Key Points
- In patients with swallowing problems, a relatively broad approach to the differential diagnosis is required, incorporating a complete review of systems.
- Connective tissue disorders commonly manifest with dysphagia. The characterization of esophageal function may help with diagnosis or classification of these disorders.
- Esophageal motility disorders associated with connective tissue disease are usually hypocontractile disorders.
- Abnormal upper intestinal motility is common in diabetics. The association of these findings with symptoms is not straightforward. Gastroesophageal reflux disease is probably more common in diabetics for many reasons.
- Chagas' disease is rare in the United States. Physicians should be aware of its cardinal findings: cardiomyopathy, megacolon, and achalasia-like esophageal motility disorder.
- The diagnosis of paraneoplastic esophageal motility disorders requires a heightened suspicion in current or former smokers with severe dysphagia or intestinal motility of relatively short duration. Specific tests focusing on serum antibodies and chest imaging are usually required for diagnosis.
- Esophageal inflammation resulting from systemic disease is usually the result of a few specific disorders: sarcoidosis, amyloidosis, and other rare skin disorders.
Introduction
It has long been known that many systemic conditions are associated with, and potentially cause, disorders of swallowing. Indeed, the medical literature contains many case reports and small series purporting an association between multiple systemic diseases and dysphagia. There are very few controlled or prospective studies that assess the association or cause of dysphagia in the setting of systemic conditions. Clinicians are often confronted with oropharyngeal and esophageal motility disorders or dysphagia of unknown cause, underlining the importance of this subject. Awareness of these conditions helps in diagnosis and potentially aids in the treatment of the underlying disease
This review summarizes the literature on those conditions in which a reasonable body of literature exists to support an association between oropharyngeal or esophageal motility disorders and a given systemic condition. The review is divided into sections based on broad categories of systemic disease (Table 1). In the discussion of each condition we address the definition, epidemiology, pathophysiology, clinical features, diagnosis, treatment, and outcome. In many instances there are large gaps in medical understanding, so some of these features are unknown for some conditions.
Connective Tissue Diseases
Systemic Sclerosis
Definition
Scleroderma refers to a group of connective tissue disorders with the common manifestation of thickened, sclerotic skin. The conditions of localized scleroderma and systemic sclerosis (SSc) are both subsets of scleroderma. Systemic sclerosis is characterized by progressive, microvascular injury and fibrosis in multiple, affected organs. The skin, blood vessels, lungs, kidney, digestive tract, and heart are most commonly affected. Localized scleroderma only affects the skin and, depending on the classification, affects the skin on different parts of the body.
The classification SSc is further divided into limited and diffuse forms. The diffuse form is characterized by more extensive skin involvement extending to the upper arm and trunk; the limited form affects only the lower arm and face. The limited form includes the CREST variant, an acronym for calcinosis, Raynaud phenomena, esophageal dysmotility, sclerodactyly, and telangiectasias.
Systemic sclerosis can occur without the typical skin involvement and is called systemic sclerosis sine scleroderma. There are several overlap syndromes that involve SSc and other connective tissue disorders such as systemic lupus erythematosus (SLE), rheumatoid arthritis, polymyositis, and dermatomyositis.
Epidemiology and Natural History
Systemic sclerosis affects every race in every country. It is rare, with an incidence between 8 and 19 cases per million per year.1, 2 The peak incidence occurs in the fourth through the sixth decade of life, affecting females four times greater than males. The prevalence is reported to be about 150 per million, with greater prevalence in nonwhite ethnic groups. The Choctaw Indians in Oklahoma have an extraordinarily high prevalence, which is evidence of a genetic link.
Systemic sclerosis occurs more commonly in those with a family history of autoimmune disease; however, there is not a strong association with possession of a human leukocyte antigen (HLA) haplotype and developing scleroderma. There is supposedly a high risk for disease if a first-degree relative is affected. Silica exposure in the mining industry is a well-known risk for scleroderma. Occupational or environmental risks associated with scleroderma-like fibrosis have been identified and include vinyl chloride, bleomycin, pentazocine, epoxy, and aromatic hydrocarbons.
Pathophysiology
The major pathophysiologic disturbance in scleroderma is the overproduction and deposition of cellular matrix proteins such as collagen, fibronectin, tenascin, fibrillin-1, and glycosaminoglycans, into affected organs, predominantly the skin. The same process likely affects the esophagus in SSc and overlap syndromes.
