Key Points
- At rest, closure of the upper esophageal sphincter (UES) is due to passive forces as well as active muscle contraction, and its relaxation requires inhibition of the cricopharyngeus and contraction of suprahyoid muscles.
- UES relaxation and opening are related, but not synonymous events.
- Manometry or electromyography are necessary to detect failed UES relaxation, as the radiographic appearances of a nonrelaxing sphincter are protean and nonspecific.
- Impaired opening of the UES can result from cricopharyngeal fibrosis, disordered neurally-mediated opening, suboptimal pharyngeal driving force, or a combination of these factors.
- The cricopharyngeal bar is a structural sphincter abnormality that is a relatively common incidental finding.
- The radiographic appearance of the cricopharyngeal bar is due to cricopharyngeal fibrosis and not due to spasm nor failed relaxation of the UES.
- Zenker's diverticulum is a consequence of a fibrosis of the cricopharyngeus muscle that results in elevated hypopharyngeal intrabolus pressures during the swallow. Its successful treatment requires disruption of the restricted cricopharyngeus muscle.
- "Incompetence" of the UES may be due to its hypotension or reflex relaxation. It results in increased esophagopharyngeal reflux that is best detected by dual pH monitoring techniques rather than esophageal motility studies.
- Reflexive UES relaxation appears to be a more important determinant of esophagopharyngeal regurgitation than UES hypotonia.
- The majority of esophagopharyngeal regurgitation events occur in the erect posture and in very close association with gastroesophageal reflux events.
Introduction
The upper esophageal sphincter (UES) serves a range of important physiologic functions. Unlike its counterpart in the distal esophagus, we are only beginning to unravel the normal workings of this sphincter and how its function is disturbed by disease. The UES provides a barrier against retrograde flow of digesta, and in so doing, serves an important protective function preventing aspiration of acidic gastric content into the respiratory tract. The sphincter also serves a barrier function by preventing the entry of air into the esophagus. The UES permits, by its physiologic relaxation, both antegrade and retrograde flow of material during swallowing, belching, and vomiting. This review focuses on two contrasting disorders of UES antegrade function. One is a failure of UES relaxation, which is a motor disorder. The other is a structural disorder of cricopharyngeus muscle leading to formation of a posterior (Zenker's) diverticulum. Zenker's is covered in some detail in this review as it is a relatively homogeneous disorder and one in which the biomechanics and structure of the cricopharyngeus have been well studied, thereby making this disorder a good model to illustrate the biomechanical issues. At the same time, it must be emphasized that there are other conditions in which UES opening is reduced in extent, duration, or timing, which is discussed in less depth (see Other Conditions Associated with Disordered Upper Esophageal Sphincter Opening, below). Disordered UES opening can be due to an intrinsic, restrictive sphincter disorder, as seen for example with the cricopharyngeal bar, either alone or in association with a Zenker's diverticulum. Alternatively, diminished UES opening can be a manifestation of disordered pharyngeal propulsion where the intrapharyngeal forces imparted by the advancing swallowed bolus are insufficient to open the sphincter to its fullest extent. In the latter circumstance the UES itself may or may not be primarily dysfunctional. If in isolation, or in addition to pharyngeal weakness, the UES fails to relax, then UES opening will be impaired also. The distinction and the biomechanical differences between disordered opening and failed relaxation are discussed, as are the normal and abnormal regulation of retrograde flow (regurgitation) (see Upper Esophageal Sphincter Incompetence, below).
Upper Esophageal Sphincter "Achalasia"
Definition and Prevalence
The term UES achalasia (literally meaning failure of relaxation of the UES) is widely misused and is probably best avoided. The vast majority of reports in the last 30 years about this entity have not actually measured (either manometrically or electromyographically) the phenomenon of UES relaxation. Rather, the term has been used to describe the radiologic appearance of incomplete UESopening that has been assumed, but not proven, to represent failure of UESrelaxation. This assumption is erroneous, as manometrically confirmed failure of UES relaxation can manifest as a wide range of radiologic appearances, none of which is specific for failed sphincter relaxation. Hence, for the purpose of this review, I have avoided the term UES achalasia, preferring instead the term failed UES relaxation.
There are many instances in the literature in which the term cricopharyngeal or UES achalasia has been used inappropriately1, 2 to describe the radiologic abnormality that is best described morphologically as a cricopharyngeal bar.3 Indeed manometric recordings from the pharyngoesophageal segment in patients with cricopharyngeal bars have shown the sphincter in this radiologic entity to demonstrate normal resting UES pressure and that the UES relaxes fully during swallowing.4 Hence, neither cricopharyngeal spasm nor achalasia underlie the radiographic appearance of the cricopharyngeal bar. Indeed in one recent study of the radiographic features of manometrically confirmed failed UES relaxation, only one of 18 cases had a cricopharyngeal (CP) bar,5 a prevalence comparable to that seen (5–19%) in unselected radiographic series.6, 7 More importantly from the clinical standpoint in a group of patients undergoing videofluoroscopy, dysphagia is no more prevalent in those individuals found to have a CP bar (13%) than it is in those without a CP bar.8 Notwithstanding these data, circumstances exist in which the CP bar does contribute to dysfunction and symptoms in the dysphagic patient (see Other Conditions Associated with Disordered Upper Esophageal Sphincter (UES) Opening). {Ed: Heading title was different}
Failure of relaxation of the UES can only be confirmed by intraluminal manometry or, arguably better, from direct electromyography (EMG) recordings from the muscle segment itself.9, 10, 11 The true prevalence of the disorder is unknown, but it is probably much less common among the dysphagia population than previously thought. A systematic review of nearly 400 consecutive detailed combined manometric and radiographic studies in patients with pharyngeal dysphagia, found manometrically confirmed failure of UES relaxation in 4.8%.5
Etiology of Failed Upper Esophageal Sphincter Relaxation
Upper esophageal sphincter relaxation, indeed the entire repertoire of pharyngeal and cricopharyngeal motor events during deglutition, originates in the medullary swallow center.12 Hence, it is not surprising that the neuroanatomic level of the majority of identifiable disorder(s) underlying failed UES relaxation can be traced to the rostral medulla. Ninety percent of cases can be attributed to either medullary lesions or Parkinson's disease (Table 1). Lateral medullary infarction (Wallenberg's syndrome) is a common cause, while approximately 25% of Parkinson's patients have failed sphincter relaxation.13, 14, 15 Interestingly, the abnormality may be detectable before the onset of dysphagia in Parkinson's disease,15 suggesting that pharyngeal propulsion can readily compensate for the disorder and that dysphagia severity is more related to the degree of associated pharyngeal dysfunction than failed UES relaxation per se (see below). This observation stands in contrast to the situation in esophageal achalasia, where relief of the resistance offered by the lower esophageal sphincter radically improves swallowing despite persistence of complete esophageal aperistalsis.
