Key Points
- Esophageal peristalsis results from sequential contraction of circular muscle, which serves to push the ingested food bolus toward the stomach. Esophageal longitudinal muscle may also play a role in peristalsis.
- Swallow-induced peristalsis is called primary peristalsis, and the peristalsis elicited by esophageal distention is called secondary peristalsis.
- Peristaltic contractions are always preceded by inhibition that, in the case of primary peristalsis, is called deglutitive inhibition.
- Peristalsis in the striated muscle part of the esophagus is dependent on central mechanisms, involving sequential activation of vagal lower motor neurons in the vagal nucleus ambiguus.
- Peristalsis in the smooth muscle of the esophagus is dependent on both central and peripheral mechanisms.
- The central mechanism involves patterned activation of the preganglionic neurons in the dorsal motor nucleus of the vagus that project onto inhibitory and excitatory neurons in the esophageal myenteric plexus.
- The peripheral mechanism involves regional differences in the inhibitory and excitatory intramural nerves and intrinsic properties of the muscle.
- Intramural inhibitory nerves act by releasing nitric oxide (NO) and vasoactive intestinal peptide, whereas the excitatory nerves release acetylcholine and substance P.
Introduction
The esophagus is a hollow muscular tube, closed proximally and distally by muscular sphincters.1 The esophageal wall is composed of distinct layers. The inner mucosal layer consists of squamous epithelium and underlying connective tissue, within which lies a longitudinally oriented muscle layer called the muscularis mucosa. The function of this muscle layer is unclear, but it likely is involved in mucosal movement. The outer muscular coat, known as the muscularis propria, is involved in bolus transport and consists of an inner layer of circularly oriented muscle fibers and an outer layer of longitudinally oriented fibers. In between these two muscle layers lies the myenteric plexus, which controls the motor function of these muscles. The upper esophageal sphincter (UES) and proximal one third of esophageal body is composed of striated muscle. There is then a transition zone where striated and smooth muscle intermix. The lower esophageal sphincter (LES) and the distal one half to two thirds of the esophageal body are composed of smooth muscle.
The main function of the esophagus is to propel swallowed food or fluid into the stomach. This occurs through sequential or "peristaltic" contraction of circular muscle in the esophageal body, in concert with appropriately timed relaxation of the upper and lower esophageal sphincters. The esophagus also must clear any refluxed gastric contents back into the stomach and takes part in vomiting and belching.
Esophageal peristalsis can be initiated by deglutition ("primary" peristalsis) or local distention ("secondary" peristalsis). Deglutition is one of the most complex reflex neural activities. The initial phase is voluntary when food is chewed, mixed with saliva and formed into a bolus before being pushed to the posterior pharynx by the tongue. Receptors in the posterior pharynx are then activated to initiate the involuntary phase of deglutition, which involves carefully sequenced contraction of numerous head and neck muscles. The food bolus is rapidly pushed toward the esophagus by the pharyngeal constrictor muscles. Concurrently, there is activation of muscles that lift the palate and close off and elevate the larynx in order to prevent misdirection of the bolus into the nasopharynx and larynx, respectively. The UES opens almost immediately upon activation of the deglutition reflex, allowing the food bolus to pass through. It then rapidly shuts to prevent retrograde passage of the bolus. Once this oropharyngeal phase has served to propel the bolus into the esophagus, the esophageal phase of deglutition takes over. This involves two major phenomena, namely the sequential contraction of circular muscle of the esophageal body, which results in a peristaltic wave that pushes the food toward the stomach, and relaxation and opening of the LES.
This review focuses on the physiologic mechanisms underlying peristalsis in the esophageal body. The reader is referred to other comprehensive reviews for detailed discussion of the physiology of the LES and oropharyngeal phase of deglutition.2, 3, 4, 5, 6
Methods of Study
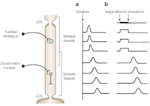
A number of methodologies have been used to study motility of the esophagus. Perhaps the simplest technique is to observe the transport of a swallowed barium bolus using videofluoroscopy (Video 1). This technique has been particularly useful to assess function and dysfunction of the oropharyngeal phase of swallowing. Esophageal manometry has been used in humans and animal models to measure contraction of the circular muscle layer of the esophagus. Catheters with either perfused side holes or strain gauge transducers can be placed intraluminally to record pressure transients caused by circular muscle contraction. These pressure sensors are spaced at fixed distances along the catheter; therefore, it is possible to record pressure transients associated with the peristaltic wave as it moves down the esophagus (Figure 1). Much of what we know now about the physiology and pharmacology of the esophagus in humans has been dependent on the use of intraluminal manometry; however, recently introduced techniques have enhanced our understanding of esophageal peristaltic function and dysfunction in humans. For instance, intraluminal impedance recording has been used to assess esophageal bolus transit in humans.7 This technique is based on monitoring changes in impedance between a series of paired electrodes spaced at predetermined intervals along an intraluminal catheter. Air, solids, and liquids each produce their own characteristic change in interelectrode impedance; therefore, the technique is capable of determining either antegrade or retrograde movement of any of these substances within the esophageal lumen. High-frequency intraluminal ultrasound has also been introduced as a novel means of monitoring contraction of different muscle layers during peristalsis by recording changes in the thickness of the muscle layer.8, 9 This may be particularly useful to record contraction of longitudinally oriented muscle, because contraction of these muscle layers does not result in a detectable change in intraluminal pressure.
Figure 1: Primary peristalsis as recorded by an intraluminal manometry catheter.
