Key Points
- There is a close functional relationship between the upper gastrointestinal tract and the airway, which ensures the safety of the airway against aspiration of material in transit through the pharyngoesophageal axis.
- This functional relationship is controlled through two major mechanisms: (1) a brainstem-programmed mechanism such as those governing the integration of the laryngeal closure into swallowing, belching, and vomiting mechanisms; and (2) brainstem reflexes emanating from the pharynx, larynx, and esophagus.
- Esophageal reflexes are mainly stimulated by distention, whereas pharyngeal and laryngeal reflexes have a different mode of stimulation, such as water stimulation. Esophageal reflexes include secondary peristalsis, esophago–upper esophageal sphincter (UES) contractile and relaxation reflexes, esophago-glottal closure reflexes, and esophageal belch. Pharyngeal reflexes include pharyngoglottal closure reflex and pharyngo-UES contractile reflex. Laryngeal reflexes include laryngo-UES contractile and laryngeal adductor reflexes.
- In addition, there are inhibitory reflexes emanating from the aerodigestive tract and esophagus, including laryngo–lower esophageal sphincter (LES) relaxation, pharyngo-LES relaxation, and esophago-LES relaxation reflexes, as well as pharyngoesophageal inhibitory reflex of primary and secondary peristalsis. Normal function of these stimulatory and inhibitory reflexes is believed to be necessary for protection of the airway, aerodigestive tract, and esophagus against injurious effects of gastric content.
Introduction
Through an elaborate reflex mechanism, a close functional relationship exists among the pharynx, larynx, and esophagus during both anterograde transit, that is, the act of deglutition, as well as retrograde transit, that is, during gastroesophageal and gastroesophagopharyngeal reflux events and eructation, which helps protect the airway against aspiration.
These reflexes (1) enhance the upper esophageal sphincter (UES) pressure, such as esophago-UES contractile reflex (EUCR), pharyngo-UES contractile reflex (PUCR), and laryngo-UES contractile reflex (LUCR); (2) close the vocal cord and introitus to the trachea, such as esophagoglottal closure reflex (EGCR), pharyngo-glottal closure reflex (PGCR), and laryngeal adductor reflex; or (3) clear the contents from the pharynx and esophagus, including secondary esophageal peristalsis and pharyngeal reflexive swallow (PS).
In addition to these stimulatory reflexes, mechanical stimulation of the larynx, pharynx, and esophagus can induce relaxation of the lower and upper esophageal sphincters and proximal stomach (fundus and corpus). This group includes (1) pharyngoesophageal inhibitory reflex (PEIR), (2) pharyngo–lower esophageal sphincter (LES) relaxation reflex (LESRR), (3) laryngo-LES relaxation reflex (LESRR), (4) esophago-LES relaxation reflex (ELESRR), (5) esophago-UES relaxation reflex, (6) laryngo-gastric relaxation reflex (LGRRR), (7) pharyngo-gastric relaxation reflex (PGRR), and (8) esophagogastric relaxation reflex (EGRR).
Because the larynx and UES are the effector organs of most of the above-mentioned stimulatory reflexes, they are briefly discussed here but are extensively covered elsewhere in this publication.
The Larynx
The basic function of the larynx is protective, respiratory, and phonation. In recent years, a number of reflexes involving the glottis have been described that are believed contribute to the protective function of the larynx.
Closure of the vocal cords is an integral part of various functions of the larynx. These functions include swallowing,1 coughing,2 straining and Valsalva maneuver,3 belching,4 and several airway protective reflexes such as esophagoglottal closure,5 and pharyngoglottal closure6, 7 as well as the laryngeal adductor reflexes.
Duration of vocal cord closure during these events has been measured by direct videoendoscopic techniques1, 4, 5 and differ depending on the function. Likewise, the closure pressure that the cords generate during these functions varies depending on the performed function.8
Vocal cord closure pressure during swallowing, along with two additional closure mechanisms, aryepiglottal adduction and epiglottal descent, provides a sealed barrier against entry of the swallowed bolus of food in transit through the pharynx.1, 7 The vocal cord closure is the most distal barrier occurring first and lasting the longest of the above-mentioned deglutitive airway closure mechanisms1, 7 (Figure 1).
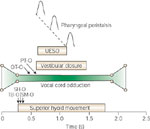
Figure 1: Relationship of deglutitive vocal cord kinetics to other events of the oropharyngeal phase of swallowing during 5-mL barium swallows.
Bolus transit through the pharynx and across the upper esophageal sphincter (UES) begins and ends while the vocal cords are at maximal adduction. TB-O, onset of tongue base movement; SH-O, onset of superior hyoid movement; SM-O, onset of submental myoelectrical activity; UESO, UES opening; OT-O, onset of bolus movement from the mouth; PT-O, arrival of bolus into pharynx. (Source: Shaker et al.1 with permission from American Gastroenterological Association)
During coughing, vocal cord closure helps to generate a high subglottic pressure. Subsequently, its sudden opening is associated with an accelerated expiratory flow2 (Figure 2). Although not perceived, the magnitude of contraction of striated muscles involved in closure of the vocal cords varies depending on the performed tasks. These varying contractions result in generation of different closure pressures commensurate with the respective task and function8 (Figure 3). Vocal cord closure is induced by contraction of adductor and tensor muscles of the larynx. The laryngeal adductor muscles include the lateral cricoarytenoid and interarytenoid muscles. Although contraction of the lateral cricoarytenoid results in median rotation of the vocalis processes of the arytenoid cartilages, completely adducting the anterior portion of the cords and closing the anterior part of the tracheal inlet, the contraction of the interarytenoid muscle results in median adduction of the arytenoids, entailing adducting the posterior part of the cords and closing the posterior part of the tracheal inlet. The laryngeal tensor muscles include the vocalis and thyroarytenoid muscles. Contractions of these muscles most probably contribute to the generation of closure pressure during various functions. A combination of various degrees of contraction of the laryngeal adductor and tensor muscles, and possibly the cricothyroid muscle, is suggested to be responsible for the production of differing task-dependent intercordal pressures (Figure 3).