Ultrastructural studies of the human esophagus have provided insights into esophageal disease in SSc. Treacy et al.,3 working with Charlie Code in 1963, were the first to correlate the extent of loss of peristaltic activity with the degree of fibrosis. There is a marked thickening of the basement membrane of capillaries and fibrosis with increased fibroblasts in the smooth muscle layer of the esophagus. Interestingly, multiple studies have shown no involvement of the nerves in the myenteric plexus.4, 5, 6
Physiologic study of the LES revealed decreased LES tone in those with abnormal peristalsis compared to normal subjects after the administration of edrophonium and gastrin I. However, direct, muscular stimulation of the LES elicited a similar response to controls using methacholine. This has been interpreted to suggest the role of neurogenic cholinergic dysfunction of the lower esophageal sphincter (LES) in SSc.7
Electromyography (EMG) combined with esophageal manometry was used to investigate SSc in subjects with recent or remote onset of dysphagia. In those with recent onset dysphagia, EMG revealed disorganized, simultaneous, and high-amplitude electrical signaling similar to that seen in diffuse esophageal spasm. In subjects with a long history of dysphagia, there were diminished spike potentials and decreased amplitude, some without any activity by EMG and aperistalsis. The authors hypothesized that ischemic changes occur early on, selectively affecting the intrinsic inhibitory neurons, and that after fibrosis occurs the activity vanishes all together.8
Autonomic dysfunction has been documented in up to 82% of those with SSc.9 This was also true in a subset with esophageal symptoms. There seems to be correlation between the severity of esophageal dysfunction and autonomic dysfunction, and Scl-70 titers.10 In one study on patients with esophageal and autonomic abnormalities, the investigators demonstrated improvement in esophageal function after the administration of clonidine.11
Gastroesophageal Reflux Disease in Systemic Sclerosis
Studies on the pathogenesis of gastroesophageal reflux disease (GERD) in SSc suggest that decreased esophageal clearance is a central pathophysiologic factor its development.12 One study demonstrated higher degrees of acid in the esophagus, both distal and proximal, in those with aperistalsis compared to those with intact peristalsis. Lower esophageal sphincter pressure was not significantly different between the two groups.13
Delayed gastric emptying can be a prominent feature in many of those with SSc.14, 15 However, to date it has been difficult to implicate delayed gastric emptying as a cause of GERD or reflux symptoms. One investigator demonstrated a correlation between delayed gastric emptying and symptoms of reflux and dysphagia.15 Others have demonstrated some improvement in the upper gastrointestinal (GI) tract, but not specifically reflux symptoms with improved gastric emptying. No association has been determined between delayed gastric emptying and esophageal clearance.16
Clinical Features
There is not good correlation between esophageal symptoms and objective evidence of esophageal dysfunction in patients with scleroderma. As many as 40% are asymptomatic despite demonstrable esophageal dysfunction.17 Symptom onset is usually gradual, although very few studies have examined esophageal symptomatology in depth in the setting of SSc. Heartburn, dysphagia, regurgitation, and postprandial bloating are the most common symptoms to be related to the esophagus.18 Because objective evidence of esophageal disease can be found in asymptomatic subjects, some authors have recommended early use of diagnostic tests in patients suspected of having SSc.19
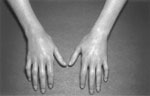
Calcinosis, Raynaud phenomena, telangiectasias, and sclerodactyly may be apparent on physical exam. Occasionally patients may have acral necrosis. Mouse-like facies and thickened sclerotic skin, especially over the dorsum of the hands, are other clues to the diagnosis (Figure 1).
Figure 1: Sclerodactyly in a patient with systemic sclerosis.
(Source: Wise JL, Murray JA. Esophageal manifestations of dermatologic disease. Curr Gastroenterol Rep 2000;4:205?212., with permission from Current Science.)
Diagnosis
Antinuclear antibodies should be obtained with emphasis on Scl-70 and anti-centromere antibodies. Some studies have evaluated whether or not certain subsets of disease are clearly different in terms of the effect on esophageal function. In general diffuse disease is often more severe, with greater degrees of visceral involvement than that of limited disease. Neither esophageal manometry nor the antibody testing can reliably determine which variant of scleroderma a patient has, as this requires incorporating the entire clinical picture. Nail bed capillaroscopy can be helpful in detecting the early changes of scleroderma.
Radiographic studies have shown absence or diminished primary peristalsis, dilation of the esophagus, esophageal strictures, gastroesophageal reflux, and hiatal hernia. Barium x-rays are far better tolerated than esophageal manometry, and therefore are a reasonable way to assess for disease progression once the diagnosis has been established by esophageal manometry.20
Findings at endoscopy include reflux esophagitis and peptic strictures. Symptomatic SSc is defined as heartburn, dysphagia, or dyspeptic symptoms; 60% of patients with SSc were found to have esophagitis and 25% had a peptic stricture.21
Esophageal manometry is considered the gold standard test for the diagnosis of scleroderma esophagus. The classic manometric pattern is that of severe disease where there is complete lack of peristalsis and very low to absent LES pressure (Figure 2). Esophageal manometry is variable in SSc depending on a predilection for esophageal disease in the individual and disease severity. A spectrum of abnormalities have been reported in the esophageal body ranging from low-normal to absent amplitude, and from ineffective peristalsis to aperistalsis.21, 22 Lower esophageal sphincter pressure ranges from low-normal to absent. Typically the proximal esophagus and pharynx are spared. Some studies have shown that esophageal manometry is a more sensitive test than barium x-ray for diagnosing SSc.23, 24
Clinical Course and Complications
The esophagus is affected in up to 90% of those with scleroderma who have had esophageal evaluations at some point in their disease process. Heartburn and dysphagia are the most common symptoms. In most series patients have strictures and hiatal hernias. Barrett's esophagus and adenocarcinoma have been reported to occur.25
Regarding disease progression, one study of repeat esophageal manometry done on 124 patients with SSc reported deterioration of function in close to half of the patients. None had improvement. However, the time interval of the reinvestigation was not accounted for in that study.26 Another study done with a median reinvestigation time of 40 months showed similar results.27
Treatment
There is no proven successful treatment for SSc, although many antiinflammatory and immunosuppressive agents have been utilized. Furthermore, the goal of treatment for foregut symptoms and signs is to ameliorate symptoms.