Pharyngeal contraction coincides with relaxation of the upper esophageal sphincter (UES). This is then followed by sequential (peristaltic) phasic contraction along the esophageal body, which propels the swallowed bolus toward the stomach. The lower esophageal sphincter (LES) relaxes soon after the initiation of the swallow and remains relaxed until the peristaltic wave arrives. (Source: Goyal and Paterson,2 with permission from The American Physiological Society.)
Video 1: Videofluoroscopy of deglutition and primary peristalsis.
The subject swallows a mouthful of barium sulfate, which is then propelled down the esophagus and into the stomach by a peristaltic contraction wave.
For more in-depth study of the neuromuscular physiology of the esophagus, in vitro studies are performed. Much has been learned by stimulating intramural nerves of muscle strips in tissue baths and recording the motor response. In addition, single smooth muscle contraction studies and intracellular electrophysiologic recordings or patch clamp recordings from single cells have been used to elucidate important information regarding the physiology of peristalsis.
General Description of Peristalsis (Figure 1)
With deglutition, the peristaltic wave follows immediately after the UES relaxation, producing a lumen-occluding contraction of the esophageal circular muscle. The contraction wave migrates aborally at a speed that varies along the esophagus. The peristaltic velocity averages about 3 cm/sec in the upper esophagus, then accelerates to about 5 cm/sec in the mid-esophagus, and slows again to approximately 2.5 cm/sec distally.10 The duration and amplitude of individual pressure waves also varies along the esophagus. The duration of the wave is shortest in the proximal esophagus (approximately 2 seconds) and longest distally (approximately 5 to 7 seconds).10 Peak pressures average 53  9 mmHg in the upper esophagus, 35
9 mmHg in the upper esophagus, 35  6 mmHg in the midportion, and 70
6 mmHg in the midportion, and 70  12 mmHg in the lower esophagus.10 These parameters can be influenced by a number of variables including bolus size, viscosity, patient position (e.g., upright vs. supine), and bolus temperature.11, 12, 13, 14, 15, 16, 17, 18, 19 For instance, a large bolus elicits stronger peristaltic contractions that migrate distally at a slower rate than a small bolus. The peristaltic velocity is also slowed by outflow obstruction or increases in intraabdominal pressure. Warm boluses tend to enhance, whereas cold boluses inhibit the amplitude of peristaltic contractions. These alterations are likely mediated by local neuromuscular reflexes as well as by vagovagal reflexes. In addition, the nature of the stimulus that triggers deglutition may affect the amplitude of the resulting peristaltic contractions.20 Furthermore, water stimulation of the pharynx inhibits a peristaltic wave already in progress.21
12 mmHg in the lower esophagus.10 These parameters can be influenced by a number of variables including bolus size, viscosity, patient position (e.g., upright vs. supine), and bolus temperature.11, 12, 13, 14, 15, 16, 17, 18, 19 For instance, a large bolus elicits stronger peristaltic contractions that migrate distally at a slower rate than a small bolus. The peristaltic velocity is also slowed by outflow obstruction or increases in intraabdominal pressure. Warm boluses tend to enhance, whereas cold boluses inhibit the amplitude of peristaltic contractions. These alterations are likely mediated by local neuromuscular reflexes as well as by vagovagal reflexes. In addition, the nature of the stimulus that triggers deglutition may affect the amplitude of the resulting peristaltic contractions.20 Furthermore, water stimulation of the pharynx inhibits a peristaltic wave already in progress.21
When pharyngeal swallows are well spaced, the esophagus responds on a one-to-one basis. However, if swallows are taken in rapid succession, esophageal activity is inhibited until the last swallow in a series.22, 23 This phenomenon is called deglutitive inhibition and reflects the predominant inhibitory discharge to the esophageal musculature, or the central inhibitory phenomenon that occurs with each swallow (Figure 2).
Peristalsis in the Striated Muscle Esophagus
Like striated muscle in other parts of the body, the striated muscle segment of the esophagus is dependent on excitatory nerve activity from lower motor neurons. The striated muscle of the esophagus is innervated by myelinated vagal lower motor neurons whose cell bodies are located in the nucleus ambiguus and nucleus retrofacialis.24, 25 A small number of cell bodies may also arise in the dorsal motor nucleus (DMN) of the vagus. These nerve fibers contain choline acetyltransferase and calcitonin gene-related peptide (CGRP) and synapse directly on the motor end plates. Acetylcholine is the primary neurotransmitter involved in activation of esophageal striated muscle. The role of CGRP is unknown. Bilateral cervical vagotomy above the origin of the pharyngoesophageal branches abolishes peristalsis in the striated muscle esophagus.1, 26 However, unilateral vagotomy has no effect on peristalsis, presumably because of extensive crossover of vagal innervation within the esophageal wall.27 Innovative experiments performed by Roman27 established that vagal efferent neurons destined for the striated muscle esophagus fire sequentially. They used the central portion of the sectioned vagus in sheep to reinnervate the sternocleidomastoid and trapezius muscles from which they were able to record electrical activity. Activation of deglutition induced sequential contraction of the reinnervated muscles that coincided with peristaltic contractions simultaneously measured by intraluminal manometry.