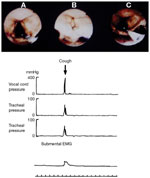
Figure 2: An example of vocal cord closure pressure during cough.
A–C: Still frames from videoendoscopic recording of a cough event during vocal cord manometry. A: Prior to initiation of cough, vocal cords are seen in an open position. The manometric catheter is seen at the lower-right quadrant of the image. The pressure gauge (white surface) is seen at the cord's level. B: Complete cord closure during cough. C: Opening of the cords following cough. Cord closure, seen in panel B (top), resulted in a closure pressure of 390 mmHg. This pressure was accompanied by an intratracheal pressure of 69 mmHg. Note that intercordal pressure has a shorter duration compared with intratracheal pressure. This is believed to be because the duration of intratracheal pressure represents the interval of cord closure as well as the interval following cord opening, when the trachea is subject to an accelerated expiratory flow. Coughing was associated with an electromyography (EMG) event. (Source: Shaker et al.8 with permission from Springer Science and Business Media.)
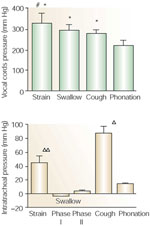
Figure 3: Comparison of intercordal and intratracheal pressure during straining, swallowing, coughing, and phonation.
Intercordal pressure during straining, coughing and swallowing was significantly higher than that of phonation (*p <.05). Straining produced pressures significantly higher than coughing (#p <.05). Intratracheal pressure during coughing induced a pressure significantly higher than all other studied events ( p <.05p). Intratracheal pressure during straining was significantly higher than those of phonation and swallowing (
p <.05p). Intratracheal pressure during straining was significantly higher than those of phonation and swallowing (
 p <.01). For all studied events, intercordal pressures were significantly higher than intratracheal pressures. (Source: Shaker et al.8 with permission from Springer Science and Business Media)
p <.01). For all studied events, intercordal pressures were significantly higher than intratracheal pressures. (Source: Shaker et al.8 with permission from Springer Science and Business Media)
The sphincteric function of the glottis, including the vocal cords and their resistance to ingress of swallowed material, is an integral part of airway protection during swallowing. Studies using cadavers have reported that artificially adducted vocal cords can prevent the ingress of air with pressures as high as 140 mmHg.9 Among the three tiers of laryngeal closure during swallowing, closure of the cords is temporally the first and anatomically the most caudad tier. Duration of complete vocal cord closure during swallowing has been reported to be about 1.7 seconds.1, 7 Vocal cord closure in the majority of instances during swallowing is followed by laryngeal elevation.1, 7 In a minority of instances the cords start their movement toward complete closure during the upward and forward excursion of the larynx.1 For this reason, even with endoscopic control, and with available pressure recording devices, it is impossible to measure the cords' pressure during the entire period of their closure. Therefore, the reported deglutitive closure pressures represent only a brief period of their closure.
Because of the short period of vocal cord closure during swallowing and coughing, only extremely sensitive recording devices with a very high rise-rate, such as strain gauge transducers, can be used to study this phenomenon. In addition to upward movement, because of subsequent approximation of arytenoids to the base of the epiglottis and epiglottal descent during swallowing, measurement of pure deglutitive vocal cord pressure, except for the brief initial phase, is at the present time virtually impossible.
The vocal cords produce a closure pressure significantly higher than the intratracheal pressure during coughing. The intercordal pressure during phonation following an initial spike-like pressure rise is minimal compared to other tasks (Figure 4). This may be attributed to a combination of significantly lesser degrees of adductor and tensor muscle contractions.1
Figure 4: Example of intercordal and intratracheal pressure during phonation.
Both productions of continuous AAAA and EEEE vowels were associated with a spike-like pressure, 220 mmHg for AAAA and 120 mmHg for EEEE, respectively, followed by a flat pressure of about 10 mmHg. These events were accompanied by a negligible increase in intratracheal pressure. (Source: Shaker et al.8 with permission from Springer Science and Business Media)
Nasopharynx
The mechanism of nasopharyngeal closure (NPC), which prevents nasal regurgitation, is different during swallowing and belching.10 During swallowing, NPC has two tiers of closure: palatal elevation and proximal pharyngeal muscle adduction. During belching, it consists of only palatal elevation.10 Nasopharyngeal closure is tightly coordinated with oral-pharyngeal bolus transit during swallowing and biomechanical events, such as vocal closure and laryngeal excursion during belching. Similar to deglutitive glottal closure, the duration of NPC during swallowing is not modified by bolus volume.10 Velopharyngeal closure during swallowing and speech has been studied using various radiographic techniques and has identified different patterns of closure during these functions. Studies using videoendoscopy10 have shown that the mechanism of deglutitive NPC, in addition to palatal elevation and its contact with the posterior pharyngeal wall, includes the adduction of the most proximal part of the lateral pharyngeal wall, identifying a second function for these pharyngeal muscles, in addition to their known role in generating pharyngeal peristalsis and bolus transport (Figure 5).
