Key Points
- Dysphagia can result from many diverse conditions.
- Attention to detail is necessary in the radiographic evaluation of the dysphagic patient.
- To evaluate the swallow, it is necessary to understand its anatomy and physiology.
- The physiology of the oral and pharyngeal swallow can be subdivided into several phases.
- Analysis of these six phases results in diagnosis of the normal and abnormal findings in a patient with dysphagia.
Introduction
Dysphagia can result from many diverse conditions, including structural and motility disorders. This review focuses on normal and abnormal motility of the organs involved in the oral and pharyngeal phases of deglutition, but does not discuss the structural causes of dysphagia or esophageal motility.
Motility disorders can result from many neuromuscular conditions affecting any level of the neural system: cortex brainstem, cerebellum, basal ganglia, cranial nerves, peripheral nerves, myoneuronal junction, and even the muscles of deglutition themselves.1, 2, 3 Thus, dysphagia can be one of the principal symptoms in brain tumors,4 CVA,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 amyotrophic lateral sclerosis (ALS),21, 22, 23, 24 Parkinson's,25, 26, 27, 28, 29, 30, 31, 32, 33 multiple sclerosis,34, 35, 36 myasthenia gravis,37, 38, 39 muscular dystrophy,40, 41, 42 and myopathies or myositis.43, 44, 45 Other neurologic diseases such as Alzheimer's46 and Huntington's disease47, 48 can also produce dysphagia.
Systemic diseases such as dermatomyositis can affect swallowing, as the muscles of swallowing are striated muscles43; scleroderma, a disease of smooth muscle, usually spares the pharynx but can affect the oral phase of swallowing owing to a decrease in salivary flow associated with accompanying Sjögren's syndrome.49, 50 Medications can exacerbate swallowing difficulties by causing local effects such as oral or pharyngeal inflammation51 or by affecting the neuromuscular transmission of peristalsis.52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64
Although it is an artificial separation, I have taken the liberty of discussing this topic under two categories: doing the study and analyzing the study. Naturally this is such a dynamic process that "doing" and "analyzing" are integrally intertwined and interwoven during the actual study. Flexibility and continuous diagnosis of abnormalities or decompensations is essential; there is no real "cookbook" approach, but constant consultation and discussion among the team are necessary.
Principles of Examination: Doing the Study
Tailoring the Examination to Each Patient65, 66
A brief relevant history should be taken prior to the examination; the history then guides the study. The basic routine can be modified in an attempt to reproduce symptoms and detect an abnormal swallow by provocative tests. The clinical condition of the patient is also important: the sicker the patient, the less routine and more tailored the examination. The examination is also varied depending on whether it is the initial diagnostic procedure or a therapeutic study directed at gauging the effect of various bolus consistencies or maneuvers on aspiration or other decompensations. Under these circumstances the study is performed jointly by the radiologist and an allied health professional trained in dysphagia management such as a speech-language pathologist (SLP) who will have evaluated the patient clinically prior to the study.
The Initial Swallow
Centering
The fluoroscope should be centered so that the entire mouth, pharynx, and larynx can be clearly seen; thus, decompensations such as drooling, premature leakage, nasopharyngeal regurgitation, and laryngeal penetration or aspiration can be appreciated.
Consistency
In general, it is best to begin with what is thought to be safest (this information may be volunteered by the patient or may be decided on by the SLP following the clinical evaluation). For example, if the clinical evaluation suggests that the patient chokes on liquids, it may be wise to begin with a small volume of purée, for example, 1/4 to 1/2 teaspoon, and "work down" through honey-thick to nectar-thick to thin liquid, whereas if there is a high suspicion of pharyngeal weakness, purée may be too thick to be handled safely and a thinner bolus may be better. If there is a suspicion of obstruction, a small volume of thin liquid may be better—again tailor the consistency to the patient.