Pathophysiology of Failed Upper Esophageal Sphincter Relaxation
Biomechanics of Normal Upper Esophageal Sphincter Relaxation and Opening
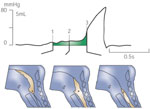
Following swallow initiation, approximately 200 milliseconds (ms) prior to the onset of UES opening, transient inhibition of vagal input to the muscular components of the UES leads to a loss of active tension in the sphincter region, leading to a drop in resting UES pressure to near-atmospheric levels.9, 16 A further reduction in intrasphincteric pressure results from anterior traction forces applied (by suprahyoid muscles) to the cricoid cartilage via the laryngohyoid complex. This second effect can result in transient drops in intrasphincteric pressure to subatmospheric levels, resulting in actual sphincter opening (luminal wall separation). The subsequent passage of a fluid bolus into the relaxed and opened sphincter zone will restore intrasphincteric pressure to supraatmospheric levels (onset of the so-called intrabolus pressure domain).17 Thus, the residual or nadir intrasphincteric pressure, at a given time in the swallow sequence, may be subatmospheric, atmospheric, or supraatmospheric, depending on the balance of forces generated by UES muscle tension (active and passive), intrabolus pressure, and external traction forces on the cricoid. Based on normal data from the author's laboratory, a deglutitive UES nadir pressure <10 mmHg, when measured by a sleeve sensor and during a "dry" swallow, is considered complete UES relaxation.5, 15, 18 Subsequent to these phenomena, the degree of actual UESopening is then determined by a balance among three forces: UES muscle tension (active and passive), intrabolus pressure, and external traction forces on the cricoid cartilage.19
Biomechanics of Failed Upper Esophageal Sphincter Relaxation
In contrast to the normal process outlined above, there is a persistence of high residual active sphincter muscle tension during the swallow. As a consequence UES opening may still occur to a variable (at times normal) degree in the presence of increased hypopharyngeal intrabolus pressure.5 Consequently, in this context, the relative integrity of the pharyngeal constrictor muscle response is of paramount importance in determining both the degree of ultimate UES opening and the severity of dysphagia. A comparison between the well-characterised UES restrictive disorder of Zenker's diverticulum and neurogenically mediated failed UES relaxation serves to highlight the major differences in biomechanical properties between failed UES relaxation and subnormal UES opening. The nonrelaxing sphincter retains the normal, essentially linear, relationship between swallowed bolus volume and extent of opening; the potential for complete opening is preserved; and intrabolus pressure, although supranormal, does not vary as a function of swallowed bolus volume (Figure 1). In summary, the stenosed sphincter in Zenker's relaxes completely during deglutition. The sphincter then rapidly reaches the limit of its opening capacity, and the intrabolus pressure thereafter continues to rise steeply as a function of bolus volume because flow rates across the stenosed sphincter are the same as those across the normal sphincter.20 In contrast, the persistent tonicity within the nonrelaxing sphincter applies a resistance to pharyngeal outflow that remains roughly constant irrespective of the sphincter diameter achieved.5
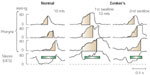
Figure 1: Intrabolus pressure and maximal sagittal upper esophageal sphincter (UES) diameter expressed as a function of swallowed bolus volume.
Intrabolus pressure (top) and maximal sagittal UES diameter (bottom) both expressed as a function of swallowed bolus volume in patients with failed UES relaxation, compared with measures from patients with a fixed, structural UES opening disorder (Zenker's) and healthy aged controls. a: Intrabolus pressure is supranormal in both patient groups. The significant volume-pressure gradient seen in normals and Zenker's, however, is not observed in those with failed UES relaxation. b: Maximal sagittal UES diameters were diminished in both types of sphincter disorder, but the normal, bolus volume-dependent increase in sagittal diameter is preserved in patients with failed UES relaxation and in healthy controls. The capacity for increased UES opening of a nonrelaxing UES approaches normal for swallowed bolus volumes of 20 mL. In contrast, the extent of UES opening in Zenker's rapidly peaks at 5 mL but changes very little with further increases in swallowed bolus volume. (Numbers in parentheses denotes subjects able to swallow bolus volume specified.) (Source: Williams et al.,5 with permission from American Journal of Physiology.)
Clinical Features
Symptoms and Physical Findings
Failed UES relaxation is a phenomenon, not a clinical diagnosis. The entity is generally seen in the context of pharyngeal dysphagia, usually in association with other neurologic symptoms and signs. There are no swallow-related symptoms specific for failed UES relaxation. Patients present with the typical features of neuromyogenic pharyngeal dysfunction including delayed swallow initiation, repetitive swallows to clear the pharynx, postnasal regurgitation (deglutitive velopharyngeal incompetence), deglutitive cough (aspiration), weight loss, and respiratory infections. Symptom onset may be either acute (e.g., brainstem stroke) or insidious (e.g., Parkinson's; syringobulbia).