Figure 5: An example of temporal relationship of deglutitive (10-mL barium swallow) nasopharyngeal closure (NPC) with other swallowing events.
NPC-O, onset of NPC; NPC-C, complete NPC; NPO-O, onset of nasopharyngeal opening; NPO-C, complete nasopharyngeal opening; VPC-O onset of velopharyngeal closure; VPC-C, complete velopharyngeal closure; VPO-O, onset of velopharyngeal opening; VPO-C, complete velopharyngeal opening; UESR-O, onset of upper esophageal sphincter relaxation; BA-PX, arrival of barium at the pharynx; VC-O, onset of vestibular closure; VC-C, complete vestibular closure; VO-O, onset of vestibular opening; VO-C, complete vestibular opening. As shown, endoscopically identifiable onset of NPC occurs earlier (0.1  0.02 second) than fluoroscopically seen at onset of velopharyngeal closure, and UES opening occurs while nasopharynx is closed. (Source: Dua et al.10 with permission from the American Physiological Association)
0.02 second) than fluoroscopically seen at onset of velopharyngeal closure, and UES opening occurs while nasopharynx is closed. (Source: Dua et al.10 with permission from the American Physiological Association)
Nasopharyngeal closure during swallowing begins from the proximal pharynx and extends caudally. This pattern of closure is the opposite of swallow-related laryngeal closure, which begins from the most caudad layer and extends orad. During belching there is no adduction of the proximal pharyngeal muscles, and NPC is primarily owing to palatal elevation and velopharyngeal contact10 (Video 1). During belching, participation of the nasopharynx includes elevation of the soft palate. This suggests the contraction of the levator veli palatini and possibly other palatal muscles such as tensor palatini, which are innervated by branches of the vagus and mandibular nerves, respectively.4
Video 1: a: Nasopharynx function during belch. b: Nasopharynx function during swallow.
Video demonstrating first nasopharynx function during belching then nasopharynx function during swallow. Nasopharynx function during belch: concurrent videoendoscopic and videofluoroscopic recording of nasopharynx during belching induced by interesophageal air injection (20 mL). During belching, there is no adduction of the proximal pharyngeal muscles, and nasopharyngeal closure (NPC) is primarily attributed to palatal elevation and velopharyngeal contact. Nasopharynx function during swallow: in contrast, NPC during swallowing includes the adduction of proximal pharyngeal muscles in addition to palatal elevation.
The onset of UES relaxation during swallowing precedes the onset of NPC. Upper esophageal sphincter relaxation occurs after the onset of vocal cord closure,11 indirectly suggesting that NPC also occurs after the onset of deglutitive vocal cord closure. Studies indicate that the arrival of the barium bolus head into the pharynx occurs  0.2 second after the complete closure of the nasopharynx.10 It is conceivable that nasal regurgitation may occur in individuals in whom this coordination is disturbed.
0.2 second after the complete closure of the nasopharynx.10 It is conceivable that nasal regurgitation may occur in individuals in whom this coordination is disturbed.
Duration of NPC is not influenced by the volume of the swallowed bolus, suggesting that this part of the swallowing cascade is stereotypically programmed and is not under a modulatory sensory feedback control.10 Interestingly, similar findings have been reported on the duration of the deglutitive glottal closure.11, 12 Studies indicate that certain aspects of swallowing, such as duration of UES opening (13), duration of hyoid bone movement,13, 14 amplitude of pharyngeal peristalsis,1, 15 and velocity of lingual peristalsis,16 can be modified by volume or consistency of swallowed boluses. Although the airway-protective aspect of oral-pharyngeal swallowing is stereotypically controlled, its bolus transit function is subject to modification by sensory feedback from the swallowed bolus.
Upper Esophageal Sphincter
The UES provides a high-pressure zone between the pharynx and esophagus. The major component of the UES—the cricopharyngeus muscle—attaches to the posterior aspect of the lamina of the cricoid cartilage like a C-clamp. Muscle fibers from inferior pharyngeal constrictors and the proximal esophagus contribute to the genesis of the UES high-pressure zone.
The UES provides the most proximal physical barrier of the gastrointestinal tract against pharyngeal and laryngeal reflux of gastric content. In addition, by maintaining a basal pressure, it prevents entry of air into the esophagus during inspiration.
