized institutional support, such as skilled nursing, respiratory therapy, and intensive care units, that evolve in high-volume centers.
None of the studies to date has demonstrated a survival advantage with the use of preoperative radiotherapy.84 Similarly, after initial enthusiasm for neoadjuvant chemotherapy as an adjunct to surgery, a well-performed randomized controlled trial showed that neoadjuvant chemotherapy before resection did not improve survival.137
More promising have been the results of studies combining neoadjuvant chemotherapy with radiation therapy. Walsh et al.138 randomized 113 patients with adenocarcinoma to undergo either surgery alone or neoadjuvant chemotherapy and radiation with surgery afterward. The chemotherapy regimen was 5-fluorouracil (5-FU) and cisplatin based, and 40 Gy of radiation was delivered. These investigators found a significant downstaging of tumors in the multimodality group; fewer subjects in this group had stage III or IV disease at the time of surgery. Additionally, the 3-year survival was improved in the multimodality group (32% vs. 6%), and median survival was significantly longer in the multimodality group (16 vs. 11 months). However, six other randomized trials assessing the value of preoperative chemotherapy and radiotherapy reported thus far have failed to demonstrate any survival benefit.
With the high pathologic response rates with radiation and chemotherapy alone, it has been questioned whether surgical therapy is needed in the treatment of patients with more advanced cancers. In a trial involving 123 patients with squamous cell carcinoma or EAC, 61 patients were randomly assigned to receive cisplatin and fluorouracil combined with 5000 cGy of radiation, and 62 were treated with 6400 cGy of radiation alone. After 5 years of follow-up, the overall survival rate was 26% in the combined therapy group, as compared with 0% in the group given radiotherapy alone. Attempts to enhance this beneficial effect by increasing the radiation dose to 6480 cGy have proved unsuccessful.139
Postoperative chemotherapy and concurrent radiotherapy are frequently offered to patients whose tumor cells extend to the surgical margin (as a result of incomplete resection). There is no documented evidence that postoperative chemotherapy or radiotherapy is beneficial in the absence of residual disease.
Palliation of Esophageal Adenocarcinoma
Despite advances in diagnosis and treatment, up to 50% of patients have incurable disease at presentation, therefore necessitating palliative measures.140 The goal of palliative therapy in patients with unresectable cancer is to ameliorate symptoms and treat complications, thereby improving their quality of life. A variety of therapies have been employed to palliate dysphagia in patients with esophageal carcinoma including esophageal dilation, radiation therapy, Nd:YAG laser, thermal electrocoagulation, PDT, and sclerotherapy of the tumor. Esophageal prostheses (stents) have also been used as a method for palliation of malignant dysphagia (Table 3). Because of improved design, materials, and deployment systems, self-expandable metal stents (SEMS) have become an attractive alternative to palliate EAC.
Surgical Therapy
For patients presenting with EAC, tumor resection should be initially considered when the surgical risk is acceptable and metastatic disease is not identified. Not only will surgery provide the potential for cure, but reliable palliation of esophageal complaints can also be achieved. Nevertheless, it is well recognized that EAC may relapse at the surgical anastomosis likely because of the high frequency of locally advanced disease.141 Also, despite negative preoperative staging, metastatic disease may be found at the time of exploration in up to 95% of patients.138 However, with the increasing use of EUS and PET scans for staging, the number of patients undergoing resection with occult metastatic disease is reduced and prognosis following resection has improved. Although surgical therapy alone usually ameliorates dysphagia, routine surgical exploration must be tempered by the poor long-term prognosis. In studies reporting follow-up after curative resection of EAC, the median survival is 10 months with a 5-year survival of 15% for those with stage III disease.142
Radiation Therapy and Chemotherapy
Several trials suggest some benefit from radiation and chemotherapy in patients with inoperable locally advanced disease. Harvey et al.143 treated 106 patients with palliation chemoradiotherapy and showed a significant improvement in dysphagia. Fifty-one percent maintained improved swallowing at last follow-up. Treatment was well tolerated with a treatment-related mortality of 6%. In another study, Keller et al.144 evaluated preoperative radiation with 5-FU and mitomycin. Overall, 18 patients (39%) achieved a complete clinical response; however, 20% developed progressive disease during chemoradiotherapy, and 20% patients did not undergo surgery for a variety of reasons which emphasizes the importance of palliation.
