The esophagus consists of two anatomically and physiologically distinct regions, namely a proximal striated muscle portion (cervical esophagus) and a distal smooth muscle portion (thoracic and abdominal esophagus). Diseases that affect these two regions are quite different.
Motor Disorders of the Esophageal Striated Muscle Segment
Diseases that affect the cervical esophagus (striated muscle) are essentially neuromuscular disorders that also affect the oropharynx. These diseases do not generally affect smooth muscle of the thoracic esophagus. Uncommonly, both striated and smooth muscles of the esophagus are affected simultaneously. These disorders include mixed connective tissue disease and amyloid deposition. By themselves, the motility disorders of the striated muscle segment are not of great clinical importance as their clinical presentation is overwhelmed by the symptoms caused by involvement of striated muscle of the oral cavity and pharynx.
Motor Disorders of the Esophageal Smooth Muscle Segment
Classification
Motor disorders of the smooth muscle esophagus have been classified in many different ways, and there is no single classification that is fully satisfactory for all users. One of the proposed classifications of the esophageal motility disorders is based on the presence of hypo- or hypermotility. This classification, however, does not facilitate pathophysiologic understanding of the disorders or provide a functional approach to these disorders. Motility disorders may be classified based on major symptom, clinical syndrome, esophageal motility findings, esophageal bolus transport, pathophysiology, or the anatomic site of major involvement.
From an anatomic perspective, motor disorders of the esophageal smooth muscle may be classified as those involving the esophageal body or the lower esophageal sphincter (LES) and those involving the longitudinal muscle.
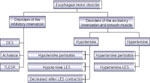
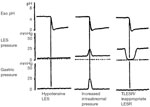
Pathophysiologic classification of esophageal motility disorders is presented in Figure 1. Based on the type of innervation affected, they can be broadly classified into disorders of inhibitory (nitrergic) or excitatory (cholinergic and noncholinergic) innervation. Disorders of inhibitory innervation may be those associated with reduced function or increased function. Disorders of decreased inhibitory nerve function include such conditions as diffuse esophageal spasm when the esophageal body is primarily involved, and achalasia when LES and esophageal body are both involved. Increased function of the nitrergic inhibitory nerves is manifested by transient lower esophageal sphincter relaxation (TLESR) and LES hypotension. Both of these abnormalities are associated with gastroesophageal reflux disease (GERD). Disorders of the excitatory innervation may also include those associated with increased or decreased function. Manifestations of decreased excitatory nerve function are similar to those of decreased myogenic function. They include hypotensive peristalsis in the esophagus and hypotensive LES and LES with poor reflex contraction. All these abnormalities are associated with GERD. Increased function of the excitatory nerves may be responsible for hypertensive esophageal peristaltic contractions (nutcracker esophagus) or hypertensive and hypercontracting LES.
Manometrically, either the thoracic esophagus or the LES may show abnormalities but often both are simultaneously involved. In the esophageal body, there may be changes in the resting (basal) or the swallow evoked activity. In the basal state resting pressure may be elevated or there may be spontaneous contractions. Esophageal contraction to swallows may be absent, peristaltic, or nonperistaltic, and their amplitude may be normotensive, hypotensive, or hypertensive. Similarly, the basal tone in the LES may be normotensive, hypertensive, or hypotensive. Lower esophageal sphincter relaxation to swallowing may be normal or impaired, and its rebound contraction may be exaggerated.. Manometric tracings of the main motility disorders are shown in Figure 2. The frequency of TLESR that occurs inappropriately without an associated swallow requires long-term study and cannot be identified by the usual esophageal motility studies. Similarly, contractions of the esophageal longitudinal muscle cannot be investigated by intraluminal manometry.
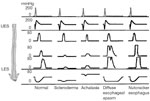
Figure 2: Motility patterns in esophageal smooth muscle disorders.
In normal individuals, the upper and lower esophageal sphincters (UES and LES) appear as zones of high pressure. With a swallow (indicated by pharyngeal contractions), pressure in the sphincters falls and a contraction wave that starts in the pharynx progresses down the esophagus. In scleroderma, the esophageal contractions as well as LES are hypotensive. In contrast in achalasia, the LES is hypertensive and fails to relax with swallows; esophageal contractions are nonperistaltic. In diffuse esophageal spasm, a large number of esophageal contractions are nonperistaltic; the amplitude of the contractions may be increased, normal or decreased. In nutcracker esophagus, the esophageal contractions are peristaltic but of large amplitude. (Source: Modified from Goyal RK. Harrison's Principles of Internal Medicine. New York, McGraw Hill, 2005. pp 1739–1745.)
Functionally, smooth muscle esophageal motor disorders may be broadly classified into three groups: poor esophageal transit, gastroesophageal reflux, and normal transit without reflux. Achalasia, diffuse esophageal spasm, and hypotensive esophageal peristalsis are associated with ineffective esophageal transport. Hypertensive peristalsis, hypertensive LES, and hypercontracting LES have normal esophageal transit. Gastroesophageal reflux (GER) or retrograde flow of gastric contents occurs when LES is hypotensive or when it shows increased frequency of TLESR. Esophageal transport abnormalities can be investigated by esophageal impedance studies. When ineffective esophageal transport is associated with GER, severe esophageal mucosal damage may occur.
Clinically, ineffective esophageal transport is associated with symptom of dysphagia. The dysphagia is primarily to solids, but in the recumbent posture when the effect of gravity is neutralized, dysphagia to liquids may also occur. However, nonperistaltic esophageal contractions and impaired LES relaxation typically cause dysphagia to both solids and liquids.
Table 1 shows correlation between various parameters in esophageal motility disorders. Another consideration that may be useful in clinical practice is whether the esophageal motor abnormality is part of a generalized systemic disease or is a localized esophageal disease. The word secondary is applied to esophageal disorders in systemic disorders such as connective tissue disease, diabetes, dermatomyositis, amyloidosis, and Chagas' disease. The words primary or idiopathic and secondary are also applied to indicate whether the etiology of the disorder is understood. Disorders that selectively affect the esophageal smooth muscle segment may be of unknown etiology, that is, idiopathic, or secondary to a known cause. Often the diseases that are specifically localized to the esophagus have no known etiology and are therefore called idiopathic or primary. Snap shots of radiologic appearances for a number of motor disorders are shown in Figure 3 and they are linked to respective videos.
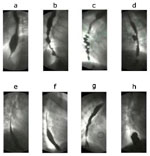
Figure 3: Radiologic appearance of some motor disorders of smooth muscle portion of the esophagus.