The UES contracts in response to segmental distention of the esophagus.17 However, this contractile response may not be present in 100% of events.4 The UES, on the other hand, relaxes either completely or partially in the majority of instances in response to abrupt generalized distention of the esophagus. In the remainder, however, it may contract or remain unaffected.18 The response of the UES to intraesophageal pH has been the subject of controversy. Although some studies report an increase in UES pressure owing to acidification of the esophageal lumen,19, 20 others have not found such a response.21, 22
Upper esophageal sphincter resting pressure decreases during sleep.23 Although stress enhances the UES resting pressure,24 it is not uncommon to find the UES resting pressure during rest and relaxation to be difficult to detect manometrically. Resting UES pressure has been the subject of multiple studies that were done using different recoding techniques such as water-perfused catheters with side holes, with strain gauges, and some with sleeve sensors. Pelemans and Vantrappen,25 using a pneumohydraulic system, found decreased resting UES pressure in subjects older than 74 years. Weihrauch et al.26 found that mean UES pressure in younger subjects (20 to 49 years) was significantly higher than that in older subjects (50 to 80 years). Shaw et al.27 did not find significant age-related changes in resting UES pressure measured with a sleeve device; however, in their study, the age of study subjects ranged only between 21 and 55 years. Similarly, Wilson et al.28 did not find age-related changes in a study of 50 healthy subjects, ages 17 to 62 years. On the other hand, Fulp et al.29 were able to show that normal elderly subjects over 62 years of age have lower resting UES pressures than younger controls. Shaker et al.18 studied the effect of aging (70 years and over) on the resting UES pressure and its response to intraesophageal air and balloon distention. The results of this study indicate that the resting UES pressure is significantly reduced in the elderly compared to the young, whereas its response to esophageal segmental and generalized distention is preserved.18 The major component of the UES, the cricopharyngeus (CP) muscle, is a striated muscle and it is conceivable that with aging it becomes subjected to loss of muscle fiber. Then again, it is also conceivable that excitation impulses that maintain the cricopharyngeal tone in the awake state is decreased in the elderly.18
The UES responses to esophageal distention by both air and balloon are variable.18 One possible explanation for this phenomenon may be the differences in the sensitivity and concentration of recruited esophageal stretch receptors during different trials of esophageal distention by air and balloon. In most species including humans the CP attaches to the posterior lamina of the cricoid cartilage and forms a C-clamp–shaped muscular band producing maximum tension in the anteroposterior compared with the lateral direction.30, 31 Two sets of muscle fibers have been identified in the human cricopharyngeus: the horizontally oriented fibers, the pars fundiforms; and an oblique band of fibers, the pars obliqua. These latter fibers extend from the lateral aspect of the cricoid cartilage to the posterior midline raphe, where they blend superiorly with the thyropharyngeus (TP) muscle.32 Unlike the TP, the pars fundiforms of the CP has no median raphe. The CP is composed of variable-sized striated muscle fibers of small to average diameter (25 to 35  m), which, in contrast to most other striated muscles, are not oriented in strict parallel fashion.32, 33, 34 The CP contains about 40% of endomysial connective tissue, much of which is elastic but has no muscle spindles.33, 34, 35 The CP muscle fibers include both the slow-twitch type I32, 34, 35 as well as fast-twitch type II32, 33, 35, 36 fibers. This arrangement provides an anatomic basis for the various functions of the UES, namely, maintaining constant basal tone and rapid relaxation and contraction during swallowing, belching, vomiting, and other reflexes.
m), which, in contrast to most other striated muscles, are not oriented in strict parallel fashion.32, 33, 34 The CP contains about 40% of endomysial connective tissue, much of which is elastic but has no muscle spindles.33, 34, 35 The CP muscle fibers include both the slow-twitch type I32, 34, 35 as well as fast-twitch type II32, 33, 35, 36 fibers. This arrangement provides an anatomic basis for the various functions of the UES, namely, maintaining constant basal tone and rapid relaxation and contraction during swallowing, belching, vomiting, and other reflexes.
Comparison of the UES intraluminal high-pressure zone with the anatomic components of the pharyngoesophageal segment30, 31, 37, 38, 39 shows an elevated pressure encompassing the proximal cervical esophagus, CP, and inferior pharyngeal constrictor (IPC) TP in animals. Although some investigators reported that the electromyographic activity of both the TP (IPC) and the CP fluctuated with changes in UES pressure and both relaxed during swallowing,39 the CP has been considered the primary muscular component of the UES by most investigators because of the finding that the peak UES pressure does indeed correspond with the CP.31 The assumption of CP being the main component of the UES is supported by the fact that most studies in humans or animals report that the CP, and not the IPC (TP),38 exhibits a continual basal tone,38, 40, 41, 42, 43, 44, 45 relaxes during swallowing,38, 40, 41, 42, 43, 44, 45, 46 and exhibits fluctuations in its activity with changes in UES pressure.38, 44, 47
The UES is a complex organ and comprises more than one muscle.48 It appears that various muscle(s) contributing to the UES, also known as the pharyngoesophageal segment high-pressure zone, are called into action depending on the physiologic function. For example, UES basal tone is produced by the CP and IPC (TP) and possibly the infracricoid esophagus.38, 39, 41, 42, 43, 44, 45, 46 Upper esophageal sphincter active relaxation during swallowing38, 41, 42, 43, 44, 45, 46, 47 and belching49 occurs primarily in the CP. The observed respiration-induced UES pressure changes39, 45, 46, 50 occur in both the CP and IPC. Upper esophageal sphincter contraction and relaxation during retching and vomiting, on the other hand, occur by the simultaneous action of the CP, IPC, and proximal cervical esophagus.41, 49 Upper esophageal sphincter contraction in response to esophageal or pharyngeal stimulation (i.e., the esophago-UES and pharyngo-UES reflexes) (see below) occurs with the CP45 rather than the IPC. Contraction of the UES during coughing and sneezing occurs in both the CP and IPC.51 Therefore, the pharyngoesophageal high-pressure zone commonly referred to as the UES may comprise the contribution of the CP, IPC, and proximal cervical esophagus depending on the physiologic state, but the CP is the one muscle that participates in all physiologic states of the UES.48
Upper Esophageal Sphincter Opening
Opening of the UES is determined by the combined effect of relaxation and distensibility of the cricopharyngeus muscle and retraction of the cricoid cartilage caused by contraction of the supra- and infrahyoid muscle groups involved in UES opening.37, 39, 52, 53, 54 Upper esophageal sphincter opening is also influenced by the distending force of the oncoming bolus traversing through the pharyngoesophageal junction.37, 39, 52, 53, 54
Esophago–Upper Esophageal Sphincter Contractile Reflex
The UES is one of the major components of the airway protective mechanisms against entry of gastroesophageal refluxate into the pharynx and larynx.18, 55 However, because UES pressure is quite variable and it decreases significantly during sleep23 as well as when one is calm and relaxed, it is conceivable that the pressure of gastroesophageal refluxate may overcome the UES if it occurs during periods of low UES pressure.