Tissue Ablation
Various methods of tissue ablation have been employed to palliate dysphagia in patients with EAC. Argon plasma coagulation is a method for ablating esophageal cancer. In a study comparing outcomes in those who underwent thermal ablation using APC vs. esophageal metal stenting, the median survival was longer for patients who underwent APC. However, the median length of hospital stay and costs were significantly higher for those palliated with APC.145 The superficial nature of the thermal energy as well as the inability to control the orientation of the tumor probes have led to limited use of thermal ablative modalities. These techniques are often used today as "salvage" methods to treat the tissue hyperplasia and tumor ingrowth/overgrowth at the margins of previously placed stents and for local control of bleeding from these tumors that tend to be vascular. Although laser therapy is useful for exophytic and polypoid lesion, it can be applied to almost any type of tumor leading to esophageal obstruction. The Nd:YAG laser is usually applied in several sessions and causes deeper injury than APC. The procedure is usually repeated 48 hours later for further treatment and debridement. Laser therapy is less successful than stenting for tumors involving the esophagogastric junction and cardia.146 It is 70% to 95% effective at relieving dysphagia.147, 148 The duration of response ranges from 1 to 2 months, but multiple sessions are usually required owing to tumor regrowth. Minor complications include chest pain, transient worsening of dysphagia from treatment related edema, and leukocytosis. Major complications include bleeding and perforation (0–6%). The laser equipment is expensive and may not be widely available, and unlike stenting, laser therapy is contraindicated in the presence of fistulas, and is not suitable for long, tortuous, circumferentially narrowed tumors.82
Ablation of tumors can also be achieved with the injection of chemicals or sclerosing agents, resulting in tumor necrosis and partial restoration of esophageal luminal patency. Tumors have been injected with cytotoxic agents, absolute alcohol, polidocanol, and sodium morrhuate. Despite the ease of technique, injection sclerotherapy has not gained in popularity for palliative therapy, as the response is partial and temporary.
Esophageal Dilation
Dilation is an effective method for providing temporary relief of dysphagia in patients with malignant esophageal stenosis. Most clinicians prefer to use over-a-wire bougie dilators (e.g., Savary-Gillard), owing to their lower incidence of esophageal perforation. The major drawbacks of peroral dilation in the setting of advanced EAC are its short-term relief of dysphagia, the need for frequent dilation sessions, and associated complications (perforation).149 Balloon dilators have several theoretical advantages over bougie dilators, the most important being that applied force is directed only radially. The ideal size to which a stricture needs to be dilated remains unclear.
Photodynamic Therapy
The use of PDT for unresectable esophageal cancer has gained popularity owing to its success in improving dysphagia, quality of life, and nutritional status, with benefits lasting from 1 to 3 months.150, 151 Because of the theoretical advantage of selective tumor destruction, PDT appears more efficacious than the Nd:YAG laser. In the largest multicenter randomized study comparing PDT to Nd:YAG laser, Lightdale et al.152 randomized 110 patients to receive PDT and 108 to Nd:YAG laser. Improvement in dysphagia was equivalent between the two treatment groups. Objective tumor response was also equivalent at 1 week, but at 1 month tumor response was 32% after PDT and 20% after laser. Nine complete tumor responses occurred after PDT and two after laser. Trends for improved response for PDT were seen in tumors located in the upper and lower third of the esophagus, in long tumors, and in patients who had prior therapy. More mild to moderate complications followed PDT, but severe complications (e.g., perforation) were more common in laser treated patients (1% vs. 7%). Other side effects from PDT are development of esophageal strictures, pleural effusions, fever, and sunburn.
Esophageal Stents
Placement of esophageal stents is a well-established, reliable, inexpensive, and durable method of palliation of malignant dysphagia. Two main types of esophageal stents are available: rigid (plastic) and expandable (metallic). Plastic stents have lost popularity because of their high complication rate and the commercial availability of the newer, easier to insert SEMS.153, 154 In a study comparing plastic stents and SEMS, 39 patients with esophageal cancer were prospectively randomized to either plastic stent (20 patients) or metallic stent (19 patients). Technical success and improvement in dysphagia scores were similar in both groups. However, complications and mortality related to deployment were significantly less frequent with metal stents than with plastic stents (complications 0% vs. 21%, mortality 0% vs. 15.8%). Late complications (food obstruction, tumor ingrowth and migration) were not significantly different between the groups. The higher acute complication rate and mortality were associated with the technique of stent insertion, which required greater esophageal dilation prior to plastic stent insertion.154
There are a large variety of SEMS, each with its own characteristics, and the patient's underlying pathology (e.g., malignant stricture at the gastroesophageal junction, tracheoesophageal fistula, etc.) dictates the type or types of stent that will be required. Types of SEMS include Wallstents (Microvasive/Boston Scientific Inc, Natick, MA), Z-stent (Wilson-Cook Medical, Winston-Salem, NC), Ultraflex stent (Microvasive/Boston Scientific Inc, Natick, MA), and Esophacoil stent (Medtronic InStent Inc, Eden Prairie, MN). Successful deployment is reported in 85% to 100% of series, although up to 22% require a second prosthesis at the time of initial stenting.155, 156, 157 Covered stents are associated with prolonged patency when compared with uncovered ones.158 Several uncovered and most covered stents are useful to palliate malignant tracheoesophageal fistulas secondary to esophageal cancer. Stents with flange diameters of 25 mm or larger are associated with lesser degrees of prosthetic migration.159 An improvement in quality of life as well as cost-effectiveness has been shown from the use of SEMS.160
Before stent insertion, an appropriate esophageal luminal diameter is required to introduce the delivery catheter. If the esophagus cannot be dilated to the minimum diameter, damage to the esophagus may occur. Conversely, overdilation may increase the chance of stent migration. Relative contraindications include uncooperative patients, tracheobronchial compression (especially with cuffed stents), significant coagulopathy, recent myocardial infarction, and presence of fixed cervical spine and cervical arthritis. If the esophageal tumor has significant necrosis, the chances of perforation and stent migration increase.
