Key Points
- Antireflux barriers prevent reflux of gastric contents into the esophagus, and their dysfunction leads to gastroesophageal reflux disease and or dysphagia.
- The antireflux barriers include two sphincters, namely, the lower esophageal sphincter (LES) and the diaphragmatic sphincter, and the unique anatomic configuration at the gastroesophageal junction.
- The two sphincters maintain tonic closure and augmented reflex closure of the sphincter mechanism.
- They both relax upon swallowing but can also relax without a swallow, as a part of the reflex called transient LES relaxation (TLESR).
- The LES is composed of smooth muscles, and it maintains tonic contraction owing to myogenic as well as neurogenic factors. It relaxes due to vagally mediated inhibition involving nitric oxide as a neurotransmitter.
- The diaphragmatic sphincter is composed of striated muscles that also exhibit tone and contracts due to the excitatory nerves. It relaxes with swallowing and TLESR.
- The loss of inhibitory mechanisms leads to esophageal achalasia, and increased frequency of TLESR is associated with gastroesophageal reflux disease.
Introduction
The anatomy and functioning of the sphincter at the lower end of the esophagus is fairly complex, and there has been tremendous progress in our understanding of all aspects of it since the original description of a high pressure zone by Code and Schlegel 50 years ago. It is quite clear that the high-pressure zone is more than the smooth muscles of the lower esophageal sphincter (LES), and a poor antireflux barrier is more than a low LES pressure. The beauty of complexity lies in the fact that it provides myriad possibilities for novel surgical, endoscopic, and pharmacologic approaches to treat sphincter dysfunction at the lower end of the esophagus.
A person can stand upside down after eating a large hearty meal, yet no food backs up into the mouth or the esophagus. It is intuitively clear that there must be a valve-like or sphincter mechanism at the lower end of the esophagus.1 The precise nature of the sphincter mechanism at the esophagogastric junction (EGJ) has been an area of intense investigation for last 50 years. The high-pressure zone identified in humans using intraluminal pressure sensors has contributions from the LES, crural diaphragm, as well as the intraabdominal pressure, although it is often erroneously called the LES pressure. It is clear that smooth muscles of the lower end of the esophagus (LES) and diaphragmatic sphincter constitute two active sphincters mechanism at the lower end of the esophagus.2 The neuromuscular mechanisms that maintain tonic or reflex contraction of these sphincters are essential for their antireflux behavior. Impairment of these mechanisms promotes gastroesophageal reflux (GER). The phrenoesophageal ligament anchors the lower end of the esophagus to the diaphragmatic sphincter and plays a key role in the maintenance of an intact antireflux barrier. The EGJ is located between the thorax and abdomen, and this precise location is important because anatomic displacement of the LES from the diaphragmatic sphincter, as occurs in hiatal hernia, is important in the pathogenesis of reflux disease. Relaxation of the sphincters is crucial in the transport of ingested contents from the esophagus into the stomach. On the other hand, relaxation of sphincters in the absence of swallow, so-called transient LES relaxation (TLESR), allows retrograde transport of gastric contents into the esophagus. Understanding the myogenic factors, neural circuitry, and neurotransmitters involved in the maintenance of the basal tone, sphincter relaxation, and induction of TLESR is of fundamental importance to the pharmacologic approaches for the antireflux therapy.
Lower Esophageal Sphincter
Anatomic Considerations
The smooth muscles of the esophagus are organized into two distinct layers, the circular and the longitudinal, and these layers continue into the LES. If one examines autopsy specimens, both of these muscle layers are not thicker in the LES as compared to the esophagus3 (Figures 1 and 2). The thickness of the LES muscles changes in a dynamic fashion with the change in LES tone, that is, it increases and decreases with the increase and decrease in the LES pressure, respectively,4, 5 and it is for this reason that autopsy studies fail to show LES as a distinct anatomic structure, because with the loss of muscle tone in the autopsy specimen the LES looks no different than the esophagus.
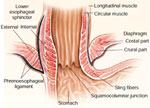
Figure 1: Anatomy of the esophagogastric junction.
The lower esophageal sphincter and the crural diaphragm constitute the intrinsic and extrinsic sphincter, respectively. The two sphincters are anatomically superimposed on each other and are anchored by the phrenoesophageal ligament. (Source: Mittal and Balaban.2 Copyright © 1997, Massachusetts Medical Society.)
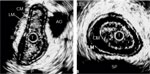
Figure 2: Ultrasonographic images of the esophagus (left) and lower esophageal sphincter (LES, right).
Images were obtained using a 12.5–MHz catheter-based transducer. Circle in the center (T) represents the ultrasound transducer. Note five layers of tissue in the esophagus and LES: MUC, mucosa; CM, circular muscle (intermuscular septum is between circular and longitudinal muscle); LM, longitudinal muscle (outside LM is adventitia); SP, spine; AO, aorta; A, anterior; P, posterior; L, left; R, right. Images were obtained in resting state. Note the asymmetry of the LES and esophagus. (Source: Liu et al.,4 with permission from American Physiological Society.)
Anatomic descriptions of the LES are limited by the fact that the LES is best identified functionally on manometry, and the anatomic dissections can only guess the identity of the LES. Nonetheless, anatomic studies suggest that LES muscle cells are not completely circular and in the lower part of the LES they are arranged in the form of incomplete "C-shaped" fibers arising from the left and the right sides that clasp each other. On the left side, the "C-shaped" fibers intermingle with the gastric sling fibers.6 The gastric sling fibers loop around the gastroesophageal junction on the left side, and its two limbs continue on the anterior and posterior walls of the stomach, respectively, parallel to the lesser curvature of stomach. The gastric sling fibers form the oblique muscle layer of the stomach and may play an important role in the formation and modulation of angle of His.7, 8 The oblique fibers can be visualized at the time of endoscopy on a retroflexed examination of the gastroesophageal junction.9, 10 The loss of function of gastric sling is felt to be associated with severe reflux disease. The excitatory and inhibitory motor neurons for the control of gastric oblique fibers are located in the stomach. On the other hand, fibers that constitute the LES are innervated by the inhibitory motor neurons located either locally within the LES or in the esophagus.8
Studies in cats and humans show functional differences between clasp fibers on the right side of the LES and the gastric sling fibers.7, 11, 12, 13 The clasp fibers maintain greater basal tone than the oblique fibers, but the latter are more sensitive to cholinergic stimulation. In addition to acetylcholine, there are differences in the sensitivity between clasp and the sling fibers to dopamine and other pharmacologic agents.13 The sling fibers that form the loop at the gastroesophageal junction may be responsible for the asymmetry of LES pressure and its regional sensitivity to anticholinergic agents.