Upper esophageal sphincter function during gastroesophageal reflux (GER) events has been of interest and the subject of several previous studies. Although some of these studies report an increase in UES pressure following experimental esophageal acidification.21, 56, 57 others have not observed a similar response19, 21 following gastroesophageal acid reflux events. Until recently, the UES function during gastroesophageal acid reflux events was not completely understood. This difficulty stems from the fact that GER results in both intraluminal pressure increase and pH changes. The UES response to intraluminal pressure increase and distention is variable depending on the intensity and nature of the stimulus, in addition to the fact that the UES response to esophageal acidification had been poorly understood. Differences in recording techniques might have also added to these difficulties. Earlier reports generally used the station pull-through technique for recording the UES pressure, whereas more recent studies have utilized a modified sleeve device for this purpose.
In 1957 Creamer and Schlegel58 described the contractile response of the UES to balloon distention and water infusion. This contractile response was found by Car and Roman40 to involve the CP. Intraesophageal instillation of local anesthetic (2% lidocaine) or removal of the mucosal layer does not affect the slow esophageal distention-induced esophago-UES contractile reflex, suggesting that distension-sensitive muscle afferent fibers are involved in activation of this reflex.59 Freiman et al.56 showed that bilateral cold block of the cervical vagus completely abolished the UES response to HCl infusion and partially blocked its response to balloon distention in dogs. Wallin et al.57 reported an increase in UES pressure following 1 minute of intraesophageal acid infusion in humans using the pull-through technique. However, this increase was not maintained 4 minutes later. Gerhardt et al.,19 using the pull-through technique, demonstrated that the UES pressure increase occurs in response to volume distention, and that this response is further enhanced by HCl but is not dependent on osmolarity of the infusate. Using a sleeve sensor, Vakil et al.21 monitored the UES pressure continuously during acid reflux events and compared the mean UES pressure of a period of 135 seconds prior to and following reflux events and did not find a significant reflux-induced UES pressure augmentation. This study corroborated an earlier report by Kahrilas et al.,23 who also did not find a sustained long duration UES pressure increase in response to gastroesophageal reflux during sleep.
The most critical time for the UES protective function during a reflux event begins with the onset of the entry of refluxate into the esophagus until its clearance from the esophagus by a secondary or primary peristalsis, namely, during the time of increased intraesophageal pressure owing to a reflux event. In normal controls and patients with reflux esophagitis, UES pressure change at the onset of reflux events was evaluated by Torrico et al.60 (Figure 6). A total of 321 reflux events were identified by the development of abrupt reflux-induced intraesophageal pressure increase (IPI); 285 events occurred in patients and 36 in controls. In control subjects, 33 of 36 and in patients 252 of 285 IPI events were associated with a pH drop. In patients and controls, 99% and 100%, respectively, of all IPI events irrespective of a pH drop were associated with an abrupt increase in UES pressure (34  2 and 27
2 and 27  6 mmHg, respectively). The average percentage of maximum UES pressure increase over pre-reflux values ranged between 66% and 96% (control subjects) and 34% and 122% (patients). These pressure increases lasted 5 to 25 seconds. These findings suggest the existence of a strong positive relationship between UES tone and intraesophageal pressure increases induced by gastroesophageal reflux events, shown previously by experimental distention. Although both acidic and nonacidic reflux events induce UES contraction, an intraluminal pH below 4 seems to augment this contractile response.
6 mmHg, respectively). The average percentage of maximum UES pressure increase over pre-reflux values ranged between 66% and 96% (control subjects) and 34% and 122% (patients). These pressure increases lasted 5 to 25 seconds. These findings suggest the existence of a strong positive relationship between UES tone and intraesophageal pressure increases induced by gastroesophageal reflux events, shown previously by experimental distention. Although both acidic and nonacidic reflux events induce UES contraction, an intraluminal pH below 4 seems to augment this contractile response.
Figure 6: Upper esophageal sphincter pressure increases in response to gastroesophageal reflux events.