Gastroesophageal reflux disease should be treated according to its severity. Several studies have documented the effectiveness of the histamine-2 receptor antagonist cimetidine.26, 28 Only a few studies have addressed the use of a protein pump inhibitor (PPI) in SSc.29, 30 Generally, patients with symptoms related to esophagitis or strictures have severe disease requiring a high-dose PPI.30
The underlying esophageal motility disturbance is not helped by cisapride or metoclopramide.16, 31, 32, 33, 34 Both of these pharmacologically distinct drugs have a minimal to modest effect in increasing LES pressure while only minimally increasing the tone of the esophageal body in SSc. Management of esophageal strictures with periodic dilation is an important component of the management of esophageal disease in SSc.
Fundoplication for GERD has been met with limited success and much less enthusiasm. Even in carefully considered cases LES pressure is not significantly augmented, nor is esophagitis completely controlled. Many physicians continue to have difficulty managing peptic strictures.35, 36 Adequate studies that evaluate the risk of fundoplication in SSc are lacking. Most physicians are reluctant to recommend fundoplication owing to concern about worsening dysphagia resulting from the surgically augmented high-pressure zone in an otherwise poorly contracting esophagus.
Raynaud Disease and Phenomena
Definition
Raynaud disease (RD) is the term used to describe blanching, cyanosis, and rubor of the fingers and toes after exposure to cold temperatures. The term Raynaud phenomenon(RP) is used as opposed to Raynaud disease when a secondary disorder (a bona fide connective tissue disorder) is determined to be the cause.
Pathophysiology
Reflex sympathetic vasoconstriction has been forwarded as the theory behind RP.  -Adrenergic agents can lessen the frequency and severity of attacks, lending some credence to this theory. The vascular disease seen in SSc and Raynaud's share similar theories for their pathogenesis.37
-Adrenergic agents can lessen the frequency and severity of attacks, lending some credence to this theory. The vascular disease seen in SSc and Raynaud's share similar theories for their pathogenesis.37
Clinical Manifestations
Raynaud disease has been reported to be associated with esophageal motility abnormalities.7, 38, 39 Other studies have shown no difference between those with RD and normal controls by scintigraphy33 or by esophageal body function and LES dynamics.23 Because RP is the sole presenting symptom in about one third of those with SSc, it isn't clear whether this is an important factor for determining the presence of esophageal function or a co-phenomenon as seen in SSc. Raynaud phenomenon is associated with the presence of esophageal dysfunction in the setting of connective tissue disease such as CREST or SSc.40
Treatment
In one study intraarterial reserpine improved esophageal function in those with RP.41 In another study peripheral exposure to cold did not induce esophageal motility disturbances.42 To our knowledge local temperature decreases within the esophagus have not been tested in those with RP.
Outcome
In a systematic study of 60 people with RD, signs of autoimmune rheumatic disease were detected in up to 50% of those in this group at 8.4 years.43 Of all the tests employed the best predictor of development of a codified connective tissue disease was nail fold capillaroscopy (odds ratio 21.1).43
Mixed Connective Tissue Disease
Definition
Mixed connective tissue disease (MCTD) is a chronic connective tissue disease considered a specific diagnostic entity owing to its association with high circulation titers of anti-U1RNP.44 This disorder is characterized by an overlap of features with SSc, SLE, polymyositis, and rheumatoid arthritis, but without meeting specific criteria for any of them.
Clinical Features
Patients may present with RP, swollen hands, arthralgias, myalgias, and fatigue. Esophageal symptoms are similar to that of SSc (see Systemic Sclerosis, above). Very few publications have addressed MCTD alone.45 A few have compared MCTD to SSc or SLE.46, 47 Heartburn and dysphagia are common. Cases of UES involvement have been reported.45
Diagnosis
Esophageal testing often shows very similar findings to SSc with decreased amplitudes of the resting LES and smooth muscle segment during contraction. The esophageal dysfunction that might occur in MCTD are indistinguishable from SSc.48, 49
Treatment
Unlike SSc some features of MCTD may respond to prednisone. Marshall et al.45 treated a series of patients with varying doses of prednisone in open-label fashion and noted statistically significant improvement in LES pressure; however, no symptom assessment was included in this study. A patient in this same series with aspiration and UES dysfunction was reported to respond rather dramatically.
Sjögren Syndrome
Definition
Sjögren syndrome (SS) is a slowly progressive autoimmune disorder characterized by lymphocytic infiltration of the exocrine glands, resulting in salivary and lacrimal gland destruction, causing xerostomia and keratoconjunctivitis sicca.50
Epidemiology
The disease predominantly affects middle-aged women (between the fourth and sixth decade of life) and has a prevalence around 1%. The incidence and prevalence of esophageal involvement isn't known. However, in published reports the proportion of SS patients with dysphagia ranges from 32% to 92%.51, 52, 53, 54, 55
Pathology and Pathophysiology
Lacrimal and salivary glands are infiltrated by active T and B lymphocytes. There is associated atrophy of the acinus and hypertrophy of ductal epithelial cells.50 Circulating autoantibodies directed against various organ antigens such as immunoglobulins are a salient feature. Autoantibodies to Ro/SS-A and La/SS-B are commonly found at diagnosis.