Continuing On with the Study
Depending on what is observed during that first swallow, the next decision is whether to stay in that position or move on to the frontal position. For example, if aspiration is seen, one may decide to try different consistencies perhaps with compensatory maneuvers to try to decrease or abolish the aspiration. Such maneuvers might include "chin-tuck" (head flexion to the chest), which may abolish aspiration, as may the "supraglottic swallow" (in which the patient breathes in through the nose, holds the breath, swallows hard, and exhales with or without cough). Unilateral pharyngeal paresis and unilateral vocal fold immobility that may be difficult to appreciate in the lateral position but can easily be diagnosed in the frontal position may be treated by turning the patient's head toward the abnormal side, thus forcing the bolus to go down the normal side.
The frontal position is especially useful for assessing vocal fold movement (ask the patient to say "eee", "cookie-cookie-cookie"; and tell the patient to "sniff like you are smelling a flower"), as well as symmetry of pharyngeal contraction and bolus passage.
Positioning
The mouth and pharynx are best evaluated in the upright lateral and frontal positions with the patient either standing or sitting. Special chairs are available that are narrow enough to be wheeled in front of the fluoroscopic table for examining incapacitated patients in the position in which they normally eat or are fed. Smaller adult patients and pediatric patients can sit on the footrest of the fluoroscopic table if necessary.
Some fluoroscopic units have cross-table capability that allows rotation of the fluoroscopic tube rather than the patient. In such units, supine or semi-supine studies can be performed if the patient is unable to sit or stand. The patient may be strapped onto the fluoroscopic table for safety.
Equipment
Dynamic recording allows the frame-by-frame analysis necessary to evaluate the normal and abnormal swallow. This is easily accomplished by hooking up a link between the fluoroscopic foot pedal or hand switch and an S-VHS recorder (VHS does not have adequate resolution) such that when the fluoroscope is on, the VCR automatically records the fluoroscopic image. The 30-frames-per-second rate allows assessment of individual structures in real time, slow motion, backup mode, and frame-by-frame analysis.
Unfortunately, the 100- or 105-mm spot-film camera with maximum frame rates of 6 to 8 frames per second is inadequate in the evaluation of swallowing because subtle but important laryngeal penetration or even aspiration may be visible on only one or two frames out of the 30/second sequence. The six to eight frames per second rate also does not allow perception of the dynamic movement of the individual structures.
Analyzing (Interpreting) the Study
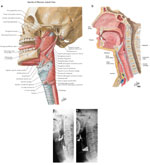
It is necessary to understand the anatomy of the mouth, larynx, and pharynx in order to be able to evaluate the physiology of the swallow67, 68, 69, 70, 71, 72, 73, 74 (Figures 1 and 2).
Figure 1: Lateral sagittal views of the pharynx comparing line drawings with contrast radiographs at rest and during phonation.
a: Line drawing indicates the constrictor and suspensory musculature of the pharynx viewed from the side. The constrictor muscles overlap, the inferior constrictor being the most external, and the superior the most internal.The middle constrictor is seen between the superior and inferior constrictor muscles. The inferior constrictor is continuous with the proximal circular muscle fibers of the esophagus. The fascicles of the cricopharyngeal portion of the inferior constrictor merge with the fascicles of the circular muscle of the esophagus. b: Sagittal line drawing showing structures in the sagittal plane including the tongue, soft palate, epiglottis, vestibule, false and true cords, arytenoid mass, cricoid cartilage, and trachea. (a,b: Source: Netter images, with permission from Elsevier Science.) c: Lateral view of the pharynx, which has now been coated by high-density barium. Structures can be seen including the soft palate (u), the dorsum of the tongue (T), the valleculae (V), the epiglottis (arrow), and the piriform sinuses (P). There is minimal retention of contrast in the valleculae and piriform sinuses. d: Lateral view during phonation, with the structures outlined by contrast. The soft palate (u) has elevated to a right angle, revealing the tonsillar fossa (arrows), expanding the valleculae (V) and piriform sinuses (P). T, tongue. (c,d: Source: Levine MS, ed. Radiology of the Esophagus. Philadelphia, WB Saunders, 1989, with permission from Elsevier Science.)