The figures are stills from the linked videos. a; Classical achalasia, showing a dilated esophageal body bird beak-like narrowing of lower esophageal sphincter (see Video 1). b; Vigorous achalasia showing diffuse spasm-like contractions in the esophageal body with closed lower esophageal sphincter (see Video 2). c; Diffuse esophageal spasm shows typical corkscrew appearance of the lower part of the esophagus (see Video 3). d: Midesophageal propulsion diverticulum with esophageal motility disorder (see Video 5). e; Normal peristaltic sequence for comparison with slow esophageal transit (see Video 6). f; Hypotensive (incompetent) esophageal peristalsis with slow esophageal transit through the esophagus (see Video 7). g; Gastroesophageal reflux (see Video 8). h; A sliding hiatal hernia (see Video 9). (Courtesy of Dr F.I. Habib)
Specific Esophageal Motor Disorders
Achalasia
Definition Achalasia is a motor disorder involving the lower two thirds (smooth muscle segment) of the esophagus, which is caused by degeneration of intramural myenteric plexus neurons. This results in impaired LES relaxation and loss of peristaltic sequencing of the esophageal contractions, producing symptoms of dysphagia, chest pain, and regurgitation.
Pathology In advanced disease, the esophagus is grosssly dilated and filled with food residues. The esophageal wall is thickened and the LES area is contracted (Figure 4). Esophageal myenteric plexus shows loss of myenteric neurons. In early stages of the disease the inhibitory neurons containing vasoactive intestinal peptide (VIP) and nitric oxide synthase (NOS) are predominantly lost, but in advanced disease excitatory neurons containing acetylcholine may also be lost.
Figure 4: Gross appearance of esophagus in classical achalasia.
(Source: Netter Image with permission from Elsevier Science)
In patients with primary achalasia, a uniform finding is an inflammatory response with associated loss of ganglion cells within the LES and esophageal body myenteric plexus. The inflammatory infiltrate consists primarily of T-cell lymphocytes. The inhibitory neurons, which contain nitric oxide (NO) and VIP, are slowly destroyed as part of the inflammatory reaction. In addition to this inflammatory myenteric plexopathy, degenerative changes in vagal trunk fibers and cell bodies in the dorsal motor nucleus of the vagus have also been described. These changes are inconsistent and most likely secondary to the enteric nervous system disease. Muscular hypertrophy with variable degrees of muscular degeneration has also been described. These too are likely secondary to the primary myenteric plexus disease. The muscle hypertrophy may due to longstanding functional obstruction at the LES. Alternatively, there is evidence that denervation per se can lead to muscle hypertrophy. The mucosa is also often abnormal in patients with achalasia. Squamous hyperplasia with nonspecific minor inflammation may be present. These mucosal changes are thought to be due to luminal stasis.
Etiology Primary achalasia is also called idiopathic achalasia because the cause is unknown. An autoimmune cause has been proposed because the predominant cell type of the inflammatory infiltrate in the myenteric plexus is the T lymphocyte, which is known to be involved in other autoimmune diseases. There is also a high prevalence of certain class II histocompatibility antigens in patients with achalasia, which are known to be associated with other autoimmune disorders. These include a higher than expected prevalence of DQW1 antigen and association with human leukocyte antigens (HLAs) DQA1*0101 and DQB1*0602. In addition, autoantibodies to myenteric plexus neurons are found in many achalasia patients.
Indirect evidence suggests that the initiating agent(s) in idiopathic achalasia may be viral. Elevated antibody titers to measles and varicella zoster have been described in a high proportion of achalasia patients. In addition, investigators have described varicella zoster DNA in esophageal tissue in a subset of achalasia patients. Importantly, these abnormalities are not uniform in patients with idiopathic achalasia.
A number of other diseases can cause so-called secondary achalasia (Table 2). One of the most common is cancer in the proximal stomach that may directly infiltrate and destroy esophageal myenteric neurons. A number of other malignancies have been described as a cause of secondary achalasia. They appear to produce immune-mediated myenteric neural damage as part of a paraneoplastic syndrome.
Chagas' disease also produces achalasia. This is due to infection with the hemoflagellate protozoa Trypanosoma cruzi. The immunologic reaction to myenteric nerve neurons infected with this parasite leads to loss of esophageal and LES intramural ganglia. Unlike primary or idiopathic achalasia, in Chagas' disease other regions of the proximal gastrointestinal (GI) tract (e.g., duodenum, gallbladder) are also involved.
Rarely, secondary achalasia can be seen in the neuropathic type of chronic intestinal pseudo-obstruction, eosinophilic gastroenteritis, amyloidosis, multiple endocrine neoplasia type II, neurofibromatosis, sarcoidosis, and Anderson-Fabrey disease. Finally, achalasia in children may be part of the so-called AAA syndrome (achalasia, alacrima, and achlorhydria).
Pseudoachalasia is a term used for lesions that cause narrowing of the esophagogastric junction (EGJ) and dilation of the esophagus resembling achalasia, particlarly on a barium swallow. However, the lesions lack the underlying pathophysiology of achalasia, and esophageal manometry does not support the diagnosis of achalasia. Pseudoachalasia may occur with a pancreatic pseudocyst around the distal esophagus, some tumors, and hematoma. Many cases that are diagnosed as postvagotomy achalasia may indeed be cases of peudoachalasia.
Pathophysiology The motor abnormality in achalasia is the result of defective function of the intramural myenteric plexus motor neurons. Functionally, the intramural motor neurons are of two types: inhibitory and excitatory. The inhibitory motor neurons relax esophageal smooth muscle by releasing NO and VIP. The excitatory motor neurons contract esophageal smooth muscle by releasing acetylcholine and substance P.
Both primary and secondary peristalsis are impaired in achalasia. The inhibitory innervation is crucial in generating an initial wave of inhibition in the circular smooth muscle of the esophagus and LES, which not only produces relaxation of the LES, but is responsible for the peristaltic sequencing of the esophageal contractions. In animal models as well as humans, drugs that interfere with NO, the primary inhibitory neurotransmitter in the esophagus, produce motility changes that mimic achalasia. Furthermore, LES muscle strips from achalasia patients contract, rather than relax, when the intramural nerves are stimulated.
Epidemiology Achalasia affects patients of all ages and both sexes. It is an uncommon disorder with an annual incidence of 1 case per 100,000. In most series, achalasia is diagnosed in about 5% of patients undergoing manometry. Achalasia is usually recognized in patients between the ages of 25 and 60 years but may be seen at any age.
Symptoms The predominant symptom of achalasia is dysphagia, but chest pain and regurgitation of food or mucoid material are also common. Patient may also report an inabilty to belch. Weight loss may occur, but is usually modest. Rarely, patients develop aspiration pneumonia.
Dysphagia appears early, occurs with both liquids and solids, and is worsened by emotional stress and hurried eating. Dysphagia to liquids as an early manifestation is characteristic. Various maneuvers designed to increase intraesophageal pressure, including the Valsalva maneuver or extending the thorax, may aid the passage of the bolus into the stomach.