A scant myenteric plexus does exist within the striated muscle esophagus, but its role in esophageal motor function is unclear. Interestingly, it has been demonstrated that motor end plates in the striated muscle esophagus are co-innervated by vagal lower motor neurons and nitrergic myenteric plexus neurons.28, 29, 30, 31 It has been speculated that this myenteric innervation may provide an inhibitory counterbalance to the predominant vagal excitatory innervation.31
Peristalsis in the Smooth Muscle Esophagus
Control of peristalsis in the smooth muscle segment of the esophagus is more complicated than in the adjacent striated muscle segment. In the latter, the central nervous system not only initiates the primary and secondary peristaltic wave, but also completely controls the sequential nature of the contraction. In the smooth muscle esophagus, the central nervous system is required for activation of primary peristalsis and exerts some control over the sequencing of the peristaltic contractions. However, peristalsis can also occur independently of the central nervous system, which highlights the importance of neuromuscular mechanisms intrinsic to the esophageal wall in the generation of the peristaltic wave. Cell bodies of vagal efferent fibers that innervate the smooth muscle esophagus are largely in the dorsal motor nucleus although at least in the cat a small proportion may also reside in the nucleus retroambiguus.25
Central Control
The observation that either bilateral cervical vagotomy or vagal cooling abolishes swallow-induced peristalsis in the smooth muscle esophagus clearly demonstrates that input from the central nervous system is required to initiate the primary peristaltic wave.32, 33, 34 In addition, Tieffenbach and Roman34 suggested that primary peristalsis in the esophageal smooth muscle is mediated by sequential activation of vagal efferent fibers. This was based on electromyographic recordings from baboon skeletal muscle that had been reinnervated by vagal efferent fibers, in which muscle spike bursts that coincided with peristaltic activity in the smooth muscle esophagus were recorded. The pattern of this muscle discharge was sequential, indicating that vagal preganglionic fibers destined for the smooth muscle esophagus were being activated by a central sequencing mechanism. It was suggested that these vagal preganglionic efferent fibers synapse on postganglionic cholinergic fibers, which in turn sequentially activate the smooth muscle. However, these studies do not explain the initial inhibitory discharge that occurs prior to peristaltic contraction in response to deglutition. Gidda and Goyal35 recorded swallow-evoked potentials from single cervical vagal efferent fibers in the opossum and were able to distinguish two types of efferent discharge based on the latency to firing. Short latency fibers begin firing within 1 second of the onset of swallowing, whereas long latency fibers had latencies ranging between 1 and 5 seconds. It was postulated that short latency discharges correlated with the initial inhibition to the esophagus and that long latency discharges correlated with peristaltic contractions. These experimental data suggested that in addition to initiating primary peristalsis, vagal efferent discharge might also modulate the speed, amplitude, and duration of the peristaltic wave.
Peripheral Neurogenic Control
The peripheral neuromuscular control mechanisms involved in peristalsis of the esophageal circular smooth muscle has been an area of intense interest and investigation for many years. A number of observations clearly establish the importance of intrinsic neuromuscular mechanisms in the generation of the peristaltic wave. As mentioned above, sequential firing does occur in vagal efferent nerves, and the vagus is needed for initiation of primary peristalsis. However, peristalsis can be induced by local distention and electrical stimulation of an esophagus devoid of extrinsic innervation. Furthermore, simultaneous electrical activation of all vagal efferent nerve fibers induces peristalsis after a variable delay, rather than an immediate simultaneous contraction36, 37, 38, 39 (Figure 3). This indicates the prime importance of peripheral neuromuscular mechanisms in the generation of peristalsis.
Figure 3: Schematic representation of esophageal contractions.
Swallowing (a) evokes a peristaltic wave of contraction that migrates smoothly from the striated to smooth muscle esophagus. Simultaneous electrical activation of all vagal efferent neurons (b) produces simultaneous contractions in the striated muscle esophagus, which would be expected based on the direct innervation of this muscle by the vagal efferent neurons. However, in the smooth muscle segment a peristaltic wave is induced. This is because intrinsic neurons activated by vagal efferent nerve stimulation are capable of evoking a peristaltic contraction without the need for centrally mediated sequencing. (Source: Goyal and Paterson,2 with permission from the American Physiological Society.)