Occasionally, failed UES relaxation can be seen in neurologically impaired individuals who have yet to develop dysphagia, although such cases usually have some radiographic evidence of impaired pharyngeal clearance.15 There are no physical signs specific to the condition. Such findings depend on the underlying neurologic cause and might include parkinsonian tremor, bradykinesia, and rigidity. Alternatively, signs consistent with brainstem disease may manifest as cranial nerve defects, dissociative sensory loss, and mixed long tract signs. It can be identified in cases of pharyngeal dysphagia with no extrabulbar signs and normal brain imaging in whom the diagnosis is not apparent. In these cases, the finding of failed UES relaxation is important as it provides indirect evidence of medullary disease (see above).
Determinants of Symptom Severity
Determinants of symptoms in the context of failed UES relaxation differ from those due to esophageal achalasia. In contrast to the pharynx, gravity is very important in effecting bolus transport through the esophagus. Despite the lack of peristalsis in esophageal achalasia, solid and liquid boluses can traverse the esophagus without causing symptoms as long as the barrier at the dysfunctional lower esophageal sphincter is removed by rendering the sphincter incompetent. That is, failed LES relaxation (not failed esophageal peristalsis) is the major determinant of dysphagia in esophageal achalasia. In contrast, the relative loss of propulsive force from pharyngeal constrictors is more important than the failure of UES relaxation in determining the severity of pharyngeal dysphagia. This hypothesis was examined systematically in a combined manometric and radiographic study of proven cases of failed UES relaxation.5 In that study, the extent of opening of the nonrelaxing UES, which varied from normal (one third) to partial (one third) to absent (one third), correlated strongly with the degree of preservation of the pharyngeal swallow response. All patients who had an intact pharyngeal swallow response had some demonstrable UES opening. Indeed 50% with an intact pharyngeal swallow response demonstrated normal maximal sagittal UES diameters during the swallow. In contrast, 65% of patients with an absent pharyngeal swallow response had a complete absence of UES opening. These findings emphasize the point that sphincter relaxation and opening, although interdependent, are not synonymous. These interrelationships among failed UES relaxation, UES opening, and the degree of preservation of the pharyngeal swallow response are illustrated in two examples showing videoradiographic images and concurrent manometric traces (Videos 1 and 2). When the pharyngeal swallow response is well preserved, extent of UES opening can be normal and dysphagia mild (Video 1). In cases in which the pharyngeal response is markedly weakened or absent, marked derangement of pressure-flow patterning is evident, resulting in markedly reduced or even absent UES opening (Video 2). Notwithstanding the strong correlation between an absent pharyngeal swallow response and impaired or absent UES opening, an absent pharyngeal response does not invariably result in failed sphincter opening. In these circumstances UES opening is possible if sufficient intrapharyngeal pressure is generated by compensatory tongue base motion. In other words, the action of the tongue base is a very important compensatory mechanism that can reduce the severity of dysphagia even if the pharyngeal response is markedly deranged. Such compensation by the tongue base is more likely to be seen in younger patients (Cook IJ, unpublished observations), presumably because it is under voluntary control.
Video 1: Videoswallow and corresponding manometric traces from a patient with mild dysphagia and failed UES relaxation due to syringobulbia.
The pharyngeal swallow response is preserved and the pharyngeal pressure wave is of normal amplitude and propagates normally. The only radiologic abnormality is a minor increase in postdeglutitive residual contrast in the hypopharynx (time T4). Note that the UES opens fully with good transsphincteric flow (T3). Note also that the intrabolus pressure (arrow) is markedly increased. The horizontal bar represents the interval of transsphincteric bolus flow determined radiologically (T3). (Source: Williams et al.,5 with permission from American Journal of Physiology.)
Video 2: Example of videoswallow and concurrent manometric tracing from a patient with severe dysphagia secondary to lateral medullary infarction causing failed UES relaxation and marked pharyngeal weakness.

Note in this example that the pharyngeal response is very feeble, giving rise to low amplitude synchronous pressure waves within the pharynx. There is very poor clearance of the swallowed bolus from the pharynx and significant aspiration of contrast. (Source: Williams et al.,5 with permission from American Journal of Physiology.)
Management and Outcome
As manometry of the pharynx is not widely available, and as manometry is necessary to detect failed UES relaxation, the initial workup of the patient with suspected failed UES relaxation is similar to that of any case of presumed neuromyogenic pharyngeal dysphagia (see below). However, a higher probability of failed UES relaxation should be suspected in the context of specific underlying neurologic disorders such as Parkinson's disease or lesions of the rostral medulla (e.g., stroke, syringobulbia, tumor). In these circumstances, manometric assessment of UES function is indicated. Identification of failed UES relaxation may influence management decisions (e.g., cricopharyngeal myotomy), although at present there is no evidence that doing so favorably influences outcomes. Conversely, when the underlying neurologic condition is unknown or uncertain, the manometric finding of failed UES relaxation should prompt a search for a medullary disorder or consideration of early Parkinson's disease if additional symptoms and signs are minimal.
Approach to Oral-Pharyngeal Dysphagia: An Overview
For in-depth coverage of diagnostic and management principles for oral-pharyngeal dysphagia, the reader is referred to several detailed reviews.21, 22, 23 Broadly speaking, the aims of management are to (1) identify and treat any underlying primary disease that might be responsible for the neuromyogenic dysfunction, (2) identify structural abnormalities that might be amenable to endoscopic or surgical therapy, (3) establish the aspiration risk in the individual and direct management to attenuate that risk, and (4) compensate for or circumvent the specific mechanical disturbances responsible for the ineffective swallow. Hence, there is no single strategy appropriate for all cases or indeed for dysphagia caused by a single disease process because the mechanical disturbances are generally neither homogeneous nor specific for a particular disease.