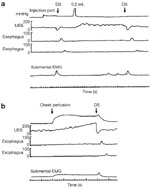
Examples of abrupt UES pressure increase in response to gastroesophageal reflux events accompanied by a clear secondary peristaltic pressure wave (SP; b) and absence of a recognizable SP (b). In both instances the intraesophageal pressure increase (IPI) encompasses the entire esophageal length, whereas a pH drop spares the proximal esophagus in b and does not reach 4.0 in a. The UES pressure increase lasts for 22 and 25 seconds, respectively. Note that although in the instance shown in a there is no change in respiration, in b there seems to be a shallow respiration at the onset of UES pressure increase and IPI. PE, proximal esophagus; ME; middle esophagus, DE, distal esophagus; EMG, electromyograph. (Source: Torrico et al.60 with permission from the American Physiological Association)
Esophagoglottal Closure Reflex (EGCR)
Some GER episodes, especially those of large volume, may cause an instantaneous increase in intraesophageal pressure that might overcome the UES. This circumstance could potentially leave the upper airway vulnerable to aspiration. Studies have documented the existence of an esophagoglottal closure reflex in humans5 (Figure 7) as well as in the feline species61 (Figure 8). Stimulation of EGCR reflex results in adduction of the vocal cords and closure of the introitus to the trachea. To evoke this reflex, esophageal distention may involve the entire body of the esophagus, such as distentions induced by air insufflation, or it may be regional, such as those caused by a short balloon. In either case, the distention must be abrupt to evoke the reflex (Figure 7b).
Figure 7: a: Example of esophagoglottal closure reflex evoked by 20 mL room air injected into mid-esophagus.
Vocal cords immediately before the onset of adduction during esophageal air injection (i). Vocal cords maximally adducted (ii). Vocal cords returned to resting position 1.42 sec after the onset of vocal cord adduction (iii). m, Manometric catheters. (Source: Shaker et al.5 with permission from American Gastroenterological Association) b: Examples of esophagoglottal closure reflex evoked by midesophageal balloon. Vocal cords immediately prior to balloon distention (i). Vocal cords maximally adducted during balloon distention (ii). Vocal cords returned to resting position (iii). Kinematics of the cords are similar to those of esophagoglottal closure reflex evoked by esophageal air distention. c: Examples of esophagoglottal closure reflex evoked by a spontaneous reflux event. As seen, vocal cords closed and opened (top of the panel) following a reflux event, resulting in a pH drop in the distal and proximal esophagus as well as common cavity. Development of common cavity following the stimulation of esophagoglottal closure reflex also resulted in secondary esophageal peristalsis (2° peristalsis). EGR, esophagoglottal reflex
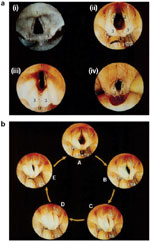
Figure 8: a: Esophagoglottal closure reflex.
One frame of videoendoscopic view of human (i), cat (ii), monkey (iii), and opossum (iv) glottis. 1, posterior epiglottis; 2, vocal cord (fold); 3, arytenoid; u, area of upper esophageal sphincter (UES) opening. B: Esophagoglottal closure reflex. Still frames from videoendoscopic recordings of glottal closure response to intraesophageal air injection in a cat. Glottis immediately before a 40-mL intraesophageal air injection (A) and 0.3 (B), 0.4 (C), 0.8 (D), and 1.0 (E) seconds after 40-mL esophageal air injection. u, Area of UES opening. Note that time intervals between frames are not equal. (Source: Shaker et al.61 with permission from the American Physiological Association)
The prime candidates for triggering the sensory signal for this reflex are the stretch receptors present in the body of the esophagus. Because vagotomy abolishes this reflex61 (Figure 9), the afferent nerves undoubtedly run in the vagus nerve, carrying the sensory impulse to the brainstem. Because studies in cats have shown that the mid-collicular decerebration does not affect the stimulation of the EGCR by intraesophageal air or balloon distention, the central control mechanism of this reflex is believed to be in the brainstem. The efferent fibers are likely vagal motor fibers to the larynx that traverse the recurrent laryngeal nerve and stimulate the adductor muscles of the glottis. The target muscles are thought to include all or some of the glottal adductors.61 These muscles include the thyroarytenoid (which is also an isometric tensor of the cords), cricothyroid (also an isotonic tensor), lateral cricoarytenoid, and, finally, interarytenoid muscle (which closes the posterior gap in the glottis). Except for the cricothyroid muscle, which is innervated by the external division of the superior laryngeal nerve, all adductor muscles are innervated by the recurrent laryngeal nerve. Because the posterior glottal gap becomes closed on direct viewing when this reflex is triggered, it seems that in addition to the lateral cricoarytenoid muscles, the interarytenoid muscle is also involved. Because the glottis, in response to esophageal distention, shortens and narrows, it is also possible that the thyroarytenoid muscles have a strong participation in this reflex.
Figure 9: Electromyographic recording from interarytenoid and lateral cricoarytenoid muscles.