Tension recording studies of isolated circular smooth muscle strips that have intrinsic but not extrinsic innervation have elegantly demonstrated an intrinsic "latency gradient" of contraction along the esophagus that appears to contribute to the generation of the peristaltic wave.40 Most of these studies have been performed in the opossum model. Short-duration electrical stimulation of the intrinsic nerves of a circular smooth muscle strip results in a contraction that occurs after the stimulus has ended (the so-called off response). The onset of this contraction relative to the stimulus increases in strips taken from more aboral segments of the smooth muscle esophagus (Figure 4). This latency gradient has been shown to relate to the initial inhibition or hyperpolarization that occurs upon nerve stimulation. In other words, with nerve stimulation there is first release of an inhibitory neurotransmitter that causes hyperpolarization of the membrane. The duration of this hyperpolarization is longer aborally,41 so that the ensuing contraction is delayed aborally. This initial hyperpolarization of the circular smooth muscle membrane potential has also been recorded in the opossum in vivo in response to swallows.42 Furthermore, a wave of initial inhibition can be recorded in humans by creating an artificial high-pressure zone using a partially distended balloon.43 The reason for the progressive increase in duration of the initial hyperpolarization in the proximal versus distal smooth muscle esophagus is unclear. It could represent a relative increase in the release or local effects of inhibitory neurotransmitter distally, or alternatively, a relative increase in excitatory neurotransmitter release or effects proximally. There is no direct evidence in support of either of these possibilities, but studies in several species44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 have shown that atropine delays the onset of peristaltic contractions, with a greater effect in the proximal than distal esophagus, whereas inhibition of nitric oxide (NO) shortens the latency of contraction, with a more pronounced effect distally than proximally (see below). To date, there has been no morphologic evidence of a gradient in the density of cholinergic or nitrergic innervation along the esophagus. This raises the possibility that intrinsic differences in smooth muscle responses along the esophagus may result in a varied response to the same quantum of released neurotransmitter. There is some evidence in support of this hypothesis. Decktor and Ryan56 noted a decrease in resting membrane potential along the smooth muscle portion of the opossum esophagus, with resting membrane potential being less negative distally. A gradient in potassium content along the opossum smooth muscle esophagus has also been reported.57 A subsequent study, however, failed to show differences in resting membrane potential at different sites along the esophagus.58 Furthermore, the electrical properties of the smooth muscle cell membrane were no different at proximal versus distal sites. More recently, Diamant and colleagues59, 60, 61, 62, 63 have reported a number of regional myogenic differences along the feline smooth muscle esophagus, which may contribute to different responses to neurotransmitters. These differences include the following: (1) A more depolarized resting membrane potential was found at 4 cm as compared to 2 cm above the LES, owing at least partly to higher sodium permeability distally.59 In addition, an increased density of voltage-dependent potassium channels was noted at the 4 cm site. (2) Regional differences in the soluble N-ethylenemaleimide sensitive factors attachment protein receptor (SNARE) protein SNAP-25 61, which appears to regulate potassium channels.62 (3) There are differences in the esophageal circular smooth muscle response to stretch and cholinergic stimulation, with strips from more proximal regions being more responsive.60 (4) There is an increased expression and current density of L-type calcium channels in the proximal versus the distal smooth muscle esophagus.61 These interesting observations explain some of the differences in contractile patterns along the esophagus and may also explain regional differences in response to neurotransmitters.
Figure 4: Electrical stimulation of intrinsic nerves in circular smooth muscle strips.
Stimulation results in phasic contraction after a variable delay. The latency to onset of this contraction increase progressively in strips taken from more aboral segments of the smooth muscle esophagus. This demonstrates the existence of an "intrinsic latency gradient" of contraction along the esophagus, which contributes to the generation of a peristaltic wave.
Although an aborally increasing gradient in the duration of the initial inhibition is an attractive model to explain peristalsis, the calculated speed of peristalsis based on intrinsic differences in the initial inhibition along the esophagus is on the order of 10 cm/sec,41 which is much faster than peristalsis in vivo. Thus, there must be mechanisms other than the intrinsic latency gradient to explain peristalsis. Experiments in which simultaneous electrical and mechanical activity were recorded in both the proximal and distal opossum smooth muscle esophagus have helped clarify the discrepancy between the in vitro and in vivo observations.42 In this study, it was shown that an initial monophasic inhibitory potential occurs along the esophagus with either swallowing or balloon distention. In keeping with the electrophysiologic and muscle strip studies, the duration of the initial hyperpolarization was slightly longer distally than proximally, but this difference was insufficient to explain the marked delay of esophageal contraction in the distal versus the proximal smooth muscle esophagus. Rather, in the distal esophagus the initial monophasic inhibitory potential was followed by a second wave of hyperpolarization before the membrane potential rebounded into depolarization and initiation of spike potentials (Figure 5). It was suggested that this secondary hyperpolarization is likely due to reactivation of descending inhibitory neurons by distention or contraction of the more proximal esophagus in the course of peristalsis (Figure 6). This suggests that intramural descending inhibitory pathways are crucial in generating the peristaltic wave. Subsequent studies in the opossum have demonstrated that localized distention appears to directly activate intrinsic nitrergic inhibitory neurons that send long aboral projections.64 In these experiments, a triple chamber organ bath was used to chemically and electrically isolate the intact opossum smooth muscle esophagus into proximal, middle, and distal segments. The peristaltic reflex was then activated by distending an intraluminal balloon in the proximal segment, whereas electrical and mechanical responses were recorded in the distal segment. Interestingly, whereas tetrodotoxin placed in the intermediate chamber abolished the descending peristaltic reflex, removing calcium and adding high concentrations of magnesium to the intermediate chamber, which blocks synaptic transmission, had no effect, indicating that the intramural neurons mediating this response traveled for at least 3 cm without synapsing on interneurons. Furthermore, although removing calcium from the distending chamber inhibited the response, this was likely due to an effect on muscle tension generation in response to stretch, as application of antagonists of all known esophageal neurotransmitters to the distending chamber had no effect. Also, the calcium channel blocker nifedipine, when placed in the oral (distending) chamber, decreased the muscle tension generated in response to distention and also inhibited the distal response.65 These long tension-activated descending neurons provide an important intrinsic mechanism to ensure that the distal esophagus remains inhibited as the bolus traverses the esophagus, irrespective of the speed of bolus transit.
Figure 5: Simultaneous recording of electrical and mechanical activity in opossum smooth muscle esophagus.
a: The response to swallowing is marked by the mylohyoid muscle electromyogram. With primary peristalsis, note that the delay in onset of depolarization, spike burst, and esophageal contraction in the distal esophagus relates to a marked secondary hyperpolarization (arrow). b: With mid-esophagus balloon distention in the left tracing, the peristaltic velocity is abnormally fast, and it is apparent that the slight delay in onset of contraction at the distal vs. proximal site is due to an initial hyperpolarization of slightly longer duration. However, when balloon distention induces a secondary peristaltic wave of normal velocity (right panel), the marked delay in onset of contraction distally correlates with a secondary hyperpolarization (arrow) that is likely due to reactivation of intrinsic descending inhibitory pathways by contractions occurring upstream. (Source: Paterson,44 with permission.)