In addition to the history and examination of neuromuscular function, laboratory tests (including inflammatory markers, muscle enzymes, and autoantibodies), should be done to help identify treatable metabolic diseases (e.g., thyrotoxicosis, Cushing's, drugs), inflammatory myopathies, and myasthenia. Electromyographic studies and muscle biopsy may be considered if myopathy or myasthenia is in the differential.
Structural disorders are generally readily detected by radiographic or endoscopic evaluation. Identification of a neoplasm or a Zenker's diverticulum dictates surgery. A cervical web or a cricopharyngeal stenosis prompts dilatation or cricopharyngeal myotomy. The evidence supporting the efficacy of mechanical disruption (usually surgical) for structural disorders is consistently favorable.22
The best means of establishing the aspiration risk is by videoradiography. This is the primary determinant as to whether and when nonoral feeding should be instituted. The decision on the advisability of gastrostomy feeding is also influenced by the likelihood that therapeutic maneuvers, which may be tested during videofluoroscopy, will reduce or eliminate aspiration; the natural history of the underlying disease; and the patient's cognitive function.
After exclusion of structural lesions and underlying treatable diseases, and having established the safety of oral feeding, specific therapies should be considered. The therapeutic options open are dietary modification, swallow therapy, surgery, or a combination of all three.22
The Role of Cricopharyngeal Myotomy or Botulinum Toxin Injection in Treating Failed Upper Esophageal Sphincter Relaxation
Cricopharyngeal Myotomy
Although it is attractive to think that detection of failed UES relaxation might prompt UES disruption by cricopharyngeal myotomy or perhaps botulinum toxin injection, the efficacy of either therapy in this condition is unproven. In contrast to the high efficacy of cricopharyngeal myotomy in Zenker's diverticulum (see below), the overall response rate of this procedure in pharyngeal neuromyogenic dysphagia of any cause is only around 60%, with an operative mortality of 1.5% and a complication rate of 6%.22 Furthermore, efficacy of myotomy, specifically in proven cases of failed UES relaxation, has not been examined. Although enthusiasm for sphincter myotomy is justified in esophageal achalasia, for the reasons outlined above, this certainly cannot be transposed to the management of failed UES relaxation in which the severity of dysfunction depends so heavily on the state of the pharynx and not on the UES itself.
Botulinum Injection
Botulinum toxin injection into the cricopharyngeus, either endoscopically or transcutaneously, has been reported in small case series to be of benefit in unspecified pharyngeal dysphagia.24, 25, 26, 27 Although these studies attempted to target cricopharyngeal disorders, optimal identification of failed cricopharyngeal relaxation (using EMG or even manometry) was not adopted in those studies. Diffusion of the toxin to adjacent muscles may worsen dysphagia, and vocal cord dysfunction has also been observed following botulinum toxin injection. More systematic evaluation of safety and efficacy is much needed before this therapy might be used to target particular dysphagic populations. However, cases of manometrically proven failed UES relaxation, with relatively preserved pharyngeal propulsive function, ought to be prime candidates for this therapy. Although the studies are yet to be done, it is hoped that appropriate patient selection for such efficacy trials will be randomised and controlled, and that patients will be selected on the basis of proven failure of UES relaxation.
Summary and Future Directions
Failure of UES relaxation is not a diagnosis as such, rather it is a functional abnormality caused by neuronal dysfunction within the central nervous system that may be a manifestation of one of a number of neurologic syndromes. This entity is not synonymous with the very common radiologic anomaly, the cricopharyngeal bar, which is a structural lesion and not a motor disorder. This author believes that the term cricopharyngeal achalasia should be dropped in favor of more precise descriptors, preferably qualified by the mode of establishing failure of UES relaxation (either manometric or electromyographic). The biomechanics of and conceptualization of the determinants of dysphagia severity differ markedly from esophageal achalasia. In the context of failed UES relaxation, symptom severity is dictated by pharyngeal reserve. Although conceptually appealing, cricopharyngeal myotomy or botulinum toxin injection remains unproven therapy in this condition as systematic efficacy studies of these modalities in cases of proven failure of UES relaxation have not been done. Such studies, in carefully selected cases with proven failure of UES relaxation should be the focus of future research.
Zenker's Diverticulum
Historical Background and Definition
The posterior hypopharyngeal pouch, Zenker's diverticulum, arises from herniation through the posterior hypopharyngeal wall at an area of relative muscular weakness (Killian's dehiscence) just proximal to the upper margin of the cricopharyngeus muscle (Figure 2). The entity was first described by Ludlow28 in 1764. Zenker and von Zeimssen29 in 1878 described the anatomic findings of 22 cases. They postulated that the pressure exerted by the pharynx through the swallowed bolus against the area of muscular thinning (Killian's dehiscence) just above the cricopharyngeus, eventually results in herniation of the mucosa through this region to form the pouch. In addition to the notion of the zone of muscular weakness, Zenker also postulated as follows: "If there is already a stenosis of the upper end of the oesophagus before the occurrence of any of these causes, it may be readily understood that this would favour the formation of a diverticulum, for not only would a foreign body in this case be more easily detained in the canal, but also, in consequence of the stasis of food caused by the stenosis, the pressure on the weak spot must be increased." Confirmation of this increased deglutitive hypopharyngeal pressure and proof of Zenker's hypothesis was another 100 years coming (see below).