Regurgitation occurs because of retention of large volumes of saliva and ingested food in the esophagus. Characteristically, this occurs when the patient assumes the recumbent position at bedtime. Infrequently, regurgitation is complicated by aspiration.
Chest pain is usually described as a pressure or squeezing sensation, and may be confused with the pain of ischemic heart disease. The presence of GER argues against achalasia. However, heartburn-like chest pain is reported by a substantial number of achalasia patients. It is believed that this may relate to formation of lactic acid from fermentation of residual food debris in the esophageal lumen.
Mild weight loss is common. If profound weight loss occurs, one should suspect psedoachalasia secondary to carcinoma. The course is usually chronic, with progressive dysphagia and weight loss over months to years. In fact, patients usually have had gradually progressive symptoms for several years before being diagnosed.
Physical findings Infrequently, patients may have physical findings of weight loss or respiratory complications, but in general there are no abnormalities on physical examination in patients with primary achalasia. Patients with secondary achalasia may have physical findings related to the underlying disease.
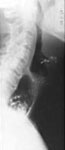
Laboratory findings Chest x-ray may show absence of the gastric air bubble and sometimes a tubular mediastinal mass beside the aorta. An air-fluid level in the mediastinum in the upright position represents retained food and fluid in the esophagus (Figure 5). Air in the distended cervical esophagus may be seen to push the contracted cricopharyngeus upward (Figure 6).
Figure 5: Chest film of a patient with longstanding achalasia.
a; anterior-posterior view showing mediastinal shadow on the right side due to dilated esophagus. b; lateral view. Note that the dilated fluid-filled esophagus displaces the tracheobronchial tree anteriorly and presents a posterior mediastinal mass superimposed over the right heart. (Courtesy of Dr F.I. Habib)
Figure 6: Cricopharyngeal contraction in achalasia.
Laterolateral film of the cervical spine showing air-fluid-solid level in the esophagus pushing the closed cricopharyngeus upward. (Courtesy of Dr F.I. Habib)
Barium swallow usually shows esophageal dilation, and in advanced cases the distal esophagus may become massively dilated. In classical achalasia, terminal part of the esophagus shows a persistent beak-like narrowing representing the nonrelaxing LES (Video 1). On fluoroscopy, normal peristalsis is lost in the lower two thirds of the esophagus. In up to 20% of achalasia patients, however, these classic x-ray findings are not present. In so called vigorous achalasia prominent diffuse esophageal spasm-like contractions in esophagus with non-relaxing LES are seen (Video 2)
Video 1: Classic achalasia.
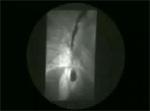
In classic achalasia, the esophagus is dilated with poor contractions. The lower esophageal sphincter remains contracted producing a bird beak appearance. Achalasia must be differentiated from other cases of narrowing at the lower end of the esophagus, like carcinoma of the esophagogastric junction and peptic strictures with scleroderma.
Video 2: Vigorous achalsia.
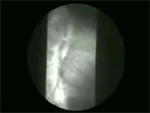
In patients with vigorous achalasia, repetitive non-peristaltic contractions are observed along with non-relaxing lower esophageal sphincter.
Computed tomography (CT) scan of the thorax often demonstrates a dilated fluid/food-filled esophagus with thickening of the esophageal wall.
Esophageal manometry classically reveals an elevated (hypertensive) basal LES pressure, but in many patients LES pressure may be normal (normotensive), or rarely reduced (hypotensive). Swallow-induced LES relaxation is either absent or is reduced in degree, duration, and consistency. Resting pressure within the esophageal body is often elevated. In response to swallows, peristalsis in the cervical (striated muscle) esophagus is normal, whereas peristalsis in the thoracic (smooth muscle) esophagus is replaced by nonperistaltic simultaneous-onset pressure elevations. In advanced achalasia with esophageal dilation, very low amplitude simultaneous pressure elevations are seen. These represent "common cavity" pressure transients caused by the bolus entering the esophagus, which is closed by the nonrelaxing LES, and not actual muscle contractions. However, in less advanced disease true nonperistaltic contractions occur, which may be of low amplitude (classic achalasia) or of large amplitude and duration (vigorous achalasia).
Injection of cholecystokinin (CCK), which normally causes a fall in LES pressure, causes paradoxical contraction of the LES (the CCK test). This paradoxical response occurs because, in achalasia, the neurally transmitted inhibitory effect of CCK is absent owing to the loss of inhibitory neurons. CCK can then directly stimulate the muscle leading to contraction.
Amyl nitrate, which is a potent relaxer of smooth muscle, has been used to help differentiate primary from secondary achalasia; when administered during manometry to patients with primary but not secondary achalasia, the LES pressure rapidly falls.
Endoscopy is helpful in excluding secondary causes of achalasia, particularly carcinoma of the gastric cardia. The esophageal lumen is often filled with saliva or food debris, and the region of the EGJ is closed as the endoscope approaches. However, the EGJ will then pop open when gentle pressure is applied, allowing the scope to advance to the stomach without resistance. Secondary achalasia, owing to a neoplasm in the cardia, should be suspected if significant resistance to scope passage is encountered.
Diagnosis The diagnosis of achalasia should be suspected in patients with symptoms of dysphagia to both solids and liquids, regurgitation of tasteless mucoid secretion or food eaten several hours earlier, and chest pain. In early cases all these symptoms may not be present together. Diagnosis is confirmed by a charcteristic barium swallow and esophageal motility findings. Manometric abnormalities in various disorders of esophageal motility are summarized in Table 3.
Differential diagnosis Infrequently, the symptoms of achalasia may be confused with those of GERD, especially if the patient describes chest pain of a burning quality. However, a careful history should help differentiate the two conditions. It is particularly important to clarify the nature of the patient's regurgitation, which arises from the stomach in acid reflux disease and the esophagus in achalasia. If there is any doubt, the barium x-ray findings, endoscopy, and manometry will clearly differentiate the two disorders. Other structural causes of dysphagia may also occasionally be confused with achalasia, but usually the history of intermittent dysphagia to both liquids and solids points to motility disorder as the cause. The barium x-ray findings often help differentiate achalasia from other motor disorders, but in up to 20% of patients barium x-ray does not show characteristic features. When scleroderma is complicated by peptic stricture, the barium x-ray findings can sometimes be confused with achalasia, but the history, physical exam, and endoscopic findings readily distinguish between these two conditions.
Once a diagnosis of achalasia is made, it is necessary to distinguish primary (idiopathic) achalasia from secondary (pseudo-) achalasia. Achalasia secondary to cancer at the EGJ needs to be especially considered in patients with clinical evidence of achalasia who present with recent onset and rapidly progressive dysphagia associated with marked weight loss. Careful endoscopic examination of the cardia using the retroflexed view is essential to exclude neoplasms of the cardia. In some cases CT scanning or endoscopic ultrasound may also be required.