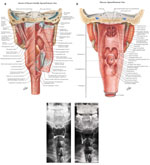
Figure 2: Comparisons between line drawings and radiographs in the coronal (frontal) plane.
a: The constrictor and lateral suspensory muscles of the pharynx viewed from the posterior aspect, showing: the pharyngeal raphe, and the superior, middle and inferior pharyngeal constrictor muscles, the thyropharyngeus muscle and the cricopharyngeus muscle. Note again that the constrictor muscles overlap, the inferior being the more external and the superior the more internal. Note also that the fibers of the cricopharyngeus or horizontal fibers of the inferior constrictor muscle merge with the fascicles of the proximal circular muscle of the esophagus. b: The structures in the anterior wall of the pharynx as viewed from the posterior aspect. Note the uvula, epiglottis, piriform sinus and tongue. The mucosa remains intact. This drawing shows the contours of the valleculae and piriform sinuses and demonstrates the relationship of the valleculae to the base of the tongue and epiglottis. (a, b: Source: Netter images, with permission from Elsevier Science.) c: The pharynx has now been coated with high-density barium outlining the valleculae (v) and piriform sinuses (p). In this patient the epiglottis is not clearly visualized on this view at rest. d: The patient demonstrates "blowing up a balloon." The pharynx is now expanded especially in the region of the proximal portions of the piriform sinus. There is more symmetric distention proximally than distally because the superior portion lacks the support of the thyroid cartilage. (c,d: Source: Jones B, Donner MW. Examination of the patient with dysphagia. Radiology 1988;167:319–326, with permission from the Radiological Society of North America.)
Individual structures are analyzed in the radiographic study (such as tongue thrust, epiglottic tilt, hyoid elevation, etc.). Therefore, each individual structure needs to be analyzed, first in isolation and then in combination with others. Individual structures that should be analyzed include the tongue, palate, pharyngeal constrictor wave, epiglottis, hyoid elevation, and cricopharyngeal opening and closing.
One should also be looking for signs of decompensation, such as drooling or premature leakage, nasopharyngeal regurgitation, retention in the oral cavity, laryngeal penetration or aspiration, to name some of the decompensations that indicate a compromised swallow.
In other words, sometimes information is gained by analyzing the structures themselves. At other times the shape or position of the bolus indicates abnormality: Does the bolus fragment? Does it pool in abnormal places such as the floor of the mouth or cheek? Is it misdirected such as into the nasopharynx or larynx?
Functional Evaluation of the Normal and Abnormal Swallow
Figure 3: Line drawing of the normal swallow.
a: The bolus is held in the oral cavity by apposition of the soft palate and tongue with the surrounding laryngeal structures and airway at rest. b: The bolus is conveyed into the oropharynx by the tongue and the soft palate has elevated. The airway and surrounding larynx have elevated in preparation for swallow. c: The bolus is descending into the hypopharynx on either side of the cricoid prominence. The epiglottis has tilted down to cover the closed laryngeal aditus. The larynx has reached its maximum elevation. d: The bolus continues through the hypopharynx, through the open cricopharyngeal segment, and into the esophagus, with the peristaltic wave descending behind it. The nasopharynx begins to open and opening progresses in a descending sequence. e: The wave of pharyngeal contraction has obliterated the oropharyngeal cavity and moved into the hypopharynx, with the bolus descending further through the open cricopharyngeal segment into the esophagus. The nasopharyngeal airway continues to open in a cephalocaudal fashion. f: The bolus has disappeared into the esophagus. All structures have returned to the open position: the normal contours of the airway are again visible, although the larynx has not yet completed its descent to its original resting position. Barium is coating the valleculae and piriform sinuses.(Source: Netter images, with permission from Elsevier Science.)
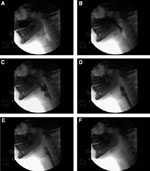
Figure 4: Selected stop-frame prints from a cinepharyngogram demonstrate several stages of a normal swallow.