Figure 2: Barium radiographs of a typical posterior pharyngeal (Zenker's) diverticulum.
Lateral view on left, anteroposterior view on right.
Clinical Features
Patients are usually elderly, with the median age at presentation being in the eighth decade.20 Presenting symptoms include dysphagia combined with varying degrees of regurgitation, depending on the size of the pouch. Regurgitation of food particles ingested many hours earlier is often reported. Patients frequently describe an audible gurgle during the swallow. When symptomatic, weight loss is common. Aspiration is not uncommon, particularly if the pouch is large, and recurrent chest infections can be a significant and dangerous clinical feature.
Pathophysiology
Since Zenker and von Ziemssen's29 original hypothesis in 1878, a large number of theories have been debated in regard to pathogenesis. Initially it was believed that UES incoordination, specifically premature sphincter closure, in some cases combined with early UES relaxation, was the cause of the proposed elevated hypopharyngeal pressure.30, 31, 32 The validity of those early observations is questionable, however, because UES relaxation profiles were recorded with a discrete side hole positioned within the sphincter without appreciation of its deglutitive axial mobility.33 Other theories proposed included UES spasm,34 failure of UES relaxation,14 and a second swallow against a closed sphincter.31 However, there was no consistent demonstration of any of these phenomena and subsequent carefully conducted manometric studies reported normal or low basal sphincter tone and normal pharyngosphincteric coordination.20, 35 Furthermore, UES opening has now been shown to be restricted due to an intrinsic myopathic process affecting the cricopharyngeus muscle36, 37, 38 and the increased deglutitive hypopharyngeal pressure, as originally postulated by Zenker, has indeed been shown to be markedly elevated.20, 38
Simultaneous videoradiography and pharyngeal manometry enables the investigator to quantify the pressures imparted via the bolus to the hypopharynx (intrabolus pressure) during bolus flow across the pharyngoesophageal segment (Figure 3). Hypopharyngeal intrabolus pressure is the low-pressure domain (2–10 mmHg) within the bolus itself and which is registered before the onset of the major pressure upstroke caused by the advancing hypopharyngeal contraction (>100 mmHg). From this figure it can be appreciated that intrabolus pressure will vary as a function of the resistance to bolus flow offered by the UES, and that of the many variables impacting pressure-flow relationships, the major determinant of such resistance is sphincter diameter during flow. In Zenker's, combined radiographic and manometric studies of this type have confirmed normal pharyngosphincteric coordination, normal resting UES tone, and complete UES relaxation during the swallow.20 Those studies demonstrated that opening of the UES was restricted, and as a result that hypopharyngeal intrabolus pressure is increased (Figure 4). Hence, as originally suggested by Zenker, diverticulum formation is due to a poorly compliant but normally relaxing UES that cannot fully distend during the process of sphincter opening. Further evidence supporting this hypothesis is the finding that cricopharyngeal myotomy, effectively a curative surgical procedure, normalizes both the extent of UES opening and hypopharyngeal intrabolus pressure.39
Figure 3: Hypopharyngeal intrabolus pressure is an indirect measure of UES compliance.
The pressure record shown is from a recording site approximately 1 cm above the upper margin of the UES. The cartoons below are derived from video frames at three time points before, during, and after passage of the bolus in relation to that recording site. Note that the mid-intrabolus pressure (center) is that pressure recorded from within the bolus as the bolus is traversing the open UES. The intrabolus pressure domain is relatively low and precedes the much higher pressure resulting from the arrival of the pharyngeal contraction coincident with the tail of the bolus. The magnitude of the intrabolus pressure is most dependent on the resistance to flow offered by the UES, in turn determined mainly by the UES diameter during opening and the intrinsic tissue elasticity or compliance within the sphincter.
Figure 4: Example of increased hypopharyngeal intrabolus pressure in a patient with Zenker's diverticulum compared with a normal on the left.
During the 10-mL swallow (center), note the markedly increased intrabolus pressure recorded 1 cm above the UES during bolus flow (represented by the hatched bar) across the open sphincter. The subsequent swallow coping with the much reduced residual bolus, shows a much smaller intrabolus pressure domain. Note also the complete UES relaxation in response to small volume (right) or dry (not shown) swallows. (Source: Cook et al.,20 with permission from American Gastroenterological Association.)
Why is UES opening restricted in these patients? Histopathologic studies of the cricopharyngeus and inferior constrictor muscles retrieved at the time of cricopharyngeal myotomy shows myopathic features including muscle fiber necrosis, phagocytosis, and increased fibroadipose tissue replacement confined to the cricopharyngeus muscle (Figure 5).37 These morphologic changes in the cricopharyngeus muscle do influence the contractile and elastic properties of the muscle and probably account for its restricted opening.36
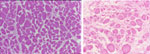
Figure 5: Normal cricopharyngeus muscle (left) compared to that from a patient with Zenker's (right).
When compared with the normal cricopharyngeus muscle, the muscle from patient with Zenker's demonstrates fiber dropout (due to necrosis, as evidenced by scattered degenerative fibers), greater fiber size variability, and marked increase in fibroadipose tissue replacement. (Source: Cook et al.,37 with permission from Blackwell Publishers.)