Ultrastructural studies of the sphincter muscle in the opossum suggest that muscle cells from the LES are of larger diameter and form fewer gap junctions than do those of the esophageal body. The sphincter muscle cells also have irregular surfaces and evaginations that are not seen in the esophageal body. These evaginations are likely to be related to the tonically contracted state of the sphincter muscle.14 The LES can also be distinguished from the esophageal body, by the presence of more numerous intermuscular spaces containing blood vessels and connective tissue. Mitochondria and the smooth endoplasmic reticulum mass are greater in the LES than in the esophageal body.15
Lower Esophageal Tone and Contraction
The difference between LES and esophageal muscle is that the former maintains higher basal tone than the latter, and in the in vivo situation the LES in humans is recognized as a zone of high pressure, 2 to 4 cm in length. What is responsible for this tone? A multitude of myogenic, neural, and neurohumoral factors can either increase or decrease LES tone16, 17 (Table 1). The myogenic elements responsible for LES tone maintenance may be due to differences in the structural protein as described by Szymanski et al.,18 who reported that the LES has proportionally more  -actin and basic essential light chains LC17b, and less of a seven amino acid–inserted myosin isoform and caldesmon than the esophageal body circular muscle. There are also distinct intracellular signaling pathways in the LES as compared to the esophageal body. The unique signaling pathway may contribute to the tonic contraction of the LES.
-actin and basic essential light chains LC17b, and less of a seven amino acid–inserted myosin isoform and caldesmon than the esophageal body circular muscle. There are also distinct intracellular signaling pathways in the LES as compared to the esophageal body. The unique signaling pathway may contribute to the tonic contraction of the LES.
From an electrophysiologic point of view, the LES muscle is in a state of greater depolarization than the esophageal muscle, as evidenced by a higher resting membrane potential than the esophageal muscle.19 The depolarized state of the sphincter smooth muscle is suggested to be due to the resting chloride conductance.20 Periodic spike bursts or increase in the depolarization result in an increase in the LES tonic activity. Tonic LES contraction is both spike dependent and spike independent.21
The relative contribution of myogenic tone to the LES pressure differs in different species. In the opossum, administration of tetrodotoxin (a nerve poison) does not affect LES pressure in vivo, suggesting that the basal LES tone is entirely myogenic.22 Some investigators believe that in cats, dogs, and humans, neural cholinergic drive contributes significantly to the basal LES tone.23, 24, 25 Atropine, for example, in the dose of 15  g/kg, reduces LES pressure by 50% to 70% in humans.26 Brookes et al.8 identified a large population of cholinergic neurons in the guinea pig LES. Immunohistochemical staining of the myenteric plexus of the LES shows the presence of acetylcholine and substance P, the two likely candidates for excitatory neurotransmissions. These nerve cells are likely to be located in the parasympathetic pathway to the LES. Electrical stimulation of the sympathetic nerves also results in LES contraction that is mediated by
g/kg, reduces LES pressure by 50% to 70% in humans.26 Brookes et al.8 identified a large population of cholinergic neurons in the guinea pig LES. Immunohistochemical staining of the myenteric plexus of the LES shows the presence of acetylcholine and substance P, the two likely candidates for excitatory neurotransmissions. These nerve cells are likely to be located in the parasympathetic pathway to the LES. Electrical stimulation of the sympathetic nerves also results in LES contraction that is mediated by  -adrenergic receptors.27, 28
-adrenergic receptors.27, 28  -adrenergic stimulation, on the other hand, leads to LES relaxation, an effect that could be mediated through
-adrenergic stimulation, on the other hand, leads to LES relaxation, an effect that could be mediated through  1,
1,  2, or a recently described
2, or a recently described  3 receptor.29, 30
3 receptor.29, 30  3-receptor stimulation, unlike
3-receptor stimulation, unlike  1 and
1 and  2, does not cause any cardiovascular side effects, which may be relevant for the treatment of esophageal motor disorders associated with LES hypertension (achalasia, diffuse esophageal spasm, and nutcracker esophagus). It has been suggested that interstitial cells of Cajal (ICCs) that are present in the LES and may serve as intermediary to amplify actions of neurotransmitters on the LES smooth muscle cells.31, 32, 33
2, does not cause any cardiovascular side effects, which may be relevant for the treatment of esophageal motor disorders associated with LES hypertension (achalasia, diffuse esophageal spasm, and nutcracker esophagus). It has been suggested that interstitial cells of Cajal (ICCs) that are present in the LES and may serve as intermediary to amplify actions of neurotransmitters on the LES smooth muscle cells.31, 32, 33
Slow phasic contractions in concert with the gastric component of the migrating myoelectrical complex (MMC) occur in the LES of humans and animals.34, 35, 36, 37 During the first phase of the MMC, the LES pressure is relatively stable, but during late phase II and throughout phase III, large-amplitude phasic contractions occur without major change in the basal pressure. The MMC-related contractions are abolished by atropine and anesthesia.35, 38 Motilin, a neurohumoral agent released into circulation from the specialized cells in the wall of intestine, is responsible for phasic LES contraction.36 Phasic contraction of the proximal half of the LES also occurs following swallow- or esophageal distention–induced LES relaxation. Postrelaxation contraction is coordinated with esophageal peristalsis and is atropine sensitive. A similar behavior is seen in the muscle strips in vitro.39 Post-relaxation contraction is related to the level of baseline tone. Human LES circular muscle strips show prominent after-contraction when basal tone is low, but as tone increases the after-contraction is diminished.