a: The bolus (B) is retained in the mouth by apposition of the posterior aspect of the tongue and the relaxed soft palate (U). A small amount of contrast has been introduced through the nose and is coating the posterior nasopharyngeal wall and the superior portion of the soft palate. b: As the bolus is propelled into the oropharynx, the soft palate elevates to a right angle and apposes to the converging posterior pharyngeal wall, Passavant's cushion (pc). c: The soft palate (U), posterior pharyngeal wall, and dorsum of the tongue (T) remain closely in contact until the bolus has cleared from the oropharynx. The proximal stripping wave (sw) has begun. d: The posterior stripping wave (sw) has become more pronounced and is proceeding distally. The epiglottis has completely inverted and the vestibule is closed as the bolus passes through the hypopharynx. Note the closed larynx with the appearance of the conus (arrow) and the tracheal air column (T). The cricopharyngeus is completely open to allow easy passage of the bolus. There is a tiny amount of contrast in the entry to the vestibule, which is extruded as swallowing proceeds. e: The stripping wave (sw) has proceeded further. There is a small amount of bolus (B) still trapped in the hypopharynx. The larynx is still closed. (a-d: Source: Jones B, Kramer SS, Donner MW. Dynamic imaging of the pharynx. Gastrointest Radiol 1985;10:213–224, with permission of the authors.)
Each swallow may be divided into several phases:
- Oral phase
- Tongue/palate seal
- Nasopharyngeal (soft palate/superior constrictor) seal
- Compression and propulsion of bolus
- Hyoid/larynx/epiglottic tilt (laryngeal complex)
- Cricopharyngeal opening
- Esophageal peristalsis
Phases 1 to 6 are discussed here.
Oral Phase
The normal oral phase (Figures 3a and 4a) The lips engulf the bolus, which is held at rest toward the front of the mouth in a midline groove. With a larger bolus, the bolus sits further toward the back of the oral cavity. The average normal bolus size for thin liquids is 21 mL [standard deviation (SD)  5 mL).75 Boluses commonly used in clinical practice are 5 and 10 mL.67
5 mL).75 Boluses commonly used in clinical practice are 5 and 10 mL.67
There are two types of normal oral phase, based on where the bolus is held at the beginning of swallow: the "tipper" and the "dipper."76 In the more common "tipper swallow" (95%), the bolus is held above the tongue in a central groove and is then transferred into the pharynx by a progressive upward and posterior movement of the tongue blade, a movement that completely obliterates the oral cavity behind the bolus, the tongue progressively abutting the hard palate.
In the less common "dipper swallow" (5%), the bolus is held under the tongue in the floor of the mouth at rest; a downward scooping movement of the tongue blade transfers the bolus into the oral cavity and on into the pharynx in the same way as a tipper swallow. An individual's swallow is always either a "tipper" or a "dipper" type.
Occasionally one will see a "chipmunk swallow," when a large bolus is partially held in the cheeks and the patient then swallows several times to clear the oral cavity, each swallow decreasing the amount held in the cheeks. However, pooling in one or both cheeks after completion of a swallow indicates weakness, for example, after a stroke.
The abnormal oral phase Weakness of the lip seal results in drooling. Both bolus and tongue movements can suggest tongue weakness. Malpositioning or fragmenting of the bolus, difficulty propelling the bolus, multiple tongue blade movements to gradually propel the bolus, tremor or myoclonus, undulations of tongue blade, failure to obliterate the oral cavity, atrophy of the tongue, and low hyoid position can all be signs of tongue weakness. In the frontal view, asymmetry of the tongue or asymmetry of bolus position and preservation of air in the apex of the mouth during bolus transfer are also signs of weakness.
Also, failure to maintain the bolus in the central tongue groove with loss of bolus in the floor of the mouth is a sign of tongue weakness (Figure 5a). The bolus has now fragmented and may be left behind in the floor of the mouth after the swallow. Do not confuse this with a "dipper" swallow.
Figure 5: Oral decompensations with abnormal retained bolus.
Two different patients demonstrate pooling in the floor of the mouth (a) (arrows) and in the cheek (b) (arrow). (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 2003, with permission.)
Pooling in the cheek after the swallow is abnormal, indicating weakness, as is ballooning of the cheek (Figure 5b).
Tongue/Palate Seal
Normal (Figure 3a, b) Normally a bolus can be maintained in the mouth and does not leak out anteriorly, owing to the competence of the lip seal and tongue to the soft palate seal posteriorly. Normally, at rest the soft palate apposes the posterior tongue blade.