The pathologic mechanisms responsible for the solid-food dysphagia in SS are not understood. Most patients do not have esophageal motility abnormalities; however, in some cases nonspecific esophageal motility abnormalities have been documented.51, 52, 56In two studies of SS patients with dysphagia, there was no explanation for the dysphagia.54, 57This has led to the assumption that dysphagia is the result of the lack of adequate salivary flow. However, surrogates of decreased salivary function have not correlated with dysphagia or esophageal motility either.53, 58Direct measurement of salivary production has not been studied in relation to dysphagia.
Most patients with SS do not have heartburn. There is only one original publication to our knowledge to date that explores the role of GERD in SS patients.59This showed no significant GERD in SS patients with esophageal symptoms. Various studies report LES pressures higher, lower, or no different from normal.56, 58, 59No data exist on outcomes from acid-suppressive management of these patients.
Clinical Features
Xerostomia and solid food dysphagia are the symptoms of interest in relation to the esophagus. Almost all SS patients have a dry mouth, and approximately three quarters in large series of SS report dysphagia. Occasionally, patients with SS present to the gastroenterologist with the burning mouth or burning throat syndrome. A high suspicion of SS is needed to make a diagnosis for these patients. Patients may develop significant renal, pulmonary, or neurologic disease from SS.
Diagnosis
Endoscopy has been recommended as the first test carried out for the evaluation of solid food dysphagia.60 Barium studies or esophageal manometry are often employed when endoscopy is negative. Usually no cause for dysphagia is found, although there have been reports of proximal esophageal webs in SS.57
Treatment
There is no treatment that is effective in altering the underlying disease process in SS. Treatment is nonspecific. The use of sialogogues has not been studied in dysphagia in SS, but they are commonly prescribed. Ample fluids immediately preceding and during swallowing and treatment of underlying reflux disease, if present, would be warranted. Pills should be crushed and liquid medicines administered where possible.
Systemic Lupus Erythematosus
Definition
Lupus is another idiopathic connective tissue disorder with a clinically recognized pattern of multisystem disease. The skin, joints, blood, lungs, and kidneys are usually affected most commonly. Criteria for diagnosing SLE have been reviewed elsewhere.61
Pathophysiology
The major feature of the pathophysiology of lupus is the deposition of immune complexes in various affected organs resulting from pathogenic production of autoantibodies.164 The mechanisms responsible for esophageal motility derangements in SLE have not been studied.
Clinical Features
Lupus commonly affects the GI tract but is often overshadowed by other organ system involvement.65 Dysphagia occurs in 1.5% to 13% of lupus patients and heartburn in up to 50%.63, 64
Diagnosis
Assessments of patients in whom esophageal symptoms have been a major feature demonstrate similar findings to those of SSc and MCTD (see earlier sections). Hypotensive LES and hypoperistalsis or aperistalsis have been documented. The degree of manometric abnormality appears to be less than that seen in MCTD47 or SSc.65 As with SSc correlation between esophageal function by radiologic and manometric study is poor.65, 66 Sjögren syndrome is associated with SLE as a secondary phenomenon and also could explain dysphagia in these patients.
Swallowing difficulties are rarely the result of severe esophageal mucosal disease in association with epidermolysis bullosa acquisita, which has been associated with SLE and other disorders such as multiple myeloma.67 Very few studies have focused on oropharyngeal or esophageal motility.47
Treatment
Immunomodulating agents have been used successfully to treat affected organ systems in SLE. The possibility exists that dysphagia might also improve. There are no data to our knowledge that examine the response of the swallowing unit to treatment for SLE. Potent immunosuppressive regimens can induce dysphagia by causing opportunistic esophagitis.
Rheumatoid Arthritis
Definition
Rheumatoid arthritis (RA) is a chronic multisystem disorder of unknown etiology with a immune basis that manifests most commonly as a destructive symmetric polyarthritis.68
Pathophysiology
The most prominent theory is that RA is an immunologically mediated event. Infiltration of inflammatory myeloid and mononuclear cells into the synovium is a hallmark of RA. Plasma cells within the synovium are responsible for the production of rheumatoid factor. Esophageal involvement is theorized to occur as a result of vasculitis or amyloidosis (see Systemic Amyloidosis, below).
Clinical Features
Rheumatoid arthritis patients may develop oral and pharyngeal motility disorders. Dysphagia related to cricoarytenoid joint dysfunction has been reported.69 There has been documented pharyngeal segment immobility and dysfunction owing to inflammation and destruction of the cervical spine and mandible as well.70 Xerostomia may also contribute to dysphagia in these patients.71
Secondary amyloidosis can cause various oral, pharyngeal, and esophageal motility disorders. Dysphagia in RA is most commonly related to pill-induced mucosal disease from nonsteroidal antiinflammatory drugs (NSAIDs) or bisphosphonates used to retard bone loss secondary to corticosteroid use.
Diagnosis
Because there are various possibilities for what might cause swallowing difficulties in RA, evaluation should include tests that assess oral, pharyngeal, and esophageal motor function. Pill-induced mucosal injury should be excluded prior to diagnosing a secondary motility disorder attributed to RA.
Treatment
No studies have been done, to our knowledge, addressing the effectiveness of disease-modifying treatment on oral, pharyngeal, or esophageal dysfunction. The treatment of the underlying RA may have benefit for the dysphagia; however, complications of that therapy can induce esophagitis especially when there are potent immunosuppressive agents.