The LES pressure increases in response to increases in intraabdominal pressure. However, considerable controversy exists as to whether the rise in LES pressure during abdominal compression is due to reflex LES contraction or merely a passive transmission of the increased intraabdominal pressure.40, 41 It is unlikely to be a passive transmission because a contracted muscle is relatively noncompliant.42 Studies in the opossum show that the LES abdominal compression reflex is a true reflex that is mediated via the vagus nerve and is dependent on cholinergic neurons.43 In the human, increases in abdominal pressure during abdominal compression or other physical maneuvers result in reflex contraction of the LES as well as the diaphragmatic sphincter.26, 44
The brainstem contains the central control mechanism for the LES, which is closely integrated with the swallow pattern generator. There is a topographic representation of neurons representing LES in the dorsal motor nucleus of the vagus nerve (DMV).45, 46 The rostral cells are involved in the excitatory and caudal cells in the inhibitory innervation to the LES. The afferent information from the sensory nucleus of the tractus solitarius (NTSs) is relayed to the DMV motor neurons via the interneurons. Glutamate is the neurotransmitter of the sensory afferents. The motor neurons of the DMV contain acetylcholine, nitric oxide, dopamine, and epinephrine.47 Inhibition of transient LES relaxation by 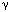 -aminobutyric acid (GABA)-B agonist48 and cannabinoid (CB1) receptors49 is mediated at the level of both NTSs and the DMV. The swallow pattern generator, located in the reticular formation or the interneurons, is under the influence of GABA, and stimulation of the GABA-B receptor results in a reduction in its threshold and therefore of the swallow frequency.50
-aminobutyric acid (GABA)-B agonist48 and cannabinoid (CB1) receptors49 is mediated at the level of both NTSs and the DMV. The swallow pattern generator, located in the reticular formation or the interneurons, is under the influence of GABA, and stimulation of the GABA-B receptor results in a reduction in its threshold and therefore of the swallow frequency.50
Diaphragmatic Sphincter
Anatomic Consideration
The mammalian diaphragm is primarily a respiratory muscle. However, it should be considered as two separate muscles consisting of the crural and the costal diaphragms.51, 52 The costal diaphragm is a respiratory muscle, but the crural diaphragm has two functions, respiratory and gastrointestinal. Respiratory function relates to ventilation, and gastrointestinal to the sphincter like action at the lower end of the esophagus. During human development myoblasts originating in the body wall and derived from the cervical segments invade two pleuroperitoneal membranes and form the costal diaphragm. On the other hand, the two crura develop in the mesentery of the esophagus.53 The costal diaphragm originates primarily from the ribs, and the crural diaphragm from the lumbar vertebra, as two pillars (right and left crura). The esophageal hiatus is formed primarily by the right crus, but there are anatomic variations, and in approximately 20% of cases the left crus also contributes partially to its formation.54 The fibers of the crus are oriented in the craniocaudal direction. According to Delattre et al.,54 the esophageal hiatus resembles a veritable canal, a two-staged canal; the upper part is fully muscular and measures 2.5 cm in length, but the lower part forms a gutter that is open anteriorly and surrounded by the muscles of the right crus on the posterior and lateral aspects. The central fibers have a relatively circular arrangement, but the peripheral fibers are oriented in a craniocaudal direction. The unique arrangement of its muscle fibers results in two different types of actions on the esophagus when it contracts: a vertical or craniocaudal motion, and a circumferential squeeze.
Phrenic nerves formed by the branches of C5, C6, and C7 nerve roots provide both motor and sensory innervation to the crural and costal diaphragm. In the dog, the C5 root has been reported to innervate almost exclusively the costal diaphragm, whereas the C7 root innervates the crural diaphragm.55 In the cat, Sant'Ambrogio et al.56 found that electrical activity of a hemidiaphragm requires intact C4–6 rootlets, with the vertebral (or crural) region mainly receiving its innervation from C6 and the costal region from C5. However, later more refined topographic mapping studies in the cat showed that there was no such simple segmental organization, but that generally the ventral region of both muscles is innervated by the C5 neurons, and the dorsal portions are innervated by the C6 neurons.57 The use of electrically evoked glycogen depletion technique greatly increased the resolution of the cervical root and phrenic nerve branch innervation territories.58 Advances have been forthcoming not only in the study of peripheral projection of phrenic nerve roots and branches but also in the analysis of the central origin of the crural and costal motoneurons in the spinal cord.59 Retrogradely labeled motoneurons, when three different tracers were applied to cat phrenic nerve branches, were found to be mixed throughout the full extent of the phrenic motor nucleus. Therefore, although there is a highly delineated strip-like peripheral projection to muscle fibers, the motoneurons in the spinal cord are relatively intermingled rostrocaudally, dorsoventrally, and mediolaterally.59 The phrenic nerve is also responsible for the sensory innervation of the crural diaphragm. The proprioception function transduced by the muscle spindles located in the crural diaphragm is likely to be important in the reflex contraction of the diaphragmatic sphincter secondary to stretch exerted on it during increases in intraabdominal pressure.
Diaphragmatic Contraction
Measuring the contribution of diaphragmatic sphincter pressure to the EGJ is problematic in humans for three reasons: (1) The LES and diaphragmatic sphincter are anatomically superimposed on each other, and therefore it is difficult to discern whether the intraluminal pressure is related to LES or diaphragmatic sphincter contraction. (2) The craniocaudad movements of the diaphragm result in difficulty in maintaining the pressure sensor in the EGJ location. (3) The diaphragmatic sphincter, being a skeletal muscle, can contract very rapidly and requires high-fidelity pressure sensors to record its activity.60 These limitations are partially overcome by recording pressure and electromyogram (EMG) activity simultaneously in the LES, using a reverse perfused sleeve sensor equipped with electrodes.61 Contraction of diaphragmatic sphincter provides a powerful sphincter mechanism at the lower end of the esophagus. It contributes to both tonic (sustained) and phasic pressure increases at the level of the LES. It is widely believed that end-expiratory pressure at the lower end of the esophagus is due to tonic contraction of the LES, and the increase in pressure with inspiration is due to the contribution from the diaphragmatic sphincter. In the cat, paralysis of the diaphragmatic sphincter by curare results in the loss of inspiratory pressure oscillations or phasic contraction at the LES.62 The best evidence for the tonic contraction of the diaphragmatic sphincter is provided by studies in patients with a completely absent LES (the latter resected because of cancer at the distal esophagus or proximal stomach).44 Klein et al.44 found a high pressure zone that had both end-expiratory and inspiratory pressure oscillations in these patients. In humans, tidal inspirations cause a 15- to 20-mmHg increase in the LES pressure, and with forceful inspiration the increase in LES pressure can be 100 to 150 mmHg63 (Figure 3). The diaphragmatic sphincter also contracts reflexively during all those physiologic maneuvers that are associated with an increase in intraabdominal pressure (Figure 4). Studies in normal subjects as well as patients with an absent LES demonstrate increases in pressure at the lower end of the esophagus, with an increase in intraabdominal pressure as a result of abdominal compression, straight leg raise maneuver, coughing, and Valsalva maneuver.26, 44
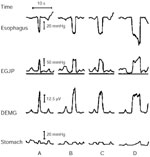
Figure 3: Esophagogastric junction pressure (EGJP) during diaphragmatic contraction recorded by a reverse perfused sleeve sensor equipped with electrodes to record electromyographic activity of the crural diaphragm.