Abnormal A kink in the palate in the erect position is abnormal and a sign of soft palate weakness or an indication of palatal compensation for a weak tongue (Figure 6). In the supine position, however, the palatoglossus muscle contracts to maintain the bolus within the mouth, producing a kink in the soft palate.
Figure 6: Weakness of the soft palate.
Kinking of the soft palate as a sign of weakness. Note that the superior surface of the soft palate has been coated by instillation of barium through the nose. There is a kink partway down the uvula (arrow), which in the erect position is a sign of weakness. (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 2003, with permission.)
Incompetence of the seal between the tongue and the palate results in premature leakage from the mouth into the pharynx before initiation of swallowing, with the potential for aspiration into the open, unprotected larynx (Figure 7). In the frontal position, unilateral premature leakage versus central leakage points to the side of the weakness (Figure 7b).
Figure 7: Premature leakage.
Films from two separate patients reveal the appearance of premature leakage from the mouth. a: This still frame from a cinepharyngogram in the lateral projection demonstrates contrast leaking over the dorsum of the tongue into the valleculae (black arrows). Note that the soft palate (u) is incompletely apposed to the posterior portion of the tongue. There is the potential for aspiration into the open vestibule of the larynx (white arrows). b: In the frontal projection there is unilateral leakage over the back of the left side of the tongue into the left vallecula (v) with overflow into the left piriform sinus (ps). (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 2003, with permission.)
Soft Palate/Superior Constrictor
Normal soft palate/Passavant cushion seal (Figures 3b, 4b) As the bolus is propelled into the pharynx, the soft palate elevates to a right angle, apposing Passavant's cushion, a forward moving bulge, produced by focal contraction of the upper fibers of the superior constrictor muscle.
Abnormal palate/constrictor seal Weakness of the soft palate or superior constrictor muscle can result in nasopharyngeal regurgitation (Figures 8 and 9).
Figure 8: Nasopharyngeal regurgitation.
Contrast is seen in the nasopharynx (black arrows) posterior to the uvula. Contrast is retained in the valleculae and along the back of the tongue. Contrast is seen along the posterior surface of the epiglottis (white arrows) indicating that laryngeal penetration has occurred. Note also squaring of the valleculae and prevertebral soft tissue atrophy, signs of pharyngeal paresis. (Source: Jones B, Kramer SS, Donner MW. Dynamic imaging of the pharynx. Gastrointest Radiol 1985;10:213–224, with permission from the authors.)
Figure 9: Multiple abnormalities.
There is nasal regurgitation (straight arrow). The epiglottis has remained upright during bolus passage (curved arrow), but there is no laryngeal penetration as there is normal laryngeal elevation and closure. (Source: Jones B, Donner MW. The Pharynx In: Taveras JM, Ferrucci JT, eds. Rad-Diag-Imaging-Intervention. Philadelphia: Lippincott Williams & Wilkins, 1986, with permission.)
Compression and Propulsion of Bolus
Normal (Figures 3c, d and 4c-e) Bolus is propelled and compressed by a combination of pharyngeal contraction and tongue base retraction. The tongue base and constrictors should tightly appose, obliterating the pharyngeal cavity. The constrictor stripping wave can be observed in the lateral position as a progressive forward movement of the posterior pharyngeal wall and in the frontal position as the movement of the lateral walls of the pharynx completely to the midline.
Tongue base retraction should be completely backward to appose the converging pharyngeal wall.
Figure 10: Pharyngeal paresis.
Frontal view shows asymmetry of distention, with the left piriform sinus (ps) bulging much more than the right piriform sinus because of weakness of the constrictor muscles. A small amount of contrast medium is present in the laryngeal vestibule (arrow), and a moderate flow defect (arrowheads) can be seen. (Source: Gore RM, Levine MS, Laufer I, eds. Textbook of Gastrointestinal Radiology. Philadelphia: WB Saunders, 1994, with permission from Elsevier Science.)
Pharyngeal paresis/paralysis No movement of the posterior pharyngeal wall indicates paralysis; poor movement indicates paresis or weakness. Failure of the lateral pharyngeal walls to come to the midline indicates pharyngeal paresis. No movement toward the midline or bulging during swallow indicates paralysis.