Treatment
Transcervical Surgical Approach
The mainstay of treatment for symptomatic Zenker's diverticulum is disruption of the cricopharyngeus muscle (by surgical myotomy or dilatation). Cricopharyngeal myotomy can be performed either alone or in combination with pouch resection or suspension. Myotomy can be performed through a neck incision or via a transoral approach. Simple dilatation can afford symptomatic benefit of variable duration40, 41 and can be considered in elderly patients with significant comorbidity with high operative risk. Cricopharyngeal myotomy is the key element for successful long-term relief of dysphagia and it has been established that resection of the pouch alone is inadequate treatment and that myotomy is the essential element in treatment of this condition.42, 43 Indeed excellent symptomatic results can be achieved from myotomy alone.43, 44 Regression of the intact pouch can be observed following myotomy,45 and radiologic recurrence of the pouch is reduced after myotomy.32
Transoral, Endosurgical Approach to Myotomy
Transoral treatment of the pouch, either by endosurgical stapling or laser, has gained wide acceptance in the past 10 years. The technique was first described in 1960.46 If the pouch is deep enough (>2 cm), it is possible to straddle the cricopharyngeal bridge with a diverticuloscope, and incise and staple the cricopharyngeus. This incision removes the "common wall" between the cervical esophagus and the diverticulum, thereby achieving a cricopharyngeal myotomy and facilitating drainage of the pouch without removing it. Limited numbers have also been performed using the flexible endoscope.47 Endosurgical stapling is arguably the treatment of choice for the elderly or higher risk patient and is rapidly gaining acceptance as first-line therapy for average risk cases with favorable anatomy.
Outcome Following Surgery
No controlled trials of the efficacy of surgical treatment for Zenker's diverticulum exist. For traditional transcervical cricopharyngeal myotomy, the consistency of published response rates of between 80% and 100% is in keeping with the strong clinical impression that surgery is nearly always curative in this disorder.48, 49 Similarly, no controlled efficacy data exist for endosurgical approaches to treatment. Uncontrolled series consistently report success rates of 81% to 96%, and complication rates up to 6% (mediastinitis, haemorrhage, aspiration), with an estimated mortality after combining published studies of around 0.5%.50, 51 These figures are comparable to those for open surgical approaches, but patients are generally fed sooner and incur shorter hospital stays. While the long term outcome from the traditional transcutaneous approach is generally reconised as good, long published term outcome data from the transoral approaches is sparse. The choice between the two approaches at present rests largely with the specific experience of the operator and what is available locally to the clinician.
Other Conditions Associated with Disordered Upper Esophageal Sphincter Opening
As discussed in the introduction, although the cricopharyngeal bar is frequently an incidental anomaly, this condition can make a significant contribution to symptoms in the dysphagic patient. Broadly speaking this occurs in two situations. The first is in the context of a marked reduction in the luminal caliber at the cricopharyngeus. In this circumstance, the abnormality often takes the form of a circumferential stenosis, visible clearly in both anteroposterior and lateral radiographic projections. The second is when the CP bar is present in conjunction with neuromyogenic pharyngeal weakness. In this circumstance the weakness and the bar may be related or unrelated. For example, recent studies, one of which was case controlled, of inflammatory myopathy and dysphagia noted increased prevalence of a cricopharyngeal bars and stenosis, with or without Zenker's diverticulum, in patients with dysphagia due to polymyositis or dermatomyositis.52, 53 These observations suggest that some cases of CP bar might be secondary to a focal or more generalized myopathy. Whether in isolation or in association with a weak pharynx, if the dysphagia is deemed to be related to the CP bar, then cricopharyngeal dilation can be a beneficial treatment.54
As mentioned above, virtually any neuromyogenic condition that weakens pharyngeal propulsive forces can lead to a reduction in UES opening. This radiographic observation does not automatically imply that the UES is intrinsically dysfunctional. Nonetheless, abnormalities of the timing, duration, and extent of UES relaxation have been identified in some patients with certain neurologic disorders including Parkinson's disease (see above), medullary lesions (see above), and amyotrophic lateral sclerosis (ALS). Dysphagia affects up to a third of those with ALS, and their videofluoroscopic appearances can be very variable and stage-dependent.55 Several systematic studies found no manometric evidence of UES spasm or failed relaxation.5, 56 However, a study recording EMG activity directly from the cricopharyngeus muscle in ALS patients and healthy controls found UES opening was delayed, sometimes in association with premature sphincter closure. This resulted in shortening of the UES opening interval. That study also noted unexpected motor unit bursts appeared during the usual period of electromyographic quiescence as well as discoordination between the laryngeal elevator muscles and the cricopharyngeus muscle.57
Upper Esophageal Sphincter Incompetence: Mechanisms of Esophagopharyngeal Regurgitation
Prevalence and Significance of the Problem
It has been proposed that a wide range of oral and respiratory tract diseases are associated with gastroesophageal reflux disease (GERD). Although the strength of association and causation is tenuous in some, the list includes laryngitis, subglottic stenosis, asthma, unexplained cough, pulmonary fibrosis, dental erosions, sinusitis, and obstructive sleep apnea.58, 59, 60, 61 Some reports show a prevalence of reflux as high as 78% in patients with hoarseness and in half of all patients with voice complaints.58, 59 Animal studies demonstrate exquisite sensitivity of the vocal cords to minute amounts of acid.62 Acid contact with the larynx causes fatal central and obstructive apnoea in a rabbit model similar to obstructive apnoeic episodes recognized in human infants with GERD,63 raising the possibility that acid regurgitation may be implicated in sudden infant death syndrome. Despite the obvious clinical importance of the phenomenon of pathologic regurgitation, the actual prevalence of the subsets of these diseases in which pathologic acid regurgitation is truly a causative factor remains unknown.