Figure 6: Model showing the marked delay in onset of distal esophageal contractions during peristalsis.
With the initial stimulation, be it swallowing or distention, there is a short-lived hyperpolarization that is slightly longer in more aboral region of the esophagus. Intrinsic descending inhibitory neurons can then be continuously reactivated by contraction or bolus distention as it migrates down the esophagus.
Neurotransmitters Involved in Esophageal Peristalsis: Evidence of Dual Peripheral Innervation
Vagal efferent neurons involved in esophageal peristalsis synapse on both inhibitory and excitatory myenteric neurons. Ganglionic transmission is predominantly nicotinic, although there may be associated muscarinic and serotonergic transmission as well.66, 67, 68 Immunohistochemical stains for neurotransmitter content reveal a large number of different peptide and nonpeptide chemicals within the esophageal myenteric plexus.29, 69, 70, 71 However, it appears that two types of motor nerves predominate. One stains for NO synthase and vasoactive intestinal peptide, and the other for choline acetyl transferase and substance P. Nitric oxide is the predominant inhibitory neurotransmitter, whereas acetylcholine, acting on muscarinic receptors, is the predominant excitatory neurotransmitter. Evidence for this dual innervation comes from a number of sources. In the opossum model, nerve stimulation of isolated circular smooth muscle strips produces a predominant "off" contraction (i.e., the contraction occurs after the electrical stimulation ends) that was found to be resistant to both adrenergic and cholinergic blockade.40, 72 Subsequent studies have shown that this off contraction can be blocked by a NO synthase (NOS) inhibitor.53, 73, 74 However, depending on the parameters of nerve stimulation, atropine sensitive contractions may also be induced.75, 76 Furthermore, in the presence of a NOS inhibitor, a predominant cholinergic contraction in response to nerve stimulation becomes unmasked.53 Similar observations have been made in human esophageal muscle strip studies.77
These observations are supported by studies using vagal efferent nerve stimulation.39, 51, 53 With a short train of nerve stimulation, contractions are induced along the smooth muscle esophagus after the stimulus is over. These are often peristaltic in nature, but this can be influenced by adjusting the electrical stimulus parameters. If a long stimulus train is used, however, both an intrastimulus contraction (A wave) and a poststimulus contraction (B wave) are frequently observed.39, 51, 53 The intrastimulus contraction is usually peristaltic and is blocked by atropine, whereas the poststimulus contraction is blocked by a NOS inhibitor and is either simultaneous in onset or has a very rapid peristaltic velocity. Whether A waves, B waves, or both are induced by long train vagal efferent stimulation depends on the stimulus frequency used. Low-frequency stimulation favors A waves, whereas high-frequency stimulation favors B waves, implying that intrinsic cholinergic and nitrergic neurons respond differently to different intensity stimuli. Interestingly, administration of atropine not only blocks A waves, but also unmasks or enhances B waves, whereas NOS inhibition does the opposite (Figure 7). When both atropine and a NOS inhibitor are applied together, both A and B waves are abolished.51 Similarly, both antagonists are required to completely abolish swallow-induced peristalsis in the opossum model.51 These studies provide support for the concept that vagal efferent nerve fibers innervate both inhibitory (nitrergic) and excitatory (cholinergic) neurons. It thus appears that the normal peristaltic wave is a result of blended innervation that may vary along the esophagus. Cholinergic neurons activate contraction by directly depolarizing the muscle. On the other hand, nitrergic neurons presumably cause contraction through a "rebound" depolarization following an initial hyperpolarization; that is, NO serves as both an inhibitory and excitatory neurotransmitter.
Figure 7: Evidence of dual innervation of esophageal peristalsis.
a: Vagal efferent nerve simulation using high stimulus frequency produced a B wave only (i.e., contraction after the end of stimulation). Following administration of N -nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO synthase, the B wave is abolished, and an A wave (i.e., intrastimulus contraction) is unmasked, which in turn is abolished by the administration of atropine. Subsequent administration of L-arginine (a substrate of NO synthase that reverses the effect of L-NAME), the B-wave returns. b: Following the administration of L-NAME, the amplitude of the swallow-induced peristaltic wave is diminished and peristaltic velocity increases owing to a shortening of the onset of contraction in the distal esophageal site (1 cm above the LES). Subsequent administration of atropine abolishes primary peristalsis in the opossum smooth muscle esophagus. (Source: Anand and Paterson,51 with permission from the American Physiological Society.)
-nitro-L-arginine methyl ester (L-NAME), an inhibitor of NO synthase, the B wave is abolished, and an A wave (i.e., intrastimulus contraction) is unmasked, which in turn is abolished by the administration of atropine. Subsequent administration of L-arginine (a substrate of NO synthase that reverses the effect of L-NAME), the B-wave returns. b: Following the administration of L-NAME, the amplitude of the swallow-induced peristaltic wave is diminished and peristaltic velocity increases owing to a shortening of the onset of contraction in the distal esophageal site (1 cm above the LES). Subsequent administration of atropine abolishes primary peristalsis in the opossum smooth muscle esophagus. (Source: Anand and Paterson,51 with permission from the American Physiological Society.)