Diaphragmatic contraction was induced by standardized inspiratory efforts (standardized Muller maneuver) of different durations, 1, 2, 4, and 6 seconds. Each inspiratory effort resulted in a negative esophageal pressure, an increase in the EGJP, and an increase in the integrated diaphragm EMG activity (DEMG). Note that the peak EGJP and the peak DEMG occur at the same time. (Source: Sivri and Mittal61, with permission from American Gastroenterological Association).
Figure 4: Reflex contraction of the esophagogastric junction recorded by a reverse perfused sleeve sensor equipped with electrodes to record crural DEMG activity.
Note the rapid increase in EGJP and crural DEMG activity with each of the two straight leg raises (SLRs). (Source: Sivri and Mittal61, with permission from Amercian Gastroenterological Association.)
Contractions of the inspiratory muscles of respiration produce negative intrathoracic and negative intraesophageal pressure, thus increasing the pressure gradient between the stomach and esophagus in favor of GER. Contraction of the abdominal wall and diaphragm also increases the pressure gradient between the stomach and esophagus. All of the maneuvers accompanied by contraction of the inspiratory and the abdominal wall muscles that increase gastroesophageal pressure gradients are accompanied by contraction of diaphragmatic sphincter. Thus the rapid changes in pressure gradients between esophagus and stomach, caused by skeletal muscle contraction of the chest and abdomen, are antagonized by rapidly contracting skeletal sphincter muscles of the diaphragmatic sphincter.2
Asymmetry of the Intraluminal Pressure at the Lower End of the Esophagus
Originally described by Winan, several investigators have noticed that the LES pressure is circumferentially asymmetric.7, 64, 65, 66, 67 In fact, LES pressure asymmetry is present in both the axial as well as circumferential directions. Axial asymmetry means that the pressure in the LES is asymmetric along its length. The LES pressure is distributed in a bell-shaped curve, with the highest pressure somewhere in the middle.67 Circumferential asymmetry is reflected in the form of asymmetric pressures at the same axial level in the LES. The highest LES pressure is in the leftward direction. Richardson and Welch68 found that atropine caused a greater reduction in the leftward pressure as compared to the pressure on the right. Preiksaitis et al.,7, 11 in cats and humans, demonstrated greater sensitivity of sling (oblique) fibers (located on the left) to acetylcholine, and it is very possible that the sling fibers are responsible for the circumferential pressure asymmetry. Ultrasound images of the LES show that the shape of the LES is relatively more circular toward the left,66 which could be due to either a stronger contraction of the sling fibers in vivo or to the crus of diaphragm,69 which compresses LES from the left side. From the physics point of view (Laplace's law), asymmetry in the shape of LES (Figure 2) could account for the circumferential asymmetry of the LES pressure.66
Phrenoesophageal Ligament
Loose areolar tissue surrounds the esophagus from the level of mediastinum to the upper abdomen.54 Vagus nerves and blood vessels are located in this areolar tissue. Toward the periphery, the loose areolar tissue condenses to form the phrenoesophageal ligament, which anchors the esophagus and LES to the undersurface of the diaphragmatic sphincter. Some feel that phrenoesophageal ligament originates from the undersurface of the diaphragm, and as it gets closer to the esophagus it forms two leaflets. The upper leaflet is inserted above the squamocolumnar junction and the lower leaflet is attached several centimeters below the upper leaflet. The phrenoesophageal ligament is a relatively compliant and elastic structure because it allows the esophagus and the LES to slide in and out of the hiatus several centimeters. During a swallow, the lower end of the esophagus moves approximately 2 cm in the cranial direction,70 and during TLESR the movement is even greater, 3 to 4 cm or even more.71 The margin of the crus of the diaphragm or hiatus margin also moves along with the lower end of esophagus when the LES relaxes, albeit slightly less than the esophagus.72 It may be that under physiologic conditions the LES really does not herniate into the thorax that much because the esophagus and the hiatus margin move in the cranial direction together and do not slide in relationship to each other. The weakening of the phrenoesophageal ligament, as may occur with age, results in a sliding hiatal hernia, which may be reducible or nonreducible. Nonreducible hiatal hernias are strongly associated with severe GER disease.
Relaxation of the Lower Esophageal Sphincter and the Diaphragmatic Sphincter
Lower Esophageal Sphincter Relaxation
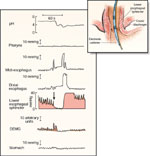
For the transport of ingested contents into the stomach, relaxation of both the LES and the diaphragmatic sphincter is essential. Deglutition and distention of the esophagus are the two major stimuli that induce EGJ relaxation. Deglutition-induced LES relaxation starts within 2 seconds of the onset of swallowing and lasts 6 to 10 seconds (Figure 5). Lower esophageal sphincter relaxation is terminated by the arrival of esophageal peristaltic contraction at the LES and is followed by an after-contraction that may last up to 10 seconds. After-contraction is seen only in the upper part of the LES; in the lower part, LES pressure simply returns to the resting pressure level. Electrical recordings show that swallow-induced LES relaxation is associated with cessation of spike activity when present.73 In the upright position, the swallowed bolus may reach the LES very quickly owing to gravity, and in that situation the bolus may be transiently held at the LES before passage into the stomach. The LES normally relaxes to a pressure very close to the intragastric pressure. Besides relaxation, the LES also opens to allow passage of the bolus into the stomach. It is important to understand the difference between relaxation and opening because manometry only measures relaxation and not the opening function of the LES.74 As long as the intraluminal diameter of the LES is greater than the size of manometry catheter, the latter records complete relaxation. However, for the bolus to pass through the LES the latter must also open to the diameter of the bolus. Lower esophageal sphincter relaxation is an active process that is mediated by neurotransmitters, but the opening is related to passive or viscoelastic properties of the LES. Bolus pressure is responsible for the opening of the LES.
Figure 5: An example of swallow-induced lower esophageal sphincter (LES) relaxation (left) and transient LES relaxation (right).