Endocrine Diseases
Diabetes Mellitus
Definition
Diabetes mellitus (DM) is a very common multisystem endocrine disorder caused by either a lack of circulating pancreatic insulin or a relative insensitivity to its effects on various body tissues. The resultant effect is hyperglycemia. Diabetes mellitus is associated with complications but less so if blood sugars can be tightly controlled.72 The GI tract is commonly affected in DM.
Pathophysiology
The main theory of how the esophagus is affected in diabetes in that of a segmental, parasympathetic demyelination and progressive axonal atrophy within the esophagus. The myenteric plexus is appears preserved.73
Evidence of sensory dysfunction of the esophagus in diabetics comes from two studies on cortical evoked potentials. In these studies diabetics with autonomic neuropathy had aberrant or absent cortical evoked potentials compared to normal subjects.74, 75
Predictable alterations in esophageal function were observed when blood glucose levels were increased by systemic infusion.76, 77 Peristaltic velocity was increased during "physiologic" hyperglycemia (8 mmol/L)77 and decreased when even higher pathologic blood glucose levels were achieved (15 mmol/L).76 Lower esophageal sphincter pressure decreased at the 15-mmol concentration and was significantly lower even after edrophonium administration (a direct cholinergic that predictably augments LES pressure) compared to euglycemia.76
Acute hyperglycemia does not seem to affect proximal esophageal or UES function. The acute effect of hyperglycemia on the esophagus of the diabetic has not been formally evaluated, to our knowledge. Acute physiologic hyperglycemia lowers sensory thresholds in the esophagus of normal subjects.77 Interestingly, evoked cortical potentials are more intense during supraphysiologic hyperglycemia in response to balloon distention in normal subjects.81 In a study on visceral afferent neuropathy in insulin-dependent diabetics, increased sensory thresholds, not decreased, and decreased cortical potentials were found, suggesting afferent nerve damage.74
Esophageal function has been evaluated79, 80, 81, 82, 83in selected populations of diabetics, both type 1 and 2. Most of these studies have demonstrated nonspecific abnormalities of peristalsis including spontaneous contractions, reduced amplitude contractions, and decreased LES resting pressure. Multiple-peaked contractions can also be seen.82
The presence and influence of autonomic neuropathy has been evaluated in part in several studies.80, 81, 82, 84 Interestingly, although there may be a decreased resting LES pressure in diabetics in general, those with neuropathy might have higher resting pressures that those without.81 Significantly decreased contraction amplitudes in the distal esophagus has been found by some authors79, 81 but not others.82, 84These manometric changes are more severe in those with autonomic neuropathy.
Gastroesophageal reflux has not been studied extensively in DM.85, 86, 87In the largest study,87 50 unselected insulin-dependent diabetics, the diabetics had a high frequency of pathologic reflux despite having no symptoms. In one study esophageal function testing and pH were performed on those with diabetes with and without neuropathy. Pathologic acid reflux was found in over half of the subjects without an association with neuropathy.85 In another study in diabetics with heartburn, compared to those without diabetes, diabetics did not have pathologic acid reflux to explain their heartburn.86
Clinical Features
The bulk of the population-based data would support that notion that GI symptoms including esophageal symptoms are more prevalent among both type 1 and type 2 diabetics.88, 89, 90, 91, 92However, other smaller studies have found no difference.93 Nonetheless, it is not widely recognized or accepted that DM is a specific cause of esophageal symptoms.
In the largest population-based study, conducted in Australia, dysphagia and heartburn were significantly increased among diabetics and seem to be correlated to self-reported control of glucose.89 The esophageal symptoms in diabetics do have a meaningful impact on health-related quality of life.94
Interestingly, many diabetics are asymptomatic with respect to esophageal symptoms but have abnormal results when esophageal function is tested. On the other hand, there is a poor correlation between the presence of esophageal function abnormalities in subjects with esophageal symptoms.95
Diagnosis
It isn't known how many diabetics present to the physician for their esophageal symptoms. Diagnosis of esophageal motility disorders in DM has been reported predominantly using manometry79, 80, 81, 82, 83or scintigraphy.96, 97Esophageal motility abnormalities are common in diabetics who are studied (Figure 3). Nonspecific motility abnormalities are often no more than a curiosity to the clinician. There are no treatments for most of these phenomena. Esophageal transit has been studied and found to be delayed in up to 63% of diabetics. Some assessment of glucose control should be taken into account, as poor control might predict altered motility in diabetics.
Treatment
Tighter glucose control could potentially improve symptoms in diabetics. However, no studies have looked at acute fluctuations in blood sugars in diabetics. Prevention of autonomic neuropathy by tight glucose control may prevent GI tract disease.98 Erythromycin has increased mean transit time and gastroesophageal transit half-time in non–insulin-dependent diabetics.99
There is a paucity of data that demonstrate a difference in efficacy of treatments for GERD among diabetics. However, one study has suggested diabetics more often have functional heartburn than those without diabetes.86
Outcome
With more advances in treatments and prevention of all types of DM, there will likely be fewer upper digestive tract motility disorders diagnosed and treated. Outcome data for these disorders is lacking, likely because it is so difficult to define when this common disorder is specifically to blame for underlying motility disorders of the mouth, throat, or esophagus.
Thyrotoxicosis
Definition
Thyrotoxicosis is defined as a state of excess of circulating of thyroid hormone. The most common causes of hyperthyroidism are Graves' disease, toxic multinodular goiter, toxic adenoma, medications, and various types of thyroiditis.100
Pathophysiology
Thyrotoxicosis causes a variety of interesting metabolic syndromes that rarely affect the function of the swallowing unit. These include a skeletal myopathy that affects the oropharyngeal phase of swallowing.101The literature on the association among oral, pharyngeal, and esophageal motility disorders with thyrotoxicosis is limited to case reports.