Detection of and Definition of Esophagopharyngeal Acid Regurgitation
Multielectrode pH monitoring, incorporating sensors in the hypopharynx and one or more sensors in the esophagus, permits detection of esophagopharyngeal acid regurgitation.58, 64, 65 An example of this is shown in Figure 6. The pH criteria defining esophageal reflux events are well accepted. In contrast, variable criteria have been adopted to define a valid acid event in the pharynx. There is good evidence that standard esophageal criteria are inappropriate to define pharyngeal acid regurgitation events. A careful mathematical analysis of "candidate" and "artifactual" pharyngeal pH events found that adoption of standard esophageal pH criteria yields a very high false-positive rate for pharyngeal acid events.65 This is a consequence of the very high prevalence of small pharyngeal pH drops 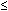 1 pH unit and the subsequent finding that many such small pH drops are caused by gaseous reflux rather than liquid regurgitation.66 The author recommends that a pharyngeal acid event be defined as a pharyngeal pH drop >2 pH units, to a nadir pH <4, with an onset of pH drop to nadir <30 seconds, which occurs during a period of confirmed esophageal acidification.65 One of the problems in confidently defining such criteria is the absence of a "gold standard" for proven acid regurgitation against which one can compare such criteria. Indirect evidence of validity has been sought from examination of the sensitivity and specificity of a positive pharyngeal pH study in predicting a favorable outcome from potent acid suppression in cases of suspected reflux-laryngitis.67, 68, 69 Unfortunately, these studies found an extremely wide sensitivity (36–80%) and specificity (40–100%) if a positive pharyngeal pH study is used as a diagnostic aid and if resolution of laryngitis on therapy is used as confirmation of an acid etiology. Clearly there are major problems with both assumptions and we remain some distance from truly validating current diagnostic criteria using dual ambulatory pH monitoring.
1 pH unit and the subsequent finding that many such small pH drops are caused by gaseous reflux rather than liquid regurgitation.66 The author recommends that a pharyngeal acid event be defined as a pharyngeal pH drop >2 pH units, to a nadir pH <4, with an onset of pH drop to nadir <30 seconds, which occurs during a period of confirmed esophageal acidification.65 One of the problems in confidently defining such criteria is the absence of a "gold standard" for proven acid regurgitation against which one can compare such criteria. Indirect evidence of validity has been sought from examination of the sensitivity and specificity of a positive pharyngeal pH study in predicting a favorable outcome from potent acid suppression in cases of suspected reflux-laryngitis.67, 68, 69 Unfortunately, these studies found an extremely wide sensitivity (36–80%) and specificity (40–100%) if a positive pharyngeal pH study is used as a diagnostic aid and if resolution of laryngitis on therapy is used as confirmation of an acid etiology. Clearly there are major problems with both assumptions and we remain some distance from truly validating current diagnostic criteria using dual ambulatory pH monitoring.
Figure 6: Tracing derived from an ambulatory dual (esophageal, pharyngeal) pH study.
In this example of a pathologic regurgitator, the distal pH electrode is in the standard position 5 cm above the lower esophageal sphincter. The proximal electrode is positioned 1 cm above the upper margin of the UES. Note the two major periods (shaded) in which some of the episodes of esophageal acidification are also associated with pH drops in the pharynx. Note also that this individual regurgitates exclusively in the upright posture during the day, but does not regurgitate at night (marked with "S")
Pathophysiology of Regurgitation
There are a number of candidate mechanisms of esophagopharyngeal regurgitation (Table 2).
Esophageal Acid Distribution
In the population with suspected reflux-related laryngitis, distal, proximal (and in some cases pharyngeal) acid exposure has been assessed using multiple pH electrodes. The consistent finding from virtually all studies is that there is no correlation between distal esophageal acid exposure and reflux laryngitis.58, 64, 70, 71, 72, 73, 74 Two of these studies found that proximal esophageal acid exposure was increased in cases with laryngitis when compared to controls, suggesting that the regional distribution of esophageal refluxate may be a risk factor for regurgitation.70, 72 This has not been a consistent finding, however, among all studies.64, 73
Body Posture (Recumbency)
It is recognized that there are probably multiple mechanisms involved, and that regurgitation can occur either day or night and in the upright or recumbent posture (Figure 7). However, somewhat counterintuitively, a 24-hour dual pH study of a group of patients at risk of regurgitation found that 85% of events proven to be pharyngeal acid regurgitation occurred during the daytime in the upright posture.65 It is likely that different circumstances facilitate regurgitation in the upright vs. recumbent postures (see below).
Figure 7: Two examples from the same patient, showing different patterns of regurgitation.
In the top tracing, there is an abrupt fall in esophageal pH, which is followed, virtually immediately, by an abrupt fall in pharyngeal pH. Both of the events in this situation occurred in the upright posture, presumably as an "explosive" phenomenon in which the acidic content travel directly from stomach to the pharynx. The bottom tracing, during sleep, shows a very different picture where one sees a fall in pharyngeal pH only following a prolonged period of esophageal acidification.
Upper Esophageal Sphincter Factors: Hypotonia
Although relative UES hypotonia may account for some regurgitation episodes, particularly those occurring in the supine posture,65 resting UES hypotonia is unlikely to be the dominant mechanism of regurgitation for a range of reasons. Basal UES pressure is not a diagnostic predictor of either (confirmed) acid regurgitation or disease states thought to be associated with acid regurgitation.64, 68, 75 Abrupt increases in intraesophageal pressure during gastroesophageal reflux generally do not exceed even the minimal resting tone of the UES high-pressure zone.75 It is unlikely that passive sphincter opening, in response to transient increases in intraluminal esophageal pressure imparted on the UES from below, would account for regurgitation. In health, UES tone falls to very low levels (<10 mmHg) during sleep without resulting in regurgitation. Finally, cricopharyngeal myotomy reduces UES tone by 50% (to around 20–30 mmHg). However, it has been shown that myotomy, even in the context of increased esophageal acid exposure, does not increase the risk of (gastro)esophagopharyngeal acid regurgitation.76 These observations imply that additional pathologic processes must be required to bring about regurgitation even when basal UES tone is low.