Complications of stent placement can occur immediately in the postprocedure period, early and late after stent deployment. Perforation is one of the most feared complications and it appears greatest in patients who have received chemotherapy and/or radiation therapy and in patients who have long strictures or inadequately dilated strictures; perforation also results from poor technique. Other complications include migration (proximal or distal), food impaction, stent occlusion, bleeding, retrosternal pain, and reflux esophagitis. The most common causes of stent-related death are perforation, bleeding, and airway compression.
Studies comparing various endoscopic modalities for palliation of malignant dysphagia are few and conflicting. Retrospective studies comparing laser therapy with stenting have shown similar outcomes with regard to relief of dysphagia but higher complication rates in the stent group. These studies are limited by their retrospective design and heterogeneous patient populations. A prospective, randomized, controlled trial of laser therapy vs. SEMS in 60 patients with previously untreated esophageal cancer demonstrated a significant improvement in the degree of dysphagia and a reduction in the reintervention rate (35.7% vs. 100%) in favor of SEMS.161 Another study of 65 patients, however, showed longer survival rates in patients undergoing thermal ablative therapy (laser predominantly) compared with those who received stenting.162
Nutritional Support
Nutritional support is often required in patients with EAC. There is evidence that enteral nutrition is beneficial in patients with dysphagia and in those receiving radiation therapies.163 Enteral nutrition is generally preferred to the parenteral approach.164 Enteral nutrition can be accomplished by feeding tubes (nasogastric or nasojejunal), although these types of tubes are associated with increased bodily concerns by patients and could limit their social interactions. Percutaneous gastrostomy or jejunostomy tubes are preferred and are better tolerated.165
Finally, palliative care of patients with EAC requires the involvement of physicians who are attentive to both physical and emotional needs. These patients require support from hospice personnel and deserve physician attention and compassion. Until esophageal cancer can be effectively treated, compassion may be one of the most important "therapies" we can offer for palliation of this disease.
Treatment Summary
According to a recent American Gastroenterological Association technical review,82 patients with early esophageal cancers confined to the mucosa should be treated with surgical resection, with consideration of EMR with adjuvant mucosal treatment for any remaining preneoplastic tissue (BE) (based on level III evidence). Patients with early esophageal cancers that penetrate into the upper third of the submucosa can be treated with endoscopic mucosal resection if surgical mortality is anticipated to be >6% (based on level III evidence). Patients with stage I and IIa disease who are good candidates for surgical therapy do not require neoadjuvant therapy before esophagectomy (based on level II evidence). Patients with stage IIb and III disease may benefit from concomitant chemotherapy and radiation therapy before surgical therapy (based on level II evidence). Patients with more advanced-stage cancer may be treated with chemotherapy and radiation therapy or be considered for palliative therapy. Endoscopic therapy is effective at alleviating dysphagia. The selection of particular endoscopic technique should be based on tumor characteristics, patient preference, and available expertise. Esophageal dilation results in short-lived palliation and is best used as an adjunct to other palliative modalities (based on level III evidence). Esophageal stenting is the preferred endoscopic modality in patients with long malignant strictures more than 2 cm from the upper esophageal sphincter or fistulas (also based on level III evidence). There is level I evidence that SEMS are superior to conventional semirigid plastic stents in the management of malignant dysphagia. Other palliative methods such as alcohol injection, laser therapy, and PDT are similarly efficacious as tumor ablative therapies (based on level II evidence).