Pathologic mechanisms for dysphagia associated with thyrotoxicosis include neuromuscular disruption and mechanical compression from a large toxic goiter. Bulbar dysfunction has been associated with isolated thyrotoxicosis, either myopathy or neuropathy or in association with periodic paralysis or myasthenia gravis.102, 103, 104, 105, 106, 107Skeletal muscle is usually weakened, and therefore the ability to propel bolus or coordinate its movement through the oropharynx is impaired. Very few reports have described abnormalities of esophageal function.107
Clinical Features
Although thyrotoxicosis can cause swallowing difficulties, primarily oropharyngeal dysphagia, the systemic features of the excessive thyroid function are much more evident. Typical signs of oropharyngeal dysphagia may be present such as nasal regurgitation, oral escape, and premature spillage of bolus into the vallecula. Aspiration has been documented in those with a bulbar palsy–like picture.103
Diagnosis
Video swallow studies can demonstrate oropharyngeal weakness and its untoward consequences such as aspiration.104, 108 Pharyngeal strength and coordination as well as UES pressure can be assessed with esophageal manometry. Esophageal body clearance can be delayed in hyperthyroidism, but the association with symptoms is unclear.109
Treatment
The dysphagia usually improves with treatment of the underlying thyrotoxicosis or the associated diseases such as myasthenia gravis.101, 103, 104, 106, 107
Hypothyroidism
Definition
Hypothyroidism is a disorder characterized by decreased thyroid gland function and usually results in a decreased quantity of circulating thyroid hormone. The most common causes of hypothyroidism include autoimmune thyroiditis, previous thyroid ablation or surgery, medications, iodine excess or deficiency, and rarely infiltrative disorders or pituitary insufficiency.100
Pathogenesis
The intestine has been reported to be affected commonly in hypothyroidism. However, very few studies or reports specifically address oral, pharyngeal, or esophageal manifestations of hypothyroidism. Ultrastructural studies of the small bowel have demonstrated infiltration of the smooth muscle and myenteric plexus by mucinous proteins.110, 111
Clinical Manifestations
Oropharyngeal and esophageal dysfunction have only been rarely reported in hypothyroidism.112, 113, 114 Oropharyngeal dysphagia as a part of myxedema coma responding to treatment has been described.113 Infiltration of proteins into the larynx has been reported as well.115 A general decrease in lower esophageal body amplitude and LES pressure that responded to thyroid hormone treatment has also been reported.114
Treatment
In the few cases reported, treatment of hypothyroidism resulted in improvement in underlying motility disturbance involving the oropharynx and esophagus.112, 114, 115
Allgrove's Syndrome
Definition
Allgrove syndrome was first described by J. Allgrove in 1978.116, 117, 118, 119The syndrome consists of achalasia, adrenal insufficiency, and alacrima. It is an autosomal recessive disorder associated with AAAS gene coding for the ALADIN protein.120 Other variants of the syndrome have been reported.
Pathophysiology
Achalasia is caused by a thickening of the intramuscular plane, loss of myenteric ganglia, and decreased nitric oxide signaling. These patients have been reported to have autonomic dysfunction121 as well as neurodegenerative processes that can affect brainstem nerves.
Clinical Presentation
Allgrove syndrome has been described primarily in children; however, cases commonly go undetected until adulthood. Achalasia is one of the hallmark features of the syndrome. The esophageal abnormalities in the Allgrove syndrome are similar to those with idiopathic achalasia. In a patient with a combination of alacrima and adrenal insufficiency this syndrome should be suspected. Allgrove syndrome may present similarly to amyotrophic lateral sclerosis.122 Various neurologic syndromes have been reported in association with the syndrome.
Diagnosis
The syndrome is diagnosed by putting together the cardinal clinical features remembered by the syndrome of the three A's: alacrima, achalasia, and adrenal insufficiency. Interestingly the adrenal insufficiency is more readily detected by insulin-induced hypoglycemia rather that adrenocorticotropin hormone stimulation.123
Treatment
There is no literature on treatment of achalasia related to Allgrove syndrome. Standard treatments for achalasia should be effective.
Infectious Diseases
American Trypanosomiasis (Chagas' disease)
Definition
American trypanosomiasis (AT) was first described by the Brazilian Carlos Chagas. All of the manifestations of AT are thought to be related to the effects of infection by the parasite Trypanosoma cruzi. This parasite is trophic for smooth muscle and infects visceral organs rich in smooth muscle such as the heart and GI tract.
The acute and chronic phases of infection have been characterized and have very different clinical presentations and implication for treatment. Only the chronic phase of the illness, in which esophageal disease manifests, is discussed in this chapter.
Epidemiology and Natural History
The affect of the AT on the esophagus is the most common cause of symptoms in chronic AT. Usually patients develop symptoms between 20 and 40 years of age. The overall incidence and prevalence for esophageal disease is unknown. It is thought to take years to develop symptoms and as such rarely presents in infants or young children. For unknown reasons, two thirds of those with esophageal disease are men.