Unilateral weakness results in asymmetry in the frontal view. The contracting normal side can push the bolus across the midline to the atonic side so the bolus passes down the paralyzed side. Without dynamic imaging, the contacting normal side may be misinterpreted as a mass, whereas the abnormality is actually on the noncontracting, bulging side.
It is not uncommon in neurologic diseases affecting the pharynx to see weakness of the superior and middle constrictor muscles with some residual constriction of the inferior constrictor muscles.77
Hyoid/Larynx/Epiglottic Tilt (Laryngeal Complex)
Normal (Figures 3c-f and 4c, d) This phase is discussed under two categories: hyoid elevation and epiglottic tilt.
Normal: Hyoid and laryngeal movement As the bolus enters the pharynx, the hyoid begins to elevate, moving upward and forward, apposing to the angle of the mandible. There is a correlation between the excursion of the hyoid bone and the volume of the bolus.78 A 2-mL bolus results in a mean elevation of 13.0 mm (females) and 13.5 mm (males), whereas a 10-mL bolus produces a 14.8-mm elevation (females) and 16.7 mm (males). The hyoid bone can elevate in one or two steps.79 Descent to the resting position occurs in a one-step fashion simultaneously with the upward tilting of the epiglottis to its resting position.
Normal epiglottic tilt Epiglottic tilt is a two-step movement in most people.
The initial movement, to the horizontal position is probably passive and related to hyoid elevation and tongue thrust. The second movement, to the completely inverted position, is an active one and is probably the result of contraction of the thyroepiglottic muscle.80
The completely inverted epiglottis looks like a seagull on the frontal view. A flow defect can occur quite commonly below the inverted epiglottis (Figure 11).
Figure 11: Normal epiglottic tilt.
The epiglottis itself can be seen as a "seagull" or inverted flat V (arrows). The tilted epiglottis has deflected the bolus into the lateral food channels, creating a filling defect or "pseudomass." (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 2003, with permission.)
Abnormal hyoid motion The hyoid may elevate incompletely or move not at all. It may elevate momentarily and not be sustained.
Aspiration can occur if there is failure of laryngeal protective mechanisms, which include laryngeal elevation, laryngeal closure, and epiglottic tilt. Laryngeal closure has at least three levels of defense: closure of arytenoids, closure of true vocal folds, and closure of the false vocal folds.
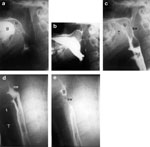
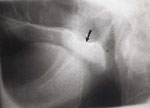
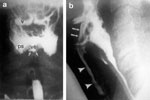
Laryngeal penetration is entry of the bolus into the laryngeal vestibule or lower in the larynx but not through the vocal folds (Figure 12). Aspiration occurs when contrast goes below the vocal folds (Figure 13).
Figure 12: Laryngeal penetration.
Curved collection of contrast is seen entering the incompletely closed vestibule of the larynx (arrow). Faint contrast is seen in the larynx and on the tracheal anterior wall caused by previous aspiration (arrowheads).
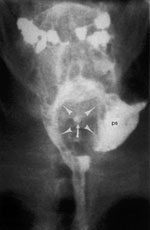
Figure 13: Aspiration.
a: Frontal view of the pharynx demonstrates aspiration of retained bolus. Note that there is retention of contrast in the valleculae (v) and piriform sinuses (ps). No swallow is taking place, yet there is entry of contrast into the laryngeal vestibule (ve) and between the vocal folds and in the ventricle (arrows). (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 2003, with permission.) b: A stop-frame print from a cinepharyngogram in the lateral position shows incomplete laryngeal closure during swallowing with laryngeal penetration (arrows) and aspiration (arrowheads) down into the trachea. The bolus is passing through the open cricopharyngeus into the cervical esophagus. Degenerative change is noted in the cervical spine. (Source: Jones B, ed. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy. New York: Springer-Verlag, 1991, with permission.)