An example of electromyographic recording from interarytenoid and lateral cricoarytenoid muscles during a 2.5-cm middle esophageal balloon distention before (a) and after (b) bilateral cervical vagotomy. As seen, EMG activities induced by balloon distention are completely abolished after bilateral cervical vagotomy. (Source: Shaker et al.61 with permission from the American Physiological Association)
Reflex connections between the digestive tract and the respiratory system have been reported previously.62 Such studies generally focus on a reflex connection between acid-sensitive receptors in the esophagus and pulmonary bronchi, such that refluxed acid may induce bronchial spasm. Coordination of the digestive and respiratory systems during swallowing is well established.1, 63 The ECGR is an example of close coordination between digestive and respiratory systems during retrograde esophageal transit. The physiologic role of the ECGR could be postulated to be one of the airway protective mechanisms during retrograde esophageal and pharyngeal transit, such as those occurring during belching, GER, regurgitation, and possibly vomiting. Studies have documented that this reflex is evoked during spontaneous GER episodes.64 Other studies have shown that this reflex is absent in about half of the patients over the age of 70 years.65
Under experimental conditions there is a direct relationship between the duration of vocal cords closure and magnitude of the esophageal distention by a balloon (Figure 10). Also this reflex is triggered significantly more frequently by proximal esophageal than distal esophageal distention.5 The more frequent elicitation of EGCR by distention of the proximal than the middle or distal esophagus could be attributed to differential distribution and the phenotype of the vagal afferent fibers innervating the different regions of the esophagus.66, 67 Another possible explanation for this phenomenon could be that the proximal esophagus also receives innervation from the recurrent laryngeal nerve (RLN).68 Therefore, stimulation of the richly innervated proximal esophagus by two branches of the vagus, that is, the cervical vagus and RLN, can further facilitate activation of ECGR.
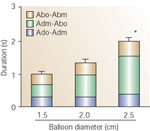
Figure 10: Relationship between the duration of vocal cords closure and magnitude of the esophageal distention by a balloon.
Duration of vocal cord adduction/abduction for a 2.5-cm balloon was significantly longer than the duration for a 1.5-cm balloon distention. This increase was caused by the increase in the duration of maximal adduction. Abo-Abm, time interval between the onset of post-adduction vocal cord opening to the maximal (resting) position of the cords; Adm-Abo, time interval between maximal vocal cord adduction and onset of the opening of the cords; Ado-Adm, time interval between the onset of vocal cord adduction to the maximal adduction. *p <.05.
(Source: Shaker et al.5 with permission from American Gastroenterological Association)
Although the function of this reflex in patients with esophagitis or supraesophageal complications of gastroesophageal reflux disease (GERD) has not been evaluated as yet, a study in cats demonstrated that acute experimental esophagitis either completely abolishes this reflex or results in significant reduction in its activation frequency.69
Pharyngeal Reflexive Swallow (PS)
Mechanical stimulation of the pharyngeal wall in animals70 and injection of water into the pharynx in humans71, 72 can trigger an irrepressible swallow, the PS. This local stimulus for the initiation of swallowing may play a role in airway protection from pharyngeal reflux of gastric contents, as well as inadvertent spillage of oral contents into the pharynx, during the preparatory phase of swallowing.
Studies have characterized the pharyngeal swallow and determined that the threshold volume of liquid required to trigger this type of swallowing in healthy elderly is significantly larger than that required for young volunteers.73 Studies have shown that the swallowing mechanism in humans can be readily activated by water stimulation of the pharynx.71, 72, 73, 74
Swallows triggered by direct stimulation of the pharynx are different from volitional or primary swallows by not inducing sequential contact of the proximal tongue with the hard palate known to occur during primary swallows.73 Therefore, PS does not result in transit of the oral bolus while it clears the pharynx (Video 2). In this regard, the pharyngeal swallow could be compared to secondary esophageal peristalsis, which usually spares the activation of the peristaltic wave from areas proximal to the point of stimulation.75, 76, 77 Except for lingual peristalsis and transit of oral bolus, the rest of the deglutitive biomechanical events during both types of swallows were found to be similar (Table 1).
Video 2: Comparison of primary and pharyngeal reflexive swallow.
Swallows triggered by direct stimulation of the pharynx are different from volitional or primary swallows by not inducing sequential contact of the proximal tongue with the hard palate known to occur during primary swallows. For this reason reflexive swallow does not result in transit of the oral bolus while it clears the pharynx. Both types of swallows are shown in real time followed by slow motion.
From a functional point of view, therefore, pharyngeal swallows may help prevent aspiration by two mechanisms: (1) Activating the swallow-induced glottal closure (which, in turn, seals off the airway and prevents possible aspiration of material that may either fall into the pharynx inadvertently during the preparatory phase of swallowing or enter the pharynx during large volume GER episodes); and (2) clearing the pharynx of materials that enter it during reflux from the esophagus.
The demonstrated need for larger volumes to trigger pharyngeal swallow in elderly patients could potentially have clinical implications. In the interval between the entry of subthreshold volume of gastric content into the pharynx and occurrence of a primary swallow, the material may be inhaled into the airway. This possibility is further enhanced by the fact that the rate of spontaneous swallowing in the elderly is lower than in the young.78, 79 Several areas within the oropharyngeal cavity have been shown to be sensitive for elicitation of the swallowing reflex. These areas include the anterior faucial pillars, the tongue, and the epiglottis, as well as the larynx and the posterior pharyngeal wall.80, 81
It has been shown that the spontaneous (primary) swallow occurs infrequently during stable sleep.78, 79, 82 The stimulation of pharyngeal swallow during sleep has not been studied. However, its nocturnal activation can potentially play an important role in preventing the contact of refluxed gastric content with laryngeal structures and its subsequent aspiration. The threshold volume for triggering the reflexive pharyngeal swallow is significantly larger in the elderly compared to the young,18 smokers compared to nonsmokers,83 and alcoholics versus nonalcoholics. Acute intravenous infusion of alcohol increases the threshold volume for triggering this reflex.84
Pharyngo–Upper Esophageal Sphincter Contractile Reflex (PUCR)
Pharyngeal mechanical stimulation in cats45 and water stimulation in humans85 induce an increase in the resting tone of the UES—the PUCR (Figure 11). It is suggested that this reflex functions as an airway-protective mechanism whereby retrograde entry of small volumes of liquid into the pharynx from the stomach can result in augmentation of UES tone, reducing the chance of further regurgitation into the pharynx.85
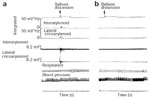
Figure 11: Effect of pharyngeal water injection on UES resting pressure.