Clinical course and complications Persistent stasis of food in the esophagus may lead to pulmonary aspiration. Nocturnal coughing spells are reported in almost 30% of the patients, and some may also develop aspiration pneumonia that is usually mild but may be severe.
The early literature suggested that esophageal squamous cell carcinoma developed in up to 7% of achalasia patients, particularly in the untreated cases. More recent series suggest that the risk is actually <2%.
Treatment Treatment is directed at decreasing the resting pressure of the nonrelaxing LES. A number of drugs are capable of doing this, including anticholinergics, nitrates, calcium channel blockers,  -blockers, and phosphodiesterase inhibitors. The anticholinergic cimetropium bromide markedly decreases resting LES tone and improves esophageal emptying in achalasia patients, but has yet to be studied in a controlled clinical trial. Isosorbide dinitrate, 2.5 to 5 mg sublingually or 10 to 20 mg orally, 20 to 30 minutes before meals may improve dysphagia. Nitroglycerin, 0.3 to 0.6 mg taken sublingually or as an oral spray just prior to meals, may also be beneficial. This may also help when taken on an as-needed basis for attacks of chest pain in achalasia patients. Unfortunately, nitrates in doses capable of relaxing the LES often cause headache and postural hypotension. Nifedipine is the calcium channel blocker with the most potency in inducing LES relaxation, and has been shown to improve dysphagia in achalasia patients in controlled clinical trials. Doses of 10 to 30 mg taken 20 to 30 minutes prior to meals are recommended. It is best to have the patient puncture the capsule and then swallow the liquid to obviate the possibility that the pill will remain stuck in the esophagus. Unfortunately, the majority of patients do not respond adequately to such agents. Not only are side effects common, but many of the drugs used do not relax the LES sufficiently to improve bolus transport. Furthermore, if they are not taken at the appropriate time before meals, their effects may have either dissipated or not yet taken effect when the patient begins eating.
-blockers, and phosphodiesterase inhibitors. The anticholinergic cimetropium bromide markedly decreases resting LES tone and improves esophageal emptying in achalasia patients, but has yet to be studied in a controlled clinical trial. Isosorbide dinitrate, 2.5 to 5 mg sublingually or 10 to 20 mg orally, 20 to 30 minutes before meals may improve dysphagia. Nitroglycerin, 0.3 to 0.6 mg taken sublingually or as an oral spray just prior to meals, may also be beneficial. This may also help when taken on an as-needed basis for attacks of chest pain in achalasia patients. Unfortunately, nitrates in doses capable of relaxing the LES often cause headache and postural hypotension. Nifedipine is the calcium channel blocker with the most potency in inducing LES relaxation, and has been shown to improve dysphagia in achalasia patients in controlled clinical trials. Doses of 10 to 30 mg taken 20 to 30 minutes prior to meals are recommended. It is best to have the patient puncture the capsule and then swallow the liquid to obviate the possibility that the pill will remain stuck in the esophagus. Unfortunately, the majority of patients do not respond adequately to such agents. Not only are side effects common, but many of the drugs used do not relax the LES sufficiently to improve bolus transport. Furthermore, if they are not taken at the appropriate time before meals, their effects may have either dissipated or not yet taken effect when the patient begins eating.
Balloon dilatation reduces the basal LES pressure by tearing muscle fibers. In experienced hands, this technique is effective in 60% to 90% of patients, although it often has to be repeated with progressively larger diameter balloons to achieve the highest success rate. Perforation is the major complication of this procedure, occurring in 2% to 5%. Bleeding may also occur but is rarely clinically significant.
Endoscopic intrasphincteric injection of botulinum toxin is effective over a short period in some patients. Although the rationale for this treatment was to block cholinergic excitatory nerves in the LES and therefore decrease resting LES pressure, the pressure decline in patients responding to this therapy is often minimal, raising the possibility that its effect involves some other mechanism(s). Most series report response rates of 60% to 70% with a median response duration of approximately 10 months. Repeat injections are therefore required, which can become quite costly. Patients with successful response to botulinum injection have been reported to have fibrotic changes develop on the outer wall of the esophagus, which may make subsequent surgical therapy more difficult. Most experts confine the use of botulinum toxin to achalasia patients who are not good candidates for more invasive therapies because of major comorbidities.
When performed by an experienced surgeon, extramucosal myotomy of the LES ("Heller" myotomy), in which the muscularis propria is incised, is effective in alleviating dysphagia in  90% of patients. The laparoscopic approach to this surgery is now preferred. It is important to extend the myotomy down onto the anterior wall of the proximal stomach to ensure that the sphincter muscles are completey incised. Many surgeons recommend performing a partial fundoplication with the myotomy in order to minimize the chance of postoperative GERD. Despite this, reflux esophagitis and peptic stricture may still follow successful treatment (more often with myotomy than with balloon dilatation). Rarely, esophagectomy with gastric pull-up or colonic interposition is performed in patients with a very dilated, sigmoid-shaped esophagus and persisting severe symptoms despite adequate ablation of the LES.
90% of patients. The laparoscopic approach to this surgery is now preferred. It is important to extend the myotomy down onto the anterior wall of the proximal stomach to ensure that the sphincter muscles are completey incised. Many surgeons recommend performing a partial fundoplication with the myotomy in order to minimize the chance of postoperative GERD. Despite this, reflux esophagitis and peptic stricture may still follow successful treatment (more often with myotomy than with balloon dilatation). Rarely, esophagectomy with gastric pull-up or colonic interposition is performed in patients with a very dilated, sigmoid-shaped esophagus and persisting severe symptoms despite adequate ablation of the LES.
Diffuse Esophageal Spasm
Definition Diffuse esophageal spasm is a disorder of inhibitory nerves that causes impaired deglutitive inhibition leading to nonperistaltic simultaneous onset contractions in the smooth muscle portion of the esophagus. The esophageal contractions may occur spontaneously or in response to swallowing. The swallow-induced contractions may be normotensive, hypotensive, or hypertensive. Lower esophageal sphincter relaxation is usually normal, although minor degrees of LES relaxation dysfunction may be present.
Pathology Little is known about the pathology of diffuse esophageal spasm. The few histopathologic studies available report patchy neural degeneration localized to nerve processes, rather than the prominent degeneration of nerve cell bodies seen in achalasia. Hypertrophy of the muscularis propria is also common. Distal esophageal diverticuli may also develop. These are called pulsion diverticuli because they form owing to marked increases in intraluminal pressure caused by frequent simultaneous contractions.
Etiology The etiology of diffuse esophageal spasm is not known. Because this disorder may progress to classic achalasia, many believe that in at least a subset of patients it also represents the early stage of a myenteric plexus denervating disease.