Both penetration and aspiration can result in a reflexive cough (normal) or not produce a cough (i.e., be silent). Silent aspirators do not know they are aspirating, and clinical prediction of aspiration, especially in silent aspirators, is notoriously inaccurate.81, 82, 83, 84
Aspiration can occur before a swallow; a classical cause is premature leakage from the mouth. Aspiration can also occur during a swallow or after a swallow (overflow of retained bolus, reflux or regurgitation).
Cricopharyngeus
Normal (Figures 3d, e and 4d, e) The cricopharyngeus muscle is the horizontal lowermost portion of the inferior constrictor muscle and arises from the cricoid cartilage. There is a zone of sparse musculature immediately above (Killian's triangle) and below (area of Laimer) the cricopharyngeus, where there is the potential for the development of a diverticulum.
The upper esophageal sphincter (UES) is longer than the cricopharyngeus and consists of a zone of high pressure consisting of some fibers of the inferior constrictor muscle, the cricopharyngeal muscle, and some circular fibers of the proximal cervical esophagus.
Between swallows, the cricopharyngeus is closed, presumably to prevent overdistention of the esophagus during respiration. The cricopharyngeus must both relax and open completely to allow unimpeded passage of bolus from the pharynx into the cervical esophagus.
Several factors open the lumen at the level of the cricopharyngeus:
1. Cricopharyngeal relaxation
2. Anterior traction from superior and anterior movement of hyoid and larynx
3. A push from above from contraction of the pharyngeal constrictor muscles (and probably from tongue-base retraction also)
4. Pressures of the bolus itself
Thus, cricopharyngeal prominence can result from failure of relaxation (probably the least common cause), a frozen larynx, or pharyngeal weakness.
Radiographically the cricopharyngeus is located at the C5-6 disk level.
Abnormal85, 86, 87, 88, 89, 90 The cricopharyngeus should open completely to allow unimpaired passage of bolus into the cervical esophagus. The cricopharyngeus may open late or incompletely or close early, sometimes trapping fluid in the sphincter segment.
Radiographically, a posterior indentation at approximately the C5-6 or C6-7 level during bolus passage indicates the level of the cricopharyngeus (Figure 14).
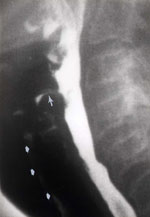
Figure 14: Multiple abnormalities.
Lateral radiograph demonstrates multiple abnormalities, including laryngeal penetration (black arrow), aspiration (white arrow), and cricopharyngeal prominence (arrowhead). Note the jet of contrast material below the prominent cricopharyngeus. (Source: Jones B, Kramer SS, Donner MW. Dynamic imaging of the pharynx. Gastrointest Radiol 1985;10:213–224, with permission from the authors.)
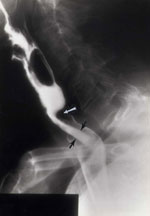
Significant luminal narrowing may produce severe dysphagia, especially with a "cricopharyngeal bar," a thumb-like indentation into the lumen from the posterior aspect (Figure 15). Cricopharyngeal bars may occur alone or in combination, for example, with Zenker's diverticulum. Cricopharyngeal prominence or bar may be seen in the elderly, as a secondary response to esophageal disease such as gastroesophageal reflux disease or spasm and, as discussed earlier, in association with pharyngeal paresis or a frozen larynx
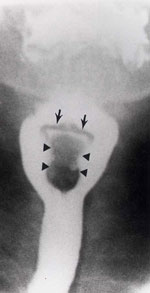
Figure 15: Cricopharyngeal bar with jet phenomenon.
There is a very prominent thumb-like indentation at the pharyngoesophageal junction (white arrow) representing a cricopharyngeal bar. A flow or "jet phenomenon" (black arrows) is seen below confirming luminal narrowing. (Source: Jones B, Kramer SS, Donner MW. Dynamic imaging of the pharynx. Gastrointest Radiol 1985;10:213–224, with permission from the authors.)
Conclusion
Videofluoroscopy and radiography of the mouth and pharynx can be extremely useful in the evaluation of functional and structural abnormalities. Careful attention to radiographic technique and the movement of structures and bolus results in a diagnosis of the normal and abnormal swallow.