Upper Esophageal Sphincter Factors: Reflexive Upper Esophageal Sphincter Relaxation
The majority of regurgitation episodes occur in the upright posture65 (Figures 6 and 7). It is likely in this context that an active relaxation of the UES is necessary to facilitate reflux of acid into the pharynx. It is recognized that the UES will relax in response to abrupt esophageal distention—the belch reflex.77, 78 It is probable that this type of non–swallow-related UES relaxation, akin to the transient LES relaxation that mediates GE reflux, underpins many if not most regurgitation episodes. This phenomenon is apparent during prolonged recordings of UES pressure (using a sleeve sensor) and multipoint pH recording from the esophagus and pharynx (Figure 8). In a preliminary prolonged manometric and pH study of seven subjects, 12 episodes of pharyngeal acidification were detected in this manner, and all were seen to occur in close association with non–swallow-related transient UES relaxations (author's unpublished observations). In the example shown, acid had been static within the esophagus for a lengthy interval before it refluxed into the pharynx. If one examines the latency between esophageal and pharyngeal pH drops during 24-hour dual pH monitoring, two distinct posture-dependent patterns are seen: a short latency pattern dominant during the upright posture, and a long latency pattern dominant seen only in the supine posture. The abrupt near-synchronous esophageal and pharyngal pH drops account for two thirds of all regurgitation events. Additionally, 90% of events occur in the upright posture.65 Hence, the majority of UES relaxations mediating pharyngeal acidification are likely to be reflexively triggered by abrupt esophageal distention, in turn caused by a gastroesophageal reflux episode. The remaining one third of episodes probably occur in response to a combination of factors, with or without the aid of strain (Figure 9).
Figure 8: Example of esophagopharyngeal regurgitation captured during prolonged manometric and dual pH recording.
The sleeve sensor that was monitoring LES pressure has slipped into the stomach; hence, no recording of LES pressure is visible. However, during a period of prolonged esophageal acidification, this example clearly shows a spontaneous (non–swallow-related) UES relaxation, which mediates pharyngeal acidification.
Figure 9: Example of esophagopharyngeal acid regurgitation occurring during a transient UES relaxation, but aided by strain.
Note that although acid has been present in the esophagus for some time, the UES relaxes spontaneously. Less than 1 second later, aided by strain, which raises intraesophageal pressure, acid refluxes into the pharynx. {Ed: tUESR = transient upper esophageal sphincter relaxation; UES = upper esophageal relaxation}
Neuropharmacology of Regurgitation Mechanisms?
From the above discussion, it is apparent that the focus of research to address these two key issues will be on the neuropharmacology of afferent and central interneuronal pathways that mediate reflexive UES responses to esophageal stimuli. In addition to UES relaxation, esophageal distention triggers a range of UES and laryngeal reflexive responses that are likely to serve protective functions.78 It is probable that upregulation of the esophago-UES relaxation reflex underlies pathologic regurgitation, whereas the integrity of the additional protective reflexes will dictate how much damage to the respiratory tract might result from increased regurgitation. Little is known about the neuropharmacology of these responses in humans, but such information will be vital to future pharmacotherapeutic targeting of disorders caused by pathologic regurgitation.
Retrograde labeling has demonstrated both vagal and spinal afferents supplying the esophagus. It is the vagal mechanosensitive afferents that respond to the types of stimuli that underpin UES reflexive responses, as they have a low threshold for circumferential stretch79 and are polymodal in that they can be modulated by other stimuli such as light touch, respiratory rhythm, acid, hypertonic saline, serotonin, and bradykinin.80 Mechanosensitive vagal afferents arise both in esophageal mucosa and from within the muscle layer. Intramuscular vagal mechanoreceptors have different functional characteristics and probably subserve different functions to mucosal mechanoreceptors.81 Intramucosal vagal afferents display a higher density in proximal vs. distal esophageal mucosa in some species, which might explain in part the observed increased likelihood of triggering the UES contractile response to proximal vs. distal distention.77, 78
Currently the best insights into the neuropharmacology of these esophago-UES and esophagoglottal reflexes comes from a very important study in a decerebrate cat model.81 As in humans, the cat UES relaxes in response to abrupt esophageal distention by air inflation and contracts in response to slow inflation by air or balloon. Lidocaine or capsaicin esophageal perfusion, removal of the esophageal mucosa, or intravenous baclofen blocked or inhibited the UES relaxation and esophagoglottal closure reflexes but had no affect on the UES contractile response. These findings confirmed that the UES relaxation reflex is mediated by capsaicin-sensitive, mucosal (not muscular) rapidly adapting mechanoreceptors, which are inhibited by activation of 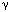 -aminobutyric acid (GABA)-B receptors. Preliminary studies in the human confirm that the UES relaxation response can be attenuated but not blocked by esophageal perfusion of lidocaine, whereas GABA-B agonism has minimal effect on the reflex (author's unpublished observations).
-aminobutyric acid (GABA)-B receptors. Preliminary studies in the human confirm that the UES relaxation response can be attenuated but not blocked by esophageal perfusion of lidocaine, whereas GABA-B agonism has minimal effect on the reflex (author's unpublished observations).
Future Directions for Research
Achieving consensus among investigators on the pH criteria used to define pharyngeal acid regurgitation events will be important to ensure comparability among studies and to facilitate better patient selection for specific therapies. The application of intraluminal electrical impedance measurement to the detection of retrograde flow may prove helpful also. Prompted by the findings in animal models, further studies specifically targeting mucosal mechanoactive vagal afferents in the human are needed to clarify the neuropharmacology of the UES and glottal responses to esophageal stimuli. In tandem, examination of the sensitivity of these responses to various stimuli in diseases proven to be caused by pathologic esophagopharyngeal regurgitation needs to be done. In this way, the subpopulations in whom future pharmacotherapy might be targeted can be identified.