To the left of the dark vertical line are normal swallow-associated events: submental EMG, pharyngeal contraction, and esophageal peristaltic contraction. The gray vertical line represents the onset of the transient relaxation. Note the associated event, that is, esophageal contractions at the onset of the relaxation. Reflux occurs during transient relaxation of the LES and results in a fall of esophageal pH from 5 to 1. Also note the difference in the duration between swallow induced and transient LES relaxation. (Source: Mittal and McCallum118, with permission American Physiological Association.)
Swallow is normally associated with LES relaxation followed by esophageal peristalsis, and as the esophageal contraction arrives at the LES, the latter recovers to baseline pressure. However, the two components (i.e., esophageal contractions and peristalsis) can disassociate. Relaxation of the LES is the more sensitive component of the swallow reflex, and it is possible to have LES relaxation without any other motor evidence of the swallow reflex. Isolated LES relaxation can be induced experimentally by applying pharyngeal tactile stimulation, which is subthreshold for producing a full swallow response.75 Similarly, electrical stimulation of the superior laryngeal nerve (SLN) with stimulus frequencies that fail to produce esophageal peristalsis cause isolated LES relaxation. Low-intensity stimulation of the swallow center in the brain stem can also cause isolated LES relaxation.76 Modifying the stimulus from pharyngeal tactile stimulation to unilateral SLN stimulation decreases the relaxation reflex.77 When repeated swallows are made in succession, as during rapid drinking, the LES remains relaxed and returns to the baseline state after the last swallow.78 The LES relaxation associated with primary peristalsis, as well as the isolated LES relaxation owing to pharyngeal or SLN stimulation, is mediated by vagal efferent nerves and abolished by bilateral cervical vagal section or vagal cooling.79, 80
During a swallow, stimuli from the oropharyngeal region traverse via the vagal afferent pathways into the nucleus tractus solitarius, which then relays information into groups of cells, rather loosely organized (reticular formation). These cells are felt to be the pattern generator for the deglutition reflex and communicate with the premotor neurons of the DMV. The rostral and caudal cells in the DMV are responsible for the excitation and inhibition of LES respectively. Nitric oxide and acetylcholine are the neurotransmitter involved in the neurotransmission at the DMV. There is also GABAergic neuron involved in the control of LES. It appears that there is a tonic inhibition of the swallow reflex by GABA. GABA-B agonist can inhibit LES relaxation. It seems that the deglutition pattern generator has a certain threshold, which can be manipulated pharmacologically. GABA-B agonist and antagonist, for example, can decrease and increase the spontaneous swallow frequency50 by altering this threshold.
Lower esophageal sphincter relaxation induced by electrical stimulation of vagal efferent nerves and esophageal distention is blocked by tetrodotoxin, which indicates that the motor neuron for LES relaxation is located with in the myenteric plexus.81, 82 These myenteric neurons may be located within the LES or several centimeters above in the body of the esophagus with processes extending into the LES.8 The transmission at the myenteric neuron can be blocked by a combination of hexamethonium and atropine, suggesting that the synaptic transmission is mediated through muscarinic and nicotinic receptors. Further studies reveal that M1 receptors are responsible for the transmission at the level of the myenteric neuron.83 Following its activation, myenteric neuron releases nonadrenergic noncholinergic (NANC) neurotransmitter or neurotransmitters that cause relaxation of the smooth muscles of LES. There are several possible candidate NANC transmitters: adenosine triphosphate (ATP), vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PCAP), nitric oxide (NO), and carbon monoxide (CO) in the smooth muscle of the GI tract.84 However, the predominant one in the LES is NO. Deglutition-induced, vagal efferent stimulation–induced, esophageal distention–induced, and direct myenteric neuron stimulation–induced relaxation are all blocked by NO antagonists.85, 86, 87, 88, 89
Distention of the striated and smooth muscle portion of the esophagus produces LES relaxation that is associated with secondary peristalsis in the esophagus. During prolonged esophageal distention, the LES recovers from relaxation despite ongoing distention.90, 91 The sphincter relaxation owing to distention in the striated muscle is centrally mediated and abolished by vagotomy, whereas the relaxation owing to distention in the smooth muscle portion is mediated by intramural nerves and remains intact after bilateral vagotomy.92 Vagus nerves may exert a facilitative influence on the LES relaxation evoked by balloon distention in the smooth muscle portion of the esophagus. As with primary peristalsis, LES relaxation is also the most sensitive component of secondary peristalsis. Thus, isolated LES relaxation without esophageal contraction occurs with distentions that are subthreshold for activation of secondary peristalsis.
Intramuscular intercellular cells of Cajal (ICC-IM) may play a role in transducing the effects of neurotransmitters released from nerve ending to smooth muscle cells.93, 94 However, studies using neural nitric oxide synthase (nNOS)-deficient mice and W/WV mutant mice that lack ICC-IM show that the LES is achalasic in nNOS-deficient mice but hypotensive with normal relaxation ability in the W/WV mice.94 W/WV mutant mice have a genetic abnormality that results in partial deficiency of c-kit and associated lack of ICC-IM. These findings suggest that ICC-IM may not serve as the mediator of inhibitory neurotransmission in the LES, but instead deficiency in the ICC-IM may impair the myogenic function of the LES.
At the intracellular level, LES relaxation is due to suppression of a resting chloride conductance by NO or activation of a potassium conductance resulting in smooth muscle hyperpolarization (electromechanical coupling). There is suppression of the Ca2+ influx, leading to cessation of myosin phosphorylation and muscle relaxation. Both cyclic adenosine monophosphate (cAMP) and cyclic guanine monophosphate (cGMP) mediate LES relaxation95, 96, 97 by activating protein kinases A and G, respectively. These signaling molecules can cause smooth muscle relaxation without causing membrane hyperpolarization (pharmacomechanical coupling). These kinases may lower the free intracellular Ca2+ by sequestering it into the endoplasmic reticulum. Both VIP and NO induce increases in the intracellular c AMP and cGMP levels. The LES relaxation induced by various physiologic stimuli is associated with an increase in the intracellular cGMP rather than cAMP, suggesting that NO is the major NANC inhibitory neurotransmitter of the LES smooth muscles.