Pathophysiology Nonperistaltic contractions are caused by dysfunction of inhibitory nerves. Indeed, studies using a partially inflated intraesophageal balloon have shown that the normal wave of inhibition that precedes the peristaltic contraction is lost in patients with diffuse esophageal spasm. Hypertensive peristaltic contractions and hypertensive or hypercontracting LES may represent associated cholinergic or myogenic hyperactivity.
Epidemiology Diffuse esophageal spasm is quite uncommon. When strict manometric criteria are used for diagnosis, it occurs in only 3% to 5% of patients undergoing manometry for suspected eophageal motor disorders.
Symptoms Patients with diffuse esophageal spasm present with chest pain and dysphagia. Regurgitation may also occur but is much less common than in achalasia. Chest pain is particularly marked in patients with esophageal contractions of large amplitude and long duration. Chest pain usually occurs at rest but may be brought on by swallowing or by emotional stress. The pain is retrosternal and may radiate to the back, the sides of the chest, both arms, or the jaw. It may last from a few seconds to several minutes. The pain may be acute and severe, mimicking the pain of myocardial ischemia. Dysphagia for solids and liquids may occur with or without chest pain and is correlated particularly with simultaneous-onset contractions.
Physical findings There are no physical findings specific for diffuse esophageal spasm.
Laboratory findings Barium swallow shows that normal sequential peristalsis below the aortic arch is replaced by uncoordinated simultaneous contractions that produce the appearance of curling or multiple ripples in the wall, sacculations, and pseudodiverticula—the "corkscrew" esophagus (Video 3). Sometimes only non-peristaltic contractions or contractions that obliterate the lumen, pushing barium away in the two directions, are seen (Video 4). Some cases show esophageal diverticulum formation (Video 5). The barium swallow may be normal in diffuse esophageal spasm because many swallows result in normal peristaltic activity.
Video 3: Corkscrew.

Simultaneous contractions that affect the smooth muscle portion of the esophagus sometimes produce the typical "corkscrew" appearance.
Video 4: Non-peristaltic contractions.
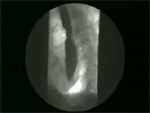
Non-peristaltic contractions are defined as simultaneous contractions that compartmentalize the esophageal lumen.
Video 5: Mid-esophageal diverticulum.
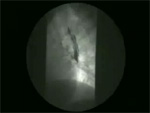
Mid-esophageal diverticula are frequently associated with diffuse esophageal spasm or vigorous achalasia.
Esophageal manometry shows spontaneous and swallow-induced contractions that are nonperistaltic in the smooth muscle portion (thoracic) esophagus. However, because these abnormalities may be episodic, the results of manometry may be normal at the time of the study. Several techniques are used to provoke esophageal spasm. Cold swallows may produce the chest pain but do not produce spasm on manometric studies. Solid boluses and pharmacologic agents, particularly edrophonium, induce both chest pain and motor abnormalities. However, correlation between induction of pain and motility changes is poor. The usefulness of pharmacologic provocative tests is limited.
Diagnosis The diagnosis should be suspected in someone with dysphagia and angina-like chest pain, especially when the dysphagia has features of a motility disorder (see above). A barium swallow x-ray showing a "corkscrew" pattern is strongly supportive of the diagnosis. Confirmation rests with esophageal manometry, which is characterized by >20% of wet swallows deteriorating into simultaneous onset contractions in the distal smooth muscle esophagus. These contractions may also be high amplitude, long duration, or multipeaked.
Differential diagnosis Diffuse esophageal spasm and related esophageal motor disorders must be differentiated from other causes of chest pain, particularly ischemic heart disease with atypical angina. A cardiac workup may be required before a noncardiac etiology is considered seriously. The presence of dysphagia in association with pain should point to the esophagus as the site of disease. Esophageal motility disorders are an uncommon cause of noncardiac chest pain, which is more commonly caused by reflux esophagitis or visceral hypersensitivity.
Clinical course and complications Patients typically have intermittent symptoms for years without progression. In fact, the frequency and severity of symptoms may decrease over time, perhaps because the patient adapts in some way to the abnormal motility. In a small subset of diffuse esophageal spasm patients (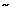 5%), vigorous or classic achalasia develops. This should be suspected if the patient develops esophageal regurgitation with worsening dysphagia.
5%), vigorous or classic achalasia develops. This should be suspected if the patient develops esophageal regurgitation with worsening dysphagia.
Treatment There are no controlled clinical trials demonstrating the efficacy of any treatment for the dysphagia associated with diffuse esophageal spasm. Open-label studies suggest that smooth muscle relaxants or anticholinergic agents used as described above for achalasia may be helpful. Many clinicians advocate empiric bougienage, as patients will often report improvement of their dysphagia for a variable period of time after this is performed. However, a study comparing bougienage with a large caliber versus a very small caliber bougie, found no difference in response rate, suggesting that any improvement may be due to the placebo effect. Similarly, there have been recent uncontrolled studies suggesting that botulinum toxin injected either into the LES or at intervals along the esophageal body may improve dysphagia and chest pain in patients with diffuse esophageal spasm.
Chest pain associated with diffuse spasm may also respond to smooth muscle relaxants, but current evidence suggests that low-dose tricyclic antidepressant therapy is a better option.
Finally, long esophageal myotomy is rarely used to treat patients with intractable dysphagia and chest pain owing to diffuse esophageal spasm, particularly if there are associated pulsion diverticula.
Hypertensive Peristalsis, Isolated LES Hypertension, and Hypercontracting LES
Definition Hypertensive peristaltis (also termed "nutcracker esophagus"), isolated LES hypertension, and hypercontracting LES are manometric diagnoses of uncertain clinical importance. These manometric abnormalities are seen in some patients with chest pain and dysphagia.
Pathology The pathology is not known. Specimens are rarely available for pathologic study. A single report describes loss of intramural neurons in the LES of patients with isolated LES hypertension; however, the methodology for identifying myenteric neurons in this study was suboptimal.
Etiology The etiology is not known.
Pathophysiology The pathophysiology of these disorders is unclear. Because the latency gradient and peristalsis are preserved, it is unlikely that these disorders entail a loss of inhibitory (nitrergic) innervation. The most likely cause of these abnormalities is overactivity of the excitatory nerves or overreactivity of the smooth muscle response to the excitatory nerves. This is evidenced by the fact that muscarinic agonists or cholinesterase inhibitors produce high-amplitude peristaltic contractions in the smooth muscle of the esophagus. Central nervous system stress can induce hypertensive contractions in the esophagus; therefore, it is possible that augmented excitatory innervation via the central nervous system may play an important role in many patients. It should be emphasized that both hypertensive peristaltis and isolated LES hypertension are manometric abnormalities and not clinical entities with predictable functional sequelae. Indeed, these manometric abnormalies may be transient phenomena related to stress (including the stress associated with esophageal intubation). Isolated hypertensive LES and hypercontracting LES may have similar pathophysiology as the hypertensive peristalsis.