As alluded to above, significant regional differences in the balance between cholinergic and noncholinergic (i.e., nitrergic) influences appear to exist along the esophagus. For instance, in vitro muscle studies using opossum esophageal circular smooth muscle have shown that atropine has a greater effect on amplitude and latency of contraction in the proximal as compared to the distal esophagus.75, 76 A similar observation has been made in vivo both in human and animal models,45, 46 where administration of atropine prolongs the latency of contraction, with a greater effect proximally than distally. Amplitude of contraction is also preferentially affected proximally. On the other hand, inhibition of NO shortens the latency and decreases contraction amplitude more so in the distal than in the proximal smooth muscle esophagus.50, 51, 52, 53, 54, 55
The physiologic role of other neurotransmitters found within the smooth muscle esophagus is unclear, as studies often fail to clearly differentiate a pharmacologic from a physiologic effect. Using an antagonist of the neurokinin-2 receptor, Krysiak and Preiksaitis78 found that tachykinins contribute to part of the noncholinergic excitatory response evoked by electrical stimulation of human circular smooth muscle strips. Enkephalins also may modulate peristalsis by presynaptic inhibition or excitation of neurotransmitters directly responsible for peristalsis.79, 80 In addition, catecholamines81 and CGRP82 inhibit esophageal contractions. Exogenously administered galanin appears to inhibit noncholinergic esophageal nerves,83 but a galanin antagonist had no noticeable effect on esophageal peristalsis.84
Role of Interstitial Cells of Cajal
In recent years there has been considerable interest in the role of interstitial cells of Cajal (ICCs) in the control of gastrointestinal GI motility. These cells are believed to be the pacemakers for the stomach and intestine. There is also evidence that they are intercalated between nerves and muscle, and therefore serve as relay stations in neuromuscular transmission. Spontaneous rhythmic contractions have been recorded from esophageal longitudinal smooth muscle at rest,85, 86 but these contractions are nifedipine-sensitive and unlikely to be due to pacing by ICCs. However, morphologic studies have reported intercalation of ICCs between nerve endings and esophageal smooth muscle cells in the opossum.87, 88 Unlike the LES and other regions of the GI tract, there is as yet no physiologic evidence that ICCs are involved in neuromuscular transmission in the esophageal body. This is largely because the ICC-deficient mouse, which has been used for physiologic studies, does not have a smooth muscle esophageal body. It has been speculated that esophageal body ICCs are involved in neuromuscular transmission, or serve as tension receptors that then relay information to intrinsic or extrinsic neurons.
Ionic and Second Messenger Mechanisms of Esophageal Circular Smooth Muscle Contraction
From the above discussion it is clear that both NO and acetylcholine are crucial neurotransmitters in generation of the peristaltic wave. Nitric oxide produces hyperpolarization of the circular smooth muscle cell membrane via a cyclic guanosine monophosphate (cGMP)-dependent pathway,89, 90 thereby causing inhibition of voltage-dependent calcium entry. There has been considerable controversy surrounding the mechanisms whereby NO induces membrane hyperpolarization.91, 92 It was initially proposed that the nitrergic inhibitory junction potential (IJP) in esophageal circular smooth muscle was due to opening of potassium channels.93, 94, 95 This was based on indirect studies, which explored the effect of altering potassium gradients across the cell membrane on the IJPs. In further support of this, NO donors were reported to activate multiple types of potassium channels and whole cell potassium currents in different smooth muscles, including esophageal.96, 97, 98, 99 These currents were sensitive to tetraethylammonium, apamin, quinine, or 4-aminopyridine. However, selective potassium channel blockers failed to abolish the nitrergic IJP in opossum esophageal circular smooth muscle,93, 94 suggesting that opening of potassium channels may not underlie the hyperpolarization caused by neurally released NO. Based on experiments utilizing chloride substitution and application of the anion channel blocker 4,4'–diisothiocyanostilbine–2,2'–disulfonic acid, Crist et al.100 proposed that the nitrergic IJP was due to closing of calcium-activated chloride channels. Subsequently, Zhang et al.,101 using the patch clamp technique, provided further evidence that NO blocks calcium-activated chloride currents in esophageal circular smooth muscle. More recently, Zhang and Paterson102 reported that the nitrergic IJP could be blocked by two different calcium-activated chloride channel blockers, namely niflumic acid and 9-anthroic acid. Furthermore, they provided evidence that the nitrergic IJP is dependent on activation of myosin light chain kinase. At rest, the membrane potential of esophageal circular smooth muscle cells shows apparently random membrane potential fluctuations of 1 to 3 mV. Inhibitors of sarcoplasmic reticulum function or blockers of chloride channels markedly attenuate these random fluctuations, suggesting that they are due to spontaneous activation of chloride channels, primed by calcium release from the sarcoplasmic reticulum.103 This data provide further support for the hypothesis that the nitrergic IJP involves blockade of chloride currents.