Pathophysiology
The esophagus is commonly affected in the course of AT. A denervation injury occurs in the myenteric plexus and can occur throughout the hollow viscera. Autopsy studies show a striking loss of myenteric neurons, similar to idiopathic achalasia. There are high levels of circulating anti acetylcholine receptor antibodies (M2).124 Tissue parasite load correlates with severity of disease.125, 126, 127Thickening of the wall of the esophagus on pathology has been observed.128
Clinical Features
Chronic AT manifests differently depending on geographic location. Most of the cases in Central America and northern South America are characterized by cardiac involvement: cardiomegaly, conduction system disturbances, and congestive heart failure. This is in contradistinction to those cases in central South America where esophageal symptoms are most common.
Chronic AT of the esophagus manifests identically to idiopathic achalasia. The onset of dysphagia, the cardinal symptom, is slow and progressive. Patients with mega-esophagus are uniformly symptomatic and have aperistalsis by manometry.129 Investigation of those with positive AT serology without mega-esophagus demonstrated abnormal manometric evaluation in about half of the subjects.130
Diagnosis
Serology is the most common way of confirming parasite infection in the past. Because AT is rarely diagnosed in the acute setting, serology is relatively specific for chronic infection.
Treatment
Esophageal treatment is similar to that of idiopathic achalasia. Heller myotomy and balloon dilation are utilized commonly. Unlike idiopathic achalasia, long-acting nitrates may have some efficacy.163 Treatment may need repeating or alternate treatment approaches attempted.
Outcome
Outcomes to treatment of the esophagus in Chagas' disease have not been well characterized. As in idiopathic achalasia, there is, as of yet, no cure for the underlying myenteric neuropathy. However, ideally with proper public health initiatives AT can be prevented.
Neoplastic Diseases
Paraneoplastic Esophageal Motility Disorders
Definition
Paraneoplastic esophageal motility disorders are rare. The diagnosis is based on the association of severe esophageal motility disorders with malignancy. The finding of a paraneoplastic antibody in the serum or a malignant neoplasm remote from the upper GI tract in the setting of a severe esophageal motility disorder is usually diagnostic.
Pathophysiology
The pathophysiology of paraneoplastic GI motility disorders appears to involve an autoimmune type mechanism.131 Pathology has revealed that lymphocytic inflammation in the myenteric plexus eventually results in the loss of the interstitial cells of Cajal.132
Unlike those tumors that directly invade the esophagus or proximal stomach, e.g., adenocarcinoma of the stomach, these tumors are remote from the esophagus and include small cell lung cancer (SCLC), malignant mesothelioma, bronchial carcinoids, and renal cell carcinoma.133, 134, 135, 136
Clinical Features
Paraneoplastic esophageal motility disorders may occur in isolation but may more commonly present as just one part of a pan-gut dysmotility syndrome.137 Usually patients have an underlying SCLC and other neurologic findings, such as a peripheral motor or sensory neuropathy.138
Symptoms include dysphagia, regurgitation, spasm-like chest pain, heartburn, nausea, early satiety, vomiting, constipation, and anorexia are common.131Neurologic symptoms are usually present such as neuropathy (sensory, autonomic, or motor), cerebellar syndromes, limbic encephalitis, and cranial neuropathies.138
Diagnosis
A high index of suspicion is required at times to make the diagnosis. Current or previous smoking is a risk factor for SCLC, the most commonly associated malignancy. Other neurologic symptoms or signs might be a clue. Paraneoplastic antibody panels are not widely available; however, they are a crucial aid in the diagnosis. This is because tumors responsible for these syndromes can be extremely small, below the detection range of conventional imaging at the onset of symptoms.139, 140Antineuronal nuclear antibodies 1 and 2 (ANNA-1 and -2) have been reported in association with the presence of SCLC and various neurologic syndromes.
Esophageal manometry is usually markedly abnormal, showing absence or near absence of peristalsis. In addition, barium esophagram may show an achalasia-like picture or esophageal spasm (Figure 4).
Because these conditions are so rare no data exist on diagnosis. High-resolution computed tomography (CT) of the chest may reveal an SCLC or malignant mesothelioma. Bronchoscopy may be positive when CT is negative. Urinary 5-hydroxyindoleacetic acid (5-HIAA) may help diagnose a carcinoid but usually isn't helpful unless there are serotonergic symptoms. Positron emission tomography scan might be a useful way of determining if a lesion is present anywhere in the body, but its use for this purpose has never been studied to our knowledge.
In some cases there is no underlying cancer discovered despite a neurologic syndrome and a circulating antineuronal antibody. In these cases esophageal symptoms may progress with or without treatment, and eventually treatments for achalasia may be helpful.
Treatment
Treatment of the underlying malignancy may be curative if this is possible. Otherwise, immunosuppression with high-dose or pulse methylprednisolone, intravenous gamma globulin, or plasma exchange has been tried in some cases where the original malignancy cannot be found. Symptomatic treatment is dependent on the precise pattern of abnormalities seen.
Outcome
In the largest outcome study to date,138 206 patients with paraneoplastic neurologic syndromes were followed. All had positive ANNA-1 status. Eighty-eight percent of these patients were eventually determined to have SCLC. The outcome will mirror those of SCLC, as this is, by far, the most commonly associated paraneoplastic malignancy. Very little is known about the outcome of treatment for esophageal motility disorder with immunosuppressive drugs. Standard treatments for achalasia and diffuse esophageal spasm such as botulinum toxin injection and laparoscopic Heller myotomy are effective.