Diaphragmatic Sphincter Relaxation
Deglutition and esophageal distention, besides causing LES relaxation, also induces selective inhibition of the diaphragmatic sphincter muscle. Deglutition-induced inhibition of diaphragmatic sphincter is usually not complete, and as a result flow across the EGJ is interrupted if the subject inspires during the period of deglutition-induced LES relaxation. In fact, alteration in the breathing pattern can cause complete arrest of the bolus in the esophagus above the EGJ.98 Esophageal distention in cats and several other species lead to diaphragmatic sphincter inhibition.99, 100, 101, 102 In awake humans, esophageal distention–induced inhibition is not as complete as the one seen in anesthetized animals.102 Complete inhibition of the diaphragmatic sphincter in humans is best seen during TLESR (Figure 6). Vagotomy abolishes esophageal distention–mediated inhibition of the diaphragmatic sphincter,99 suggesting that vagal afferents inhibit the brainstem medullary neurons responsible for diaphragmatic sphincter contraction (vagophrenic inhibitory reflex). Altschuler et al.,103 however, failed to find inhibition of the medullar inspiratory neurons during esophageal distention. Oyer et al.104 recorded EMG activity of the crural diaphragm and electrical activity (neurogram) of the phrenic nerve branch supplying the crural diaphragm, and found greater inhibition of the crural diaphragm EMG than the inhibition seen in phrenic neurogram during esophageal distention, suggesting the possibility of a distal site of inhibition. Liu et al.105 found a peripheral mechanism of inhibition (at the level of neuromuscular junction). Diaphragmatic sphincter inhibition is temporally correlated with the longitudinal muscle contraction of the esophagus, and it may be that stretch of the diaphragmatic sphincter is important in its relaxation.72 Diaphragmatic sphincter inhibition is blocked by N -nitro-L-arginine methyl ester (L-NAME), a NO antagonist.106 Nitric oxide suppresses the excitatory motor nerve junction potentials at the crural diaphragm motor end plates.107
-nitro-L-arginine methyl ester (L-NAME), a NO antagonist.106 Nitric oxide suppresses the excitatory motor nerve junction potentials at the crural diaphragm motor end plates.107
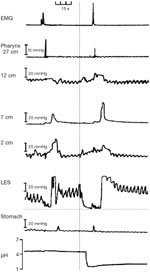
Figure 6: Physiologic record of a spontaneous, transient relaxation of the LES.
Manometric recordings were made with an electrode sleeve sensor (inset) at pressure recording ports at various locations (from the pharynx to the stomach). The vertical arrow indicates the onset of relaxation, which occurs in the absence of a swallow as shown by the absence of a pressure wave or contraction in the pharynx. There is complete LES relaxation for more than 20 seconds (horizontal line at the bottom of the tracing for the LES). Relaxation is associated with inhibition of the crural diaphragm, as indicated by the loss of inspiratory pressure oscillations at the level of the sphincter and loss of inspiratory DEMG. The contribution of the LES is shown in pink, and that of the crural diaphragm in brown. Reflux (indicated by a decrease in esophageal pH) occurs after complete relaxation of the sphincter and crural diaphragm and is associated with an increase in intraesophageal pressure. (Source: Adapted from Mittal et al159, with permission from American Gastroenterological Association.)
Lower Esophageal Sphincter and Diaphragmatic Sphincter Opening
As stated earlier, the LES and diaphragmatic sphincter relaxation and opening are quite distinct processes. Although relaxation is an active process and dependent on normal neural circuit and neurotransmitters, the LES and diaphragmatic sphincter opening is a passive process related to the viscoelastic properties of the LES, the diaphragmatic sphincter, and possibly the phrenoesophageal ligament and the surrounding abdominal viscera. Following relaxation, the LES and diaphragmatic sphincter needs to open to the size of the swallowed bolus. As per Poiseuille's equation, flow through a tube is determined by the equation: F = P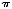 R4/8
R4/8 L, where F = flow; R = tube radius; P = driving pressure; L = tube length and
L, where F = flow; R = tube radius; P = driving pressure; L = tube length and  = viscosity.108 Therefore, the LES and diaphragmatic sphincter opening is one of the important determinants of flow or transit from the esophagus to the stomach. Under normal physiologic conditions the entire LES opening occurs following its relaxation. But LES and diaphragmatic sphincter distensibilty is increased in reflux disease. For a given distention pressure, the diaphragmatic sphincter opening is greater in patients with reflux disease as compared to normal subjects.109 Novel endoscopic and less invasive surgical approaches to treat reflux disease may improve sphincter competence by reducing its distensibility.110 A less distensible LES and diaphragmatic sphincter will result in smaller opening, which causes a relative outflow obstruction to the bolus. Under normal circumstances, in humans an opening of 13 mm is required for the solid and liquid bolus to pass freely into the stomach. Our understanding of the distensibilty at the lower end of the esophagus and its role in esophageal transit abnormality is relatively limited because the routinely used technique to study LES function (i.e., manometry) does not measure distensibilty and opening function at the lower end of the esophagus. Atropine reduces LES pressure but does not affect distensibilty or the opening functioning at the lower end of the esophagus,111 and therefore may not improve dysphagia related to poor distensibilty. Fundoplication, an effective surgical procedure to treat reflux disease, reduces distensibilty of the lower end of the esophagus and causes dysphagia.112
= viscosity.108 Therefore, the LES and diaphragmatic sphincter opening is one of the important determinants of flow or transit from the esophagus to the stomach. Under normal physiologic conditions the entire LES opening occurs following its relaxation. But LES and diaphragmatic sphincter distensibilty is increased in reflux disease. For a given distention pressure, the diaphragmatic sphincter opening is greater in patients with reflux disease as compared to normal subjects.109 Novel endoscopic and less invasive surgical approaches to treat reflux disease may improve sphincter competence by reducing its distensibility.110 A less distensible LES and diaphragmatic sphincter will result in smaller opening, which causes a relative outflow obstruction to the bolus. Under normal circumstances, in humans an opening of 13 mm is required for the solid and liquid bolus to pass freely into the stomach. Our understanding of the distensibilty at the lower end of the esophagus and its role in esophageal transit abnormality is relatively limited because the routinely used technique to study LES function (i.e., manometry) does not measure distensibilty and opening function at the lower end of the esophagus. Atropine reduces LES pressure but does not affect distensibilty or the opening functioning at the lower end of the esophagus,111 and therefore may not improve dysphagia related to poor distensibilty. Fundoplication, an effective surgical procedure to treat reflux disease, reduces distensibilty of the lower end of the esophagus and causes dysphagia.112
Transient Lower Esophageal Sphincter Relaxation (TLESR)