Symptoms When hypertensive peristalsis was first described, it generated considerable interest because it was the most common manometric abnormality found in patients with so-called noncardiac chest pain. However, it subsequently became clear that the increased contraction amplitude seen in hypertensive peristalsis usually did not correlate with pain episodes. Only rarely does chest pain coincide with hypertensive peristalsis, particularly if the contractions are markedly prolonged. It is believed the hypertensive contractions are likely an epiphenomenon, or perhaps a marker for hypersensitivity or a hyperreactive esophagus.
Theoretically, dysphagia for solids or liquids should not occur in patients with hypertensive peristalsis or isolated LES hypertension. However, dysphagia is sometimes reported by patients in whom this manometric abnormality is present. Why patients with normal peristaltic sequences would experience dysphagia is unclear. Impedence studies have shown that esophageal transit in patients with hypertensive peristalsis is generally normal. However, some studies using prolonged ambulatory manometry have found that certain patients with hypertensive peristalsis have nonperistaltic contractions during mealtime, even though only hypertensive peristaltic sequences are seen during a standard baseline manometry with wet swallows. Similarily, it is possible that some patients with isolated LES hypertension and hypercontracting LES have intermittent abnormalities of LES relaxation.
Physical findings There are no physical findings specific for these esophageal motility disorders.
Diagnosis Barium swallow shows normal sequential peristalsis and no evidence of structural esophageal disease. Esophagoscopy is also normal. The diagnosis is based on abnormalities detected during esophageal manometry. Hypertensive peristalsis is characterized by normal sequential (i.e., peristaltic) contractions in the smooth muscle esophagus of excessive amplitude or duration. The upper limits of normal for contraction amplitude and duration in the distal esophagus are <180 mmHg and <7.5 seconds, respectively; therefore, hypertensive peristalsis is typically diagnosed when contractions fall outside this range. Similarly, isolated LES hypertension is characterized by a resting LES pressure of >40 mmHg above intragastric pressure. Hypercontracting LES shows prolonged and high amplitude post-relaxation contractions.
Differential diagnosis As discussed above, ischemic heart disease must be excluded in patients presenting with chest pain. Gastroesophageal reflux is a common cause of atypical chest pain and should be excluded with either appropriate investigation or an empiric trial of proton pump inhibitor therapy. Other causes of such chest pain include chest wall origin and panic attacks.
Clinical course and complications The clinical course in these patients is highly variable. In some, symptoms are present for a few months and then inexplicably resolve. In others, symptoms persist for years and may markedly impair quality of life. Psychosocial factors may impact the clinical course.
Treatment Anticholinergics and smooth muscle relaxants are often used but are of unproven value. Controlled clinical trials have shown that low-dose tricyclic antidepressants improve the chest pain in these patients, presumably because of their ability to modulate visceral pain thresholds. Cognitive behavioral therapy has also been shown to help some patients.
Hypotensive Esophageal Contractions
Definition Hypotensive peristaltic contractions are low-amplitude (<30 mmHg) peristaltic contractions detected on esophageal motility in response to a wet swallow. They are associated with ineffective esophageal transit (Figure 7). In patients with dysphagia or GERD they are increased in frequency ( 30%). Often, the hypotensive peristaltic contractions are associated with hypotensive nonperistaltic contractions. By combining manometry with either videofluoroscopy or impedance recording, it has been shown that with contraction amplitudes of <30 mmHg, bolus transport, as well as clearance of any refluxed contents, is often impaired. Hypotensive esophageal contractions have been called 'ineffective esophageal motility'. However, this term appears to be too broad to be useful.
30%). Often, the hypotensive peristaltic contractions are associated with hypotensive nonperistaltic contractions. By combining manometry with either videofluoroscopy or impedance recording, it has been shown that with contraction amplitudes of <30 mmHg, bolus transport, as well as clearance of any refluxed contents, is often impaired. Hypotensive esophageal contractions have been called 'ineffective esophageal motility'. However, this term appears to be too broad to be useful.
Figure 7: Illustration of hypotensive (incompetent) peristaltic contractions.
These are associated with escape of barium bolus into the proximal esophagus due to a weak peristaltic contraction wave. It shows a simultaneous manometry and fluoroscopy of a barium swallow ( Source: Adapted from Kahrilas PJ, Dodds, WJ, Hogan WJ. Gastroenterology 1988;94:73)
Pathology The pathology of hypotensive esophageal contractions is not known.
Etiology In most cases, etiology of the hypotensive esophageal contractions remains unknown and therefore may be classified as idiopathic or primary. Whether it is a result of or the cause of reflux esophagitis is not known. Secondary causes of hypotensive esophageal contractions include the use of drugs with anticholinergic effects, collagen vascular disease including scleroderma, and infiltrative diseases such as amyloidosis.
Pathophysiology The pathophysiology of hypotensive peristaltic or nonperistaltic contractions may involve suppression of cholinergic excitatory activity or impaired force of the circular muscle contraction. A common cause of hypotensive esophageal contractions is the use of anticholinergic agents or drugs that have prominent anticholinergic side effects. Decreased cholinergic influence causes both reductions in the force of contraction and the loss of peristaltic sequence. In animal models of acid-induced esophagitis, inflammatory mediators have been shown to impair release of acetylcholine and also directly impair smooth muscle contraction. Thus, in many patients, hypotensive esophageal contractions may be secondary to inflammation caused by reflux or other etiologies. Hypotensive esophageal contractions are often associated with hypotensive LES. Hypotensive LES tone and weak distal esophageal contractions can lead to increased reflux and impaired esophageal acid clearance, respectively, thereby promoting the development of reflux esophagitis.
Epidemiology Hypotensive esophageal contraction is the most common manometric abnormality found in patients with GERD, occurring in 20% to 50%. In a large series, it was reported to be present in about 10% of patients undergoing esophageal manometry for any reason.
Symptoms The primary symptom in patients with hypotensive esophageal contractions is dysphagia, although in some patients dysphagia may be minimal or absent, with reflux symptoms predominating.
Physical findings There are no physical findings specific for hypotensive esophageal contractions.
Laboratory findings Barium swallow x-ray may show, as compared to normal (Video 6), a delayed transit of barium through the esophagus (Video 7). It often shows failure of the peristaltic wave in the most distal esophagus, with proximal escape of the barium bolus. Free reflux of barium from the stomach to esophagus may also be noted (Video 8). A hiatal hernia may be present (Video 9)
Video 6: Normal subject.
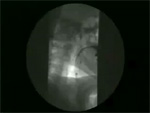
In normal subjects, swallowing is associated with opening of the upper and the lower esophageal sphincter and sequential peristaltic wave that pushes the tail of barium ahead of it. Normal esophageal clearing time is around 13 seconds.
Video 7: Delayed clearing time.