Acetylcholine affects many ionic currents in esophageal circular smooth muscle including calcium-sensitive chloride and nonselective cation currents.104, 105 A release of calcium from intracellular stores appears to be required for activation of these currents. Chloride currents cause depolarization of the smooth muscle membrane, which in turn leads to entry of extracellular calcium via voltage-sensitive calcium channels. Activation of nonselective cation channels does not lead to significant membrane depolarization. Their activation may result in calcium entry by non–voltage-dependent mechanisms.106 Acetylcholine also inhibits a number of potassium channels, which would serve to enhance its excitatory effect.105
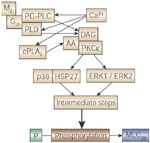
The second messenger pathways involved in acetylcholine-induced contraction are complex, and have recently been reviewed107 (Figure 8). Acetylcholine acts on muscarinic type 2 (M2) receptors that are linked to Gi3-type G proteins within the muscle membrane.108 These G proteins then activate phosphatidylcholine–specific phospholipase C and D to generate diacylglycerol, which interacts with arachidonic acid to activate protein kinase (PK) C .109 Calcium is required for activation of phospholipase and production of diacylglycerol and arachidonic acid, but PKC
.109 Calcium is required for activation of phospholipase and production of diacylglycerol and arachidonic acid, but PKC activity is calcium-independent.108, 110, 111 PKC
activity is calcium-independent.108, 110, 111 PKC is linked to two separate mitogen-activated protein kinase pathways; one involves extracellular signal-regulated kinase 1/2, and the other heat shock protein-27 linked to P38 kinase.111, 112 Either of these pathways can then ultimately lead, via activation of a series of intermediate proteins, to phosphorylation of myosin light chain kinase (MLC20), which triggers actin-myosin cross bridge cycling.
is linked to two separate mitogen-activated protein kinase pathways; one involves extracellular signal-regulated kinase 1/2, and the other heat shock protein-27 linked to P38 kinase.111, 112 Either of these pathways can then ultimately lead, via activation of a series of intermediate proteins, to phosphorylation of myosin light chain kinase (MLC20), which triggers actin-myosin cross bridge cycling.
Figure 8: Pathways involved in acetylcholine-induced contraction of esophageal circular smooth muscle.
AA, arachidonic acid ; cPLA2, cytosolic phospholipase A2; DAG, diacylglycerol; ERK, extracellular signal-regulated kinase; HSP, heat shock protein; M, muscarinic; MLC, myosin light chain ; P, inorganic phophate; PC-PLC, phosphatidylcholine phospholipase C; PKC, protein kinase C; PLD, phospholipase D; (Source: Harnett et al.,107 with permission from the American Physiological Society.)
Myogenic Mechanisms of Esophageal Peristalsis
Of interest, esophageal peristalsis can be induced in the smooth muscle esophagus in the presence of tetrodotoxin, which blocks all sodium channel–mediated action potentials in neurons.85, 113, 114 This can be evoked by long pulse duration electrical current that directly activates the muscle, esophageal distention, muscle membrane depolarization using high concentrations of K+, or pharmacologic stimulation. It is believed that there is polarization of the muscle-to-muscle communication such that depolarization of one smooth muscle cell will result in electrotonic spread of current to adjacent muscle cells in an aboral direction.115 These observations highlight the redundancy in physiologic control mechanisms in esophageal motility.
Secondary Peristalsis
Secondary peristalsis refers to peristalsis activated by esophageal distention. This can occur physiologically by food left behind after the primary peristaltic wave has passed, or by refluxed contents from the stomach. Unlike primary peristalsis, secondary peristalsis is not accompanied by deglutition with associated pharyngeal and upper esophageal sphincter motor function. In the striated muscle esophagus, distention activates a peristaltic reflex that is mediated by central mechanisms; distention activates vagal afferents, which in turn leads to sequential vagal efferent discharge to the striated musculature of the proximal esophagus.27, 116 On the other hand, secondary peristalsis in the smooth muscle esophagus is largely an intrinsic neuromuscular reflex. Indeed, luminal distention of an esophagus excised and placed in a tissue bath results in a peristaltic contraction.64, 65, 117, 118, 119 The luminal distention triggers an immediate contraction just at and proximal to the distending stimulus, which occurs in the presence of tetrodotoxin, suggesting that it is a purely myogenic contractile reflex.120 In the intact animal, however, contractions mediated by a central vagal reflex occur for a few centimeters proximal to the distending stimulus.120 Direct electrical recordings from the circular smooth muscle proximal to the distending stimulus in the opossum model have demonstrated a direct depolarization in response to balloon distention that is blocked by either bilateral cervical vagotomy or the administration of atropine.120 This provides direct evidence of separate vagal innervation of excitatory and inhibitory motor neurons. A similar, atropine-sensitive contraction orad to the balloon is also seen in humans.48 Aboral to the distending stimulus, central mechanisms do not appear to play a role. During the distending stimulus there is a descending inhibitory discharge, mediated predominantly by NO, which results in hyperpolarization and inhibition of the circular smooth muscle.51, 119, 121 This is then followed by rebound depolarization, spike bursts, and contraction. This peristaltic reflex is quite different from that described in the intestine, where the proximal excitation does not involve extrinsic innervation.122 Furthermore, in the small bowel, a series of interneurons and a number of different neurotransmitters are involved in the descending peristaltic reflex.123 However, in the opossum esophagus, interneurons do not appear to be involved. Rather, descending nitrergic neurons appear to be activated directly by the distending stimulus and send long descending inhibitory neural connections to the distal esophagus.63, 64
Role of Longitudinal Muscle and Muscularis Mucosa in Esophageal Peristalsis
Understandably, investigators have focused on the role of the circular smooth muscle in esophageal peristalsis; however, the longitudinal muscle also contracts in sequential fashion during peristalsis and appears to play a role in bolus transport. To date, studies on the physiology of the longitudinal muscle have focused entirely on the smooth muscle esophagus.