a: Rapid-pulse injection of 0.2 mL of room-temperature water resulted in augmentation of UES pressure from 55 to 137 mmHg. As seen, this augmentation persisted for 22 seconds until it returned to baseline after a dry swallow (DS). Esoph, esophageal; EMG, electromyogram. b: Slow continuous infusion of room-temperature water also resulted in an increase of preinfusion pressure of 80 to 200 mmHg measured 3 seconds before a swallow occurred. (Source: Shaker et al.85 with permission from the American Physiological Association)
Contrary to rapid water stimulation that results in an abrupt UES pressure increase, during slow, continuous water injection into the pharynx, the UES pressure increases gradually before the occurrence of the pharyngeal swallow.85 In the young and in the supine position during slow continuous injection, the UES pressure has been reported to increase from a basal pressure of 50  7 mmHg to a pressure of 94
7 mmHg to a pressure of 94  9 mmHg, a 76%
9 mmHg, a 76%  16% increase. In the elderly, despite a similar trend, the pressure difference has not reached statistical significance. Similar findings were reported for the upright position.85
16% increase. In the elderly, despite a similar trend, the pressure difference has not reached statistical significance. Similar findings were reported for the upright position.85
The afferent limb of the pharyngo-UES contractile reflex is the glossopharyngeal nerve. In animal studies,45 cutting the glossopharyngeal nerves blocked the PUCR but did not block the esophago-UES contractile reflex or the responses of the thyropharyngeus or cricopharyngeus muscles during swallowing.45 The efferent limb of the PUCR is the pharyngoesophageal nerve45 (somatomotor nerves) that branch from the vagal trunk just rostral to the nodose ganglion.86 Transection of the pharyngoesophageal nerve eliminates basal tone of the CP and blocks the CP response to all reflex stimuli—PUCR, EUCR, and swallowing—but not the response of the TP during swallowing.45 Transection of the vagus nerves at the cervical level (i.e., below the nodose ganglion) had no effect on the PUCR, but blocked the EUCR, which indicates that the recurrent laryngeal nerve (that branches from the vagal trunk in the thoracic cavity) serves no role in this reflex.45 It is possible, however, that activation of the pharyngeal receptors may affect laryngeal muscle activity mediated through the recurrent laryngeal nerves.87 A study evaluated the effect of volume, temperature, and anesthesia on the pharyngo-UES contractile reflex in humans.88 Pharyngeal water injection at a threshold volume of 0.1  0 mL invariably resulted in a significant increase in the UES pressure in all subjects. This pressure increase was not affected by volume and temperature. The threshold volume for UES pressure increase was significantly lower than that for triggering a pharyngeal swallow. Results were similar for slow continuous injection. Topical anesthesia of the pharyngeal mucosa completely abolished this reflex.88 A study of patients suspected of reflux-induced posterior laryngitis reported that the threshold volume required to evoke the PUCR was significantly higher than that of the controls.89 However, after the PUCR was stimulated, the maximum postinjection pressure in posterior laryngitis patients was similar to that of the controls, suggesting differences in the afferent, but not in the efferent, arm of the reflex in the patient group. Application of local anesthetics (2% lidocaine) to the pharyngeal mucosa blocks the contractile responses of the CP to pharyngeal stimulation, but not to its response to esophageal distention.90 This observation indicates the involvement of pharyngeal mucosal mechanoreceptors in eliciting the PUCR.
0 mL invariably resulted in a significant increase in the UES pressure in all subjects. This pressure increase was not affected by volume and temperature. The threshold volume for UES pressure increase was significantly lower than that for triggering a pharyngeal swallow. Results were similar for slow continuous injection. Topical anesthesia of the pharyngeal mucosa completely abolished this reflex.88 A study of patients suspected of reflux-induced posterior laryngitis reported that the threshold volume required to evoke the PUCR was significantly higher than that of the controls.89 However, after the PUCR was stimulated, the maximum postinjection pressure in posterior laryngitis patients was similar to that of the controls, suggesting differences in the afferent, but not in the efferent, arm of the reflex in the patient group. Application of local anesthetics (2% lidocaine) to the pharyngeal mucosa blocks the contractile responses of the CP to pharyngeal stimulation, but not to its response to esophageal distention.90 This observation indicates the involvement of pharyngeal mucosal mechanoreceptors in eliciting the PUCR.
Pharyngo-UES contractile reflex may also help to prevent pharyngo-esophageal flux of air during inspiration because studies have documented activation of this reflex by pharyngeal air injection.91 It is postulated that the contractile response of the UES to pharyngeal stimulation may counteract the inhibitory deglutitive reflexes, thus preventing pharyngeal reflux of gastric and esophageal contents. Transection of glossopharyngeal nerves in cats abolishes the pharyngo