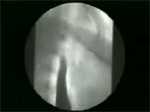
Esophageal clearing time in normal volunteers is about 13 seconds. If barium bolus is retained more than 20 seconds, clearing time is considered delayed.
Video 9: Hiatus hernia.

Normally during a swallow, a portion of the stomach moves into the chest due to contraction of the esophageal longitudinal muscle. Failure of the portion of the gastric fundus to return to its intra-abdominal location is indicative of hiatal hernia.
Esophagoscopy is either normal or shows evidence of reflux esophagitis.
Diagnosis The diagnosis should be suspected in all patients presenting with nonstructural dysphagia, particularly if there is associated acid reflux disease. It is confirmed by esophageal manometry showing low-amplitude (<30 mmHg) peristaltic or nonconducted contractions in the distal esophagus.
Differential diagnosis The main differential diagnosis is to identify secondary causes of hypotensive contractions. Medications that may suppress peristaltic contractions should be carefully excluded. Scleroderma or other connective tissue disorders involving the esophagus need to be considered in the differential diagnosis. Scleroderma patients typically have more severe abnormalities than the idiopathic hypotensive esophageal contractions. Raynaud's phenomenon is usually present and skin abnormalities may be seen. Serologic tests for autoimmune disease are helpful in differential diagnosis.
Clinical course and complications Patients with hypotensive esophageal contractions generally follow a benign clinical course. Severe reflux esophagitis with stricture formation may develop, but in general these complications respond well to intensive antireflux therapy.
Treatment There is no proven therapy for the hypotensive peristalsis, but these patients usually improve with treatment of associated reflux disease.
Scleroderma Esophagus
Definition Scleroderma esophagus is part of a connective tissue disorder, which leads to atrophy of esophageal smooth muscle with consequent loss of LES tone and esophageal peristalsis. It is one of the most important secondary motor disorders to affect the esophagus.
Pathology The pathology is characterized by atrophy of esophageal and LES smooth muscle with associated deposition of collagen in between smooth muscle fibers.
Etiology The etiology is unknown but is believed to be autoimmune in nature.
Pathophysiology In the early stages of scleroderma esophagus, there is evidence of neural dysfunction, possibly owing to impairment of microcirculation to intramural neurons. Subsequently, there is marked atrophy of esophageal smooth muscle with extensive fibrosis. This results in marked hypotension of the LES and loss of peristaltic contractions in the smooth muscle segment of the esophagus. Because of the poor LES tone and impaired esophageal clearance, free reflux usually occurs. This results in reflux esophagitis with stricture formation in advanced cases.
Epidemiology Scleroderma esophagus occurs in 75% to 80% of patients with scleroderma. It most commonly affects woman in the 30- to 50-year age group.
Symptoms The patients may have no symptoms and simply present with features of the connective tissue disorder. Esophageal involvement leads to reflux symptoms including heartburn and acid regurgitation. Dysphagia is also common, possibly owing to hypotensive esophageal contractions or nonperistaltic contractions, but it more commonly relates to superimposed stricture formation or esophageal inflammation, which results in loss of compliance of the distal esophagus. Most patients with esophageal involvement with scleroderma have associated Raynaud's phenomenon. Respiratory symptoms may also occur, owing either to direct lung involvement with scleroderma or to aspiration.
Physical findings The physical findings are those of the systemic connective tissue disease, such as scleroderma and other connective tissue diseases.
Laboratory findings Autoimmune markers such as the anti-endonuclear antigens anti-ScL-70 and anti-centromere antibodies may be present. In advanced cases, barium x-ray reveals a dilated esophagus with poor esophageal clearance and hypotensive peristaltic contractions. The region of the gastroesophageal junction (GEJ) is often wide open with free reflux. However, when reflux esophagitis with stricture ensues, narrowing at the distal esophagus may be present (Figure 9). Endoscopic examination reveals erosive esophagitis in 60% or more of patients. Stricture may also be seen. The typical manometric findings include markedly decreased resting pressures (typically <5 mmHg above intragastric pressure) and markedly low-amplitude simultaneous contractions in the distal smooth muscle esophagus.
Figure 9: Scleroderma esophagus peptic stenosis as seen in a double-contrast examination of the esophagus.
There is also a 3 cm long hiatal hernia. (Courtesy of Dr F.I. Habib)
Diagnosis The diagnosis should be suspected in patients with features of systemic scleroderma or possibly just Raynaud's phenomenon with reflux symptoms and dysphagia. The diagnosis is confirmed by demonstrating the typical manometric abnormalities.
Differential diagnosis If features of the connective tissue disorder are not present, scleroderma esophagus can be readily confused with severe idiopathic GERD. It is therefore important to ask for symptoms of Raynaud's phenomenon when evaluating patients with severe GERD. The x-ray findings in scleroderma esophagus may sometimes be confused with achalasia as both may show a dilated esophagus with narrowing at the EGJ. However, endoscopic and manometric findings can differentiate the two quite easily.
Clinical course and complications As mentioned above, patients may develop severe reflux esophagitis with stricture formation. In addition, there is a suggestion that chronic reflux with aspiration may contribute to the development of interstitial fibrosis.
Treatment There is no specific treatment for scleroderma involvement of the esophagus. However, the associated severe reflux disease is what causes most of the patient's symptoms, and this can be readily treated with proton pump inhibitors. Esophageal bougienage may also be required. In general, antireflux surgery should not be undertaken because of the risk of producing severe postoperative dysphagia. If it is performed, a partial fundoplication is generally used with a Collis procedure and esophageal lengthening.
Incompetent LES and GERD
Incompetent LES is one of most important causes of impaired antireflux barrier mechanisms at the EGJ that normally prevent GER and the development of GERD. Incompetence of theLES may be due to its hypotension, increased intraabdominal pressure that overwhelms a near normal LES and inappropriate TLESR. (Figure 8) Other factors such as decreased contractile response of the diaphragmatic sphincter and a hiatal hernia also play an important role in gastroesophageal reflux.
Figure 8: Three different mechanisms of LES incompetence in gastroesophageal reflux.
a; The LES may be hypotensive causing free reflux of acid into the esophagus. b; The LES barrier may be overwhelmed by increased intragastric pressure. This is often associated with impaired contraction of the diaphragmatic sphincter. c; LES may exhibit frequent reflex transient LES relaxation (TLESR) TLESR is a vagovagal inhibitory reflex that is different from swallowing reflex and is akin to belch reflex. (Source: Dodds WJ et al. N. Engl J Med. 1982;307(25):1547–1552)
Hypotensive LES
Definition Hypotensive LES is characterized by reduced basal tone so that the pressure gradient between the gastric fundus and the LES is less than 10 mmHg. This may cause free reflux of gastric acid into the esophagus. It may or may not be associated with hypotensive esophageal contractions.
Pathology The pathology of LES hypotension is not known. There may be atrophy of the LES smooth muscle with associated fibrosis. The myenteric plexus is thought to be normal.