Chemoprevention and Other Novel Therapies
Nonsteroidal Antiinflammatory Drugs, Aspirin, and Cyclooxygenase-2 Inhibitors
Observational data substantiate that NSAIDs are associated with a 50% or greater decrease in the risk of esophageal cancer.166, 167, 168 The exact mechanism of any chemopreventive effect is unclear, and no randomized controlled trial has confirmed this observation. However, given the poor prognosis associated with esophageal cancer, authorities have suggested the potential use of chemoprevention in the setting of BE. Recent work has concentrated on the potential use of COX-2 inhibitors as chemopreventive agents. It was hoped that a superior side-effect profile of these agents compared with nonselective NSAIDs might improve the risk-benefit ratio of chemoprevention. However, given recent revelations about the potential cardiac side effects with rofecoxib, the use of selective COX-2 inhibitors as chemoprevention is not currently advisable.
Acid Inhibition
Antireflux surgery or prolonged acid suppression using high doses of proton pump inhibitors result in the appearance of squamous islands in BE segment but have not consistently been shown to regress metaplastic epithelium or prevent EAC.169, 170, 171 Recently, however, longitudinal studies of subjects with BE have suggested that the use of proton pump inhibitors may be associated with a decreased risk of dysplasia in BE.172, 173 Currently, the effect of vigorous acid suppression on the likelihood of progression of BE is unclear.
Ablation Therapy
Ablation therapy in conjunction with acid control has been promoted for the treatment of BE to decrease the risk of EAC.174, 175 This is based on the principle that elimination of the metaplastic epithelium can result in squamous mucosal regeneration.176 Multiple ablation techniques have been proposed and involve the use of multipolar electrocoagulation (MPEC), APC, Nd:YAG laser, and PDT. Multiple case series of APC and MPEC have been reported with significant reductions in Barrett's mucosa and decrease in dysplasia, but complete ablation of BE (both endoscopic and histologic) is uncommon.177, 178 Photodynamic therapy has been approved for use in patients with HGD to decrease the risk of cancer formation.179 Unfortunately, the residual Barrett's mucosa after ablation therapy has been found to contain genetic mutations similarly to those found before ablation, suggesting that histologic improvement may not correlate with elimination of cancer risk. With the exception of PDT, these techniques are not ready for clinical application and cannot be offered outside the research arena.
According to the American Gastroenterological Association technical review,82 fundoplication should be not recommended solely to prevent esophageal cancer in patients with GERD (level II evidence). The use of high-dose proton pump inhibitors to prevent EAC has also not been established and cannot be recommended in clinical practice (level III evidence). Level II evidence suggests that ablation therapy may decrease dysplasia in BE and could potentially decrease cancer risk. The NSAIDs should not be recommended at the current time solely for the prevention of EAC but have promise as a chemopreventive agent, which might be beneficial if required for other medical indications (based on level II evidence). Patients at risk for EAC should be counseled to adapt healthier lifestyles, in particular attaining normal weight and eliminating tobacco use (based on level II evidence).
Future Challenges
It is clear that further research is needed for improvements in the early detection, prevention, and treatment of EAC. The cause of continued increase in incidence of EAC still remains undefined. The need to stratify BE patients for esophageal cancer risk is essential to implement treatment and prevention strategies. The use of biomarkers would be very attractive and enable risk stratification, allowing time and effort to be invested in patients who are truly at risk for malignant degeneration. High throughput cancer discovery techniques combined with advanced bioinformatics tools may lead to the discovery of genomic and proteomic profiles specific for BE patients at risk for developing cancer. New endoscopic imaging techniques will improve the detection of relevant lesions in these patients, enabling their endoscopic treatment. The ultimate surveillance technique for BE patients, however, involves a simple blood test for either a genetic or a proteomic abnormality.
The role of chemopreventive agents such as NSAIDs, proton pump inhibitors and dietary interventions need further research. Ablative therapy in BE patients has been applied with some promising results. The clinical significance of reduction in the amount of Barrett's mucosa, elimination of dysplasia, and development of subsquamous areas of Barrett's mucosa needs to be defined. Endoscopic therapy of early cancers seems to be achievable, but large prospective studies are needed to define the best methods and to determine the long-term outcome.





![Figure 8 : Specimen retrieval post|[ndash]|endoscopic mucosal resection using a net basket. Unfortunately we are unable to provide accessible alternative text for this. If you require assistance to access this image, or to obtain a text description, please contact npg@nature.com](/gimo/contents/pt1/thumbs/gimo45-f8.jpg)