Inflammatory Diseases
Systemic Amyloidosis
Definition
Amyloidosis is a condition caused by an abnormal folding of proteins resulting in decreased solubility and deposition in tissues anywhere in the body.141 Amyloidosis can be localized, but our treatment here refers to the systemic varieties. Amyloid disorders are commonly classified as primary, AL, usually found in association with multiple myeloma, and secondary, AA, associated with a chronic inflammatory disorder such as RA or familial Mediterranean fever.
Epidemiology
Amyloidosis is rare, and very little data exist on the epidemiology of these disorders. In large series secondary amyloidosis seemed to be more common; however, these studies were conducted before the era of disease-modifying therapy for the conditions that commonly cause secondary amyloidosis.
Pathophysiology
Amyloid infiltration occurs as a result of abnormal circulating proteins being deposited, infiltrating and damaging tissues. Recent evidence also suggests tissue damage occurs by other mechanisms besides tissue infiltration.142
Amyloidosis, when it rarely occurs, is thought to commonly affect the GI tract. Complete pathologic evaluation of the GI tract in systemic amyloidosis has revealed involvement in up to 36% of specimens in one study.143 The amyloid proteins have been found in all layers of the esophageal wall.
Esophageal manometry can show varied abnormalities in amyloidosis and include either incompetent or a hypertensive, poorly relaxing LES.144 Nonspecific esophageal body disorders and rarely an achalasia-like picture have been reported.145, 146The oropharyngeal function can be affected by amyloidosis. Most commonly the tongue is infiltrated and thickened. A case of bulbar palsy has also been reported.147
Clinical Features
Amyloidosis rarely causes symptoms consistent with esophageal motility disorders. Typically patients with GI involvement have diarrhea, malabsorption, or intestinal bleeding. We are unaware of any studies that specifically address the link between oropharyngeal dysphagia and amyloid related to cerebrovascular events.
Diagnosis
A high index of suspicion is required to make the diagnosis of amyloid in the GI tract. Amyloidosis may cause esophageal motility abnormalities; however, it is rarely associated with swallowing complaints.
Gastrointestinal tract involvement is best diagnosed by a rectal or upper intestinal biopsy, especially duodenal biopsies taken at endoscopy.148, 149 Other endoscopic findings that might be a clue include friability and scatter ulcerations or erosions in the esophagus, away from the gastroesophageal junction.149
Treatment
Therapy aimed at reducing the production of amyloid, depending underlying cause, is the primary goal of any treatment regimen. Chemotherapy and colchicine have been effective in treating primary and secondary amyloid, respectively.
The achalasia-like picture can be treated with pneumatic dilation of the LES.150 Occasionally total parenteral nutrition is needed in cases of colonic pseudo-obstruction.151
Sarcoidosis
Definition
Sarcoidosis is a chronic, systemic, idiopathic disease characterized by an abnormal accumulation of T lymphocytes and the formation of noncaseating granulomas that infiltrate and damage organs.152 Sarcoidosis can occur in all people; however, it is more common in patients in the fourth to sixth decade from northern European or African ancestry.
Pathophysiology
T lymphocytes, forming noncaseating granulomas, infiltrate almost any tissues but most commonly affect the mediastinum and pulmonary parenchyma. Pathologic studies of the GI tract in sarcoidosis reveal the stomach to be the most commonly affected organ.
Oropharyngeal and esophageal motility are usually disturbed by affecting the bulbar and myenteric nerves, respectively. When the myenteric neurons are affected, an achalasia-like picture can develop. Very little data exist on these motility disorders associated with sarcoidosis. Much of the current knowledge of these problems comes from a few case reports.153, 154, 155, 156, 157, 158, 159
Clinical Features
Dysphagia is common in esophageal sarcoidosis and might be caused by localized infiltration of the mucosal158 or extrinsic compression of the esophagus157 by hilar adenopathy. Any portion of the oral, pharyngeal, or esophageal segment can be involved.165 Several case reports detail an achalasia-like picture that can occur in association with sarcoidosis.153, 159, 161Patients with secondary achalasia from sarcoidosis might have other manifestations of sarcoidosis such as pulmonary or skin involvement.
Diagnosis
Most patients with esophageal sarcoidosis have been reported to have evidence of pulmonary sarcoid. Endoscopy might reveal mucosal ulceration, nodularity, or extrinsic compression of the pharynx or esophagus, and provides the ability to biopsy suspicious lesions. A case of sarcoid esophagus was reported where the endoscopic appearance was that of Barrett's esophagus when histologically this represented infiltration of the mucosa with noncaseating granulomas.162
Pharyngoesophagram can demonstrate both intrinsic and extrinsic disease, not to mention significant disorders of oropharyngeal or esophageal motility. Mediastinoscopy is the diagnostic test of choice in the setting of hilar adenopathy. In the presence of typical skin lesions, skin biopsies are an easy way to diagnose sarcoid. Otherwise, chest radiography might reveal the various stages of pulmonary sarcoidosis.
Treatment
Corticosteroids might resolve a patient's dysphagia with symptomatic sarcoidosis. Cook et al.157 describe resolution of dysphagia associated with distal esophageal obstruction from an enlarged lymph node, found to be involved by noncaseating granulomas, after treatment with steroids. Posttreatment esophagram demonstrated resolution of esophageal obstruction.
Botulinum toxin has been used successfully to treat the achalasia like picture associated with sarcoidosis.159