Etiology The etiology of hypotensive LES remains unknown in most cases. There are many secondary causes of LES hypotension.
Pathophysiology Although cholinergic suppression may cause LES hypotension, most cases are caused by impairment of the myogenic LES tone. LES tone may be impaired by smooth muscle relaxing drugs including calcium channel blockers, nitrovasodilators,  -adrenergic agonists, phosphodiesterase inhibitors, and certain hormones such as estrogens and progesterone. Surgical myotomy or pneumatic dilation for achalasia may also lead to hypotensive LES. Diseases such as scleroderma and other connective tissue disorders also cause LES hypotension. Inflammatory mediators in reflux-associated inflammation also cause LES hypotension. However, most cases of hypotensive LES are idiopathic without any known cause.
-adrenergic agonists, phosphodiesterase inhibitors, and certain hormones such as estrogens and progesterone. Surgical myotomy or pneumatic dilation for achalasia may also lead to hypotensive LES. Diseases such as scleroderma and other connective tissue disorders also cause LES hypotension. Inflammatory mediators in reflux-associated inflammation also cause LES hypotension. However, most cases of hypotensive LES are idiopathic without any known cause.
Epidemiology Gastroesophageal reflux disease is a very common clinical condition. It is not quite clear in what fraction of patients the GERD occurs owing to hypotensive LES; however, it is clear that severe cases of GERD often have hypotensive LES.
Symptoms The symptom complex associated with various complications of GERD is present.
Physical findings There are no physical findings unless supraesophageal complications of GERD are present.
Laboratory findings Endoscopic examination may reveal erosive esophagitis, peptic stricture, or Barrett's esophagus. Manometric findings include markedly decreased resting pressures (typically <10 mmHg above intragastric pressure). Hypotensive esophageal contractions may be present. Esophageal pH studies show increased acid reflux.
Diagnosis The diagnosis is suspected when patients present with GERD. The diagnosis is confirmed by demonstrating the typical manometric abnormalities.
Differential diagnosis Differential diagnosis includes other causes of GERD and secondary causes of LES hypotension. Absence of LES hypotension is excluded by esophageal manometry. However, other causes of GERD such as a hiatal hernia and TLESR may occur with hypotensive LES. Secondary causes of LES hypotension need to be excluded by careful history and laboratory test for connective tissue disorders.
Clinical course and complications Patients with LES hypotension and GERD may develop severe reflux esophagitis with stricture formation as well as supraesophageal complications.
Treatment Treatment for GERD regardless of the underlying cause is gastric acid suppression. Direct improvement of LES hypotension may be effected by surgical fundoplication. Surgical antireflux procedures also target other mechanisms that cause incompetence of the antireflux barriers at the EGJ. Drug therapy to improve LES tone is generally ineffective.
Transient LES Relaxation (TLESR)
Definition The TLESR reflex that inappropriately occurs in the absence of swallowing or esophageal peristalsis or other activities such as belching is called inappropriate TLESR or simply TLESR. It may be a part of the belch reflex without obvious belch. This is a centrally mediated vagovagal reflex, with the vagal afferents arising from the gastric fundus and vagal inhibitory efferent pathway projecting to the LES.
Pathology It is a functional disorder without any specific pathology.
Etiology What causes the increase in the frequency of the TLESR is not known.
Pathophysiology In experimental animals, electrical stimulation of vagal afferents arising from the gastric fundus has been shown to cause reflex relaxation of the LES. This reflex is mediated via medullary neurons in the nucleus tractus solitarius (NTS) and the caudal portion of the dorsal motor nucleus of the vagus nerve (DMV) that provide inhibitory vagal innervation to the LES. The inhibitory neural pathway consists of cholinergic preganglionic neurons and nitrergic postganglionic neurons in the myenteric plexus innervating the LES. Gastric distention increases the frequency of these reflex episodes. The underlying cause of the increased TLESR that leads to GER is not understood. The TLESRs are accompanied by episodes of GER that may lead to GERD.
Epidemiology In many patients GERD occurs owing to increased episodes of acid reflux episodes occurring during TLESRs. This is particularly true for patients with milder grades of GERD. The TLESRs may be more often associated with GER episodes in the presence of hypotensive LES.
Laboratory findings Short-term manometric studies that are usually performed for the evaluation of esophageal motility disorders are not helpful in the diagnosis of the increased frequency of TLESR.
Diagnosis Long-term manometry of the LES using the Dent sleeve is necessary for its diagnosis. However, this is not practical in clinical practice. Therefore, clinical diagnosis of TLESR is seldom made in practice.
Treatment Specific treatment to suppress episodes of TLESRs has been proposed. Block of nitrergic neurotransmission with blockers of NOS is not practical because of the generalized side effects.  -Aminobutyric acid (GABA) receptor agonists (e.g. baclofen) have been shown to suppress the reflex TLESR by either a central action in the medulla or an effect on gastric afferents.
-Aminobutyric acid (GABA) receptor agonists (e.g. baclofen) have been shown to suppress the reflex TLESR by either a central action in the medulla or an effect on gastric afferents.
Disorders of Longitudinal Muscle Contraction
The role of the longitudinal smooth muscle in esophageal functions is not well understood, mainly because activity of this muscle does not result in changes in intraluminal pressure, therefore it cannot be detected by intraluminal manometry. It has been suggested that by shortening the esophagus, longitudinal smooth muscle contraction facilitates bolus transit. Recently, high-frequency ultrasonography has been used to study contraction of the esophageal longitudinal muscle, and abnormal contraction of this muscle layer have been implicated in the pathogenesis of hiatal hernia and non-cardiac chest pain.
Hiatal Hernia and Esophageal Longitudinal Muscle Contraction
Hiatal hernia may impair antireflux barriers at the GEJ and promote GER (Video 9). This can occur in many ways including causing the LES to be incompetent because of its dislocation from the abdomen to the chest. In both animal models and humans, intraluminal acid has been shown to induce reflex contraction of the esophageal longitudinal smooth muscle. This observation has led to the speculation that such sustained acid reflux–induced longitudinal smooth muscle contraction produces esophageal shortening that might contribute to the genesis of a hiatus hernia. This line of argument suggests that hiatal hernia may develop secondary to GER and then once developed may further aggravate GERD.
Longitudinal Muscle Contraction and Chest Pain
Recent studies using high-frequency intraluminal ultrasound have provided evidence that sustained contractions of the longitudinal muscle might be the trigger for either spontaneous or acid reflux–induced chest pain in some patients. In these studies of patients with atypical chest pain, a significant proportion of spontaneous pain episodes seemed tocorrelated temporally with sustained contractions of the longitudinal muscle. Furthermore, pain induced by esophageal acid perfusion also often correlated with evoked contraction of the longitudinal muscle layer.