Intuitively, one would think that weakness of either the LES or the diaphragmatic sphincter is the cause of GER disease. Indeed, some patients with reflux disease have a weak LES, some have a weak diaphragmatic sphincter, and some have both. In the majority, however, especially in mild to moderate reflux disease, the LES and diaphragmatic sphincter pressure is normal. In fact, similar to deglutition-induced LES relaxation being a physiologic mechanism for the antegrade flow, gastric distention–induced TLESR is a physiologic mechanism for the retrograde flow of stomach contents into the esophagus. Belching, vomiting, and physiologic reflux in normal subjects occur through the mechanism of TLESR.113 A large body of data indicates that transient relaxation of the LES and diaphragmatic sphincter (TLESR) is the major mechanism of reflux in normal subjects and patients with reflux disease. Initially described as inappropriate LES relaxation,114 the name was later changed to TLESR115 because the phenomenon occurs in normal healthy situation. The term TLESR may not even be appropriate because deglutition-induced LES relaxation is also transient, and furthermore there is also concurrent and simultaneous relaxation of the diaphragmatic sphincter during TLESR. McNally et al.116 in 1964 observed non–swallow-related LES relaxation as a mechanism of belching. Dent and colleagues114 first described in 1980 the exact phenotypic description and its association with GER. The phenotype appearance of TLESR is quite distinct from the swallow-induced LES relaxation (Figures 5 and 6). Transient LES relaxation is seen as an abrupt fall in LES pressure to the level of intragastric pressure that is not triggered by deglutition, as manifested by the distinctive pattern of pharyngeal or mylohyoid muscle contraction. Transient LES relaxation is typically of longer duration than the swallow-induced LES relaxation, lasting 10 to 45 seconds. The optimal criteria for the definition of TLESR are (1) the absence of a pharyngeal swallow signal for 4 seconds before to 2 seconds after the onset of LES relaxation, or a mylohyoid EMG complex for 3 seconds before the onset of LES relaxation; (2) LES pressure fall of 1 mmHg/sec; (3) a time from the onset to complete relaxation of 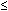 10 seconds; and (4) a nadir pressure of
10 seconds; and (4) a nadir pressure of 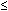 2 mmHg. Excluding LES relaxations associated with multiple rapid swallows, LES pressure drops to 2 mmHg, which has a duration of greater than 10 seconds and can also be classified as TLESRs.117
2 mmHg. Excluding LES relaxations associated with multiple rapid swallows, LES pressure drops to 2 mmHg, which has a duration of greater than 10 seconds and can also be classified as TLESRs.117
A number of events in the esophagus, stomach, and diaphragmatic sphincter accompany TLESRs. Contractions in the pharynx and mylohyoid muscle occur at the onset of 20% to 45% of TLESRs, respectively,118 but these contractions are much smaller (approximately 50%) than those associated with swallows (and may be interpreted as partial or incomplete swallows). Distal esophageal contractions occur often at the onset of TLESRs, and when recorded at more than one site usually have a synchronous onset. During the period of LES inhibition, there is also inhibition of the esophageal body as manifested by inhibition of primary peristalsis.119 The gastric fundus also relaxes during TLESR (manifesting as a drop in intragastric pressure of 2 to 4 mmHg). An important component of TLESR is also a complete inhibition of the diaphragmatic sphincter.120 Thus, TLESR is not a response localized to the LES; rather, it appears to be part of a more generalized inhibition of a number of structures within and outside of the esophagus that are conducive to the retrograde flow across the gastroesophageal junction. This pattern of inhibition during TLESR is consistent with a coordinated pattern of activity generated in the pattern generator in the brainstem.
Stimuli that Trigger Transient Lower Esophageal Sphincter Relaxation
Gastric Distention
Gastric distention is a potent stimulus for TLESR, which is not surprising given that TLESR is the mechanism by which gas is vented from the stomach during belching. Approximately 15 mL of air is delivered to the stomach with each swallow,121 and without an in-built venting mechanism, uncontrolled gastrointestinal bloating would occur. Studies in which the stomach was partitioned surgically show that the subcardiac region of the stomach is primarily responsible for triggering TLESR.122 Reduction of the compliance of this region by buttressing it with mesh reinforcement substantially reduces TLESR in dogs. Although distention of other parts of the stomach can increase the rate of TLESR, the thresholds for distention is substantially higher in these regions and the response is less marked. In humans, a volume of 750 to 1000 mL causes a fourfold increase in the rate of TLESRs within the first 10 minutes. In some studies, a similar effect has been reported after meals.123, 124
Pharyngeal Mechanism
Pharyngeal intubation increases the rate of TLESRs. In fasted patients in whom LES pressure was monitored via a gastrostomy tube, pharyngeal intubation for 1 hour increased the rate of TLESRs threefold, from 2 to 6/hour.125 Pharyngeal stimulation is usually associated with full expression of the oral, pharyngeal, and esophageal phases of deglutition. Lower esophageal sphincter relaxation without swallow can be induced by instillation of minute amounts of liquid into the hypopharynx in humans126, 127 and light stroking of the pharynx or low-frequency stimulation of the superior laryngeal nerve in the opossum.75 This reflex depends on the afferent nerve fibers from the pharynx or larynx traveling in the vagus and glossopharyngeal nerves to the NTSs.
Factors Modulating the Rate of Transient Lower Esophageal Sphincter Relaxations
In both healthy humans and dogs, the stimulation of TLESRs produced by gaseous gastric distension is almost totally suppressed in the supine posture.128, 129 In patients with reflux disease, TLESRs occur less frequently in the supine and lateral recumbent positions compared to the sitting position. Transient LES relaxation does not occur during stable sleep130; reflux episodes that do occur during the nighttime sleep periods are totally confined to periods of arousal during sleep that may last for only 10 seconds. Spontaneous TLESRs are also completely suppressed in dogs by light general anesthesia.131 Cold stress has also been shown to reduce the frequency of TLESRs.132
Effect of Antireflux Therapy on Transient Lower Esophageal Sphincter Relaxation
In patients with reflux disease, the presence or absence of endoscopically visible esophagitis does not influence the rate of TLESRs after meals. However, the effect of healing of esophagitis with acid suppressants on the rate of TLESRs is controversial; omeprazole has been reported to have no effect, whereas H2 antagonists seem to decrease the rate of TLESRs.133 At standard doses, cisapride does not appear to influence the rate of TLESRs up to 3 hours after a meal.134 Although a plethora of studies have investigated the effect of antireflux surgery on LES function, only two have examined the effect on TLESRs.135, 136 They show that fundoplication reduces TLESR frequency by 50%. The mechanisms underlying this effect include a possible a reduction in the degree of distention of the gastric cardia by the gastric wrap, which may reduce the gastric distention–induced stimulation of TLESRs.