Whereas it is easy to conceptualize how aborally progressive lumen occluding contractions of the circular muscle serve to push the bolus toward the stomach, it is less obvious how longitudinal muscle contraction might be involved in this process. It has been proposed that the longitudinal smooth muscle contraction may facilitate peristalsis by two mechanisms: (1) by shortening the esophagus, the esophageal radius must increase, thereby increasing the lumen size ahead of the oncoming bolus123; (2) longitudinal contractions tend to slide the esophagus over the bolus and increase the density of the circular muscle fibers orad to the bolus, which in turn increase the efficiency of the circular muscle contraction2. Recent studies using a mathematical model based on fluid theory have provided evidence that local longitudinal muscle contraction results in marked reduction in local pressure and shear stress in the zone of circular muscle contraction, thereby reducing the peak contractile pressure required for bolus transit.124 In addition, observations in humans125 suggest that the magnitude of the propulsive force generated on an intraluminal bolus correlates with the degree of esophageal shortening during peristalsis.
Studies in the opossum have shown that the longitudinal muscle contracts sequentially in an aboral direction during primary peristalsis.126 However, it appears that unlike circular smooth muscle, the sequential nature of the esophageal longitudinal muscle contraction in the smooth muscle esophagus is mediated centrally via vagal efferents. The duration of longitudinal muscle contraction also appears to vary along the esophagus. Similar to circular muscle, contraction is longer distally than proximally.126
In vivo studies in the opossum model have also shown that the primary neurotransmitter involved in longitudinal smooth muscle contraction is acetylcholine. The muscarinic antagonist atropine virtually abolishes longitudinal muscle contraction and esophageal shortening in response to swallowing and vagal stimulation.39, 127, 128 However, infrequent noncholinergic contractions can be evoked, but the physiologic significance of these are unclear.128
In vitro studies have also shown that longitudinal muscle contraction is predominantly mediated by cholinergic neurons; however, with certain stimulus parameters a slowly developing and sustained longitudinal muscle contraction can be evoked, which is abolished by substance P desensitization.129 Recent studies suggest that this is mediated by substance P released from capsaicin-sensitive neurons and acting via neurokinin (NK)-2 receptors.130 It is unlikely that this substance P–mediated contraction is involved in normal peristalsis. However, it may play a role in the reflex longitudinal muscle contraction that occurs with acid reflux into the esophagus.131 There has been speculation that this substance P–mediated contraction may also be involved in certain esophageal pain syndromes.132
Nitric oxide has been reported to cause paradoxical contraction of esophageal longitudinal smooth muscle,86, 133, 134 but it is unclear whether this neurotransmitter is involved in physiologic contraction of this muscle layer. Nitric oxide synthase inhibition appeared to decrease swallow-induced esophageal shortening in the cat, but evidence for a NO-mediated neural response could not be found in vitro in this species.134
Although there is evidence that the longitudinal smooth muscle may participate in deglutitive inhibition,135 there is no evidence to date that this is related to direct inhibitory innervation to the longitudinal smooth muscle. Elegant studies in which electrical activity was recorded from a flap of isolated longitudinal smooth muscle in vivo showed no evidence of an inhibitory junction potential occurring during primary peristalsis.136
Unlike the adjacent circular smooth muscle, at rest the longitudinal smooth muscle may contract in a cyclical pattern, and intracellular recordings have revealed rhythmic depolarization-repolarization with a frequency of 2 to 3/min.85, 86 This cyclic electrical activity is abolished by nifedipine.86 The physiologic significance, if any, of this cyclical activity is unknown.
Little is known about the physiologic role of the muscularis mucosa during peristalsis. It may contract primarily in response to luminal stimuli, thereby evoking movement of esophageal mucosa. It may also serve to hold the normally loosely attached overlying mucosa in place, thereby preventing excessive movement of the mucosa during bolus movement (2). Studies on the physiology and pharmacology of this muscle layer have been carried out.137, 138, 139, 140, 141 As with the longitudinal smooth muscle of the muscularis propria, contraction primarily involves cholinergic neurons acting on muscarinic receptors. There also appears to be a more sustained or tonic contraction due to release of substance P.140, 142
Conclusion
Esophageal peristalsis, which can be triggered by either swallowing or local esophageal distention, serves to propel esophageal contents into the stomach. This is orchestrated by a complicated interaction between the central nervous system and the myenteric plexus, with the latter predominating in the smooth muscle esophagus. Esophageal peristalsis consists of sequential contraction of the circular muscles of the muscularis propria, which is largely mediated by acetylcholine. This sequential contraction serves to occlude the esophageal lumen and push the bolus aborally. An important component in this process is the nitrergic inhibition of the circular smooth muscle that occurs aboral to the oncoming bolus. In addition, sequential contraction of longitudinal muscle also occurs during peristalsis. This serves to shorten the esophagus and increase the cross-sectional diameter, thereby facilitating bolus transport. There remains much to be learned about the physiologic control of esophageal peristalsis, including (1) the precise mechanisms whereby cholinergic and noncholinergic (mainly nitrergic) innervations interact to generate a peristaltic wave; (2) the cellular mechanisms involved in the nitrergic inhibition of esophageal circular smooth muscle; (3) the role of interstitial cells of Cajal in coordinating esophageal peristalsis; and (4) the role of other neurotransmitters in modulating peristalsis. Further understanding of the basic physiology underlying esophageal peristalsis will serve as a foundation for improved treatment of patients with dysphagia and chest pain due to esophageal motor dysfunction.