Neural Pathways Mediating Transient Lower Esophageal Sphincter Relaxation
Transient LES relaxation is a neural reflex with afferent and efferent pathways and a pattern generator located in the swallow center of the brainstem. It appears that the efferent pathway for TLESR is similar to the deglutition-induced relaxation of the LES (i.e. the vagus nerve).2 Transient LES relaxations are completely abolished by cooling of the cervical vagus in dogs,137 and eructation is substantially inhibited by truncal vagotomy 5 cm above the diaphragm.138 Section of splanchnic nerves (sympathectomy) does not affect the induction of TLESR.139 The absence of TLESRs in patients with achalasia suggests that TLESR shares a final common pathway with swallow-induced LES relaxation.140 The afferent pathway, to a large degree, begins with stimulation of tension receptors in the proximal stomach, particularly the gastric cardia. Penagini et al.141 found that the gastric receptors responsible for eliciting TLESR are sensitive to stretch rather than tension and should be called stretch receptors. The afferents from the gastric mechanoreceptors traverse through the vagus nerve, via nodose ganglion into the NTSs and to the DMV via interneurons. The DMV contains the cell bodies of vagal efferent neurons, which project to the LES. In the opossum, intrinsic gastric nerves independent of extrinsic nerves can induce LES relaxation.142 However, it seems unlikely that a local pathway could play a major role in induction of TLESR because of the observation that the latter is completely blocked by cervical vagal cooling.137
Selective inhibition of the diaphragmatic sphincter, which is characteristic of TLESRs, also occurs during vomiting143 and is coordinated through the brainstem. The precise mechanism by which the LES and the diaphragmatic sphincter are coordinated during TLESR is not clear.
The pathway for the triggering of TLESRs is shown in Figure 7. The basic element is a vagal reflex pathway triggered by gastric distention or pharyngeal stimulus and integration that occurs in the brainstem. The threshold for triggering TLESR may be lowered by concurrent stimulation of the pharynx (and possibly larynx) and increased potentially by the supine posture, sleep, and anesthesia. A pattern generator in the brainstem that mediates esophageal, LES, gastric, and diaphragmatic events during TLESR controls the efferent vagal output. Under usual circumstances, the pharyngeal components of deglutition are bypassed, but these can be partly activated on occasion causing the small pharyngeal and mylohyoid complexes that occur with some TLESRs.
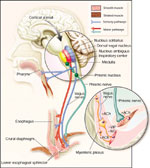
Figure 7: Neural pathways to the LES and crural diaphragm.
Esophageal peristalsis and relaxation of the LES induced by swallow result from the excitation of receptors in the pharynx. The afferent stimulus travels to the sensory nucleus, the nucleus solitarius (small insert). A programmed set of events from the dorsal vagal nucleus and the nucleus ambiguus mediates esophageal peristalsis and sphincter relaxation. The vagal efferent fibers communicate with myenteric neurons that mediate LES relaxation (large inset). The postganglionic transmitters are nitric oxide (NO) and vasoactive intestinal peptide (VIP). Transient lower esophageal sphincter relaxation (TLESR), the principal mechanism of reflux, appears to use the same efferent neural pathway as the swallow reflex. The afferent signals for TLESR may originate in the pharynx, larynx, or the stomach. The efferent pathway is in the vagus nerve, and nitric oxide is the postganglionic neurotransmitter responsible for LES relaxation. Contraction of the crural diaphragm is controlled by the inspiratory center in the brainstem and the nucleus of the phrenic nerve. The crural diaphragm is innervated by right and left phrenic nerves through nicotinic cholinergic receptor acetylcholine (Ach). +, excitatory effects; –, inhibitory effects. (Source: Mittal and Balaban2. Copyright © 1997. Massachusetts Medical Society.)
Neurotransmission in the Neural Circuit of Transient Lower Esophageal Sphincter Relaxation
The efferent neural circuitry and neurotransmitters for deglutition-induced LES relaxation and TLESR are the same. Nitric oxide is the final mediator of the LES and diaphragmatic sphincter inhibition. Various neuropharmacologic agents that have been shown to influence TLESR frequency are listed in Table 2. Nitric oxide antagonist decreases TLESR frequency in dogs, cats, and humans.144, 145, 146 Sensitivity of gastric afferent pathways can be manipulated to affect TLESR frequency. Baclofen, a GABA-B agonist reduces the frequency of TLESR dramatically in all species studied to date, including humans.147, 148, 149, 150 It seems that the inhibitory effect of baclofen on TLESR is mediated at several levels; it reduces afferent discharges of gastric mechanoreceptors at the peripheral level.151, 152 At the central nervous system GABA-B activity exerts a tonic inhibitory influence on the premotor neurons of the DMV, and baclofen facilitates this inhibitory influence.153, 154 Baclofen also inhibits synaptic transmission in the inhibitory motor neuron of the myenteric plexus located within the wall of the esophagus.155 Glutamate, working through mGlu group II and mGlu group III receptors, reduces afferent nerve sensitivity of the gastric mechanoreceptors.156 Glutamate is synthesized in the nodosa ganglion and transported peripherally to the afferent nerve endings in the stomach. Galanine also has affects on the mechanosensitivity of gastric afferents.157 More recently, the cannabinoid receptor (CR1) agonist has been found to be a potent inhibitor of TLESR, but in the case of CRI the effects are mediated at the level of the brainstem49 and not at gastric afferents. The cholecystokinin (CCK)-A receptor antagonists,158 atropine,159 and morphine160 also reduce TLESR frequency. The effect of CCK-A antagonists is mediated through a peripheral rather than a central mechanism. Infusion of CCK-A increases the frequency of TLESRs, and this increase can be abolished by administration of a nitric oxide antagonist.145 Atropine's effect appears to be mediated at the level of the brainstem.161 The effect of morphine is reversed by naloxone, suggesting that the effect is mediated through  receptors.
receptors.
It should be mentioned that none of the pharmacologic agents abolishes TLESR completely. Rather, they all reduce the frequency of TLESR, which is ideal from the point of view of clinical usefulness. Complete blockade would result in undesirable side effects such as bloating, difficulty belching, and difficulty vomiting (similar to fundoplication). The therapeutic potential, however, for most of the pharmacologic agents that inhibit TLESR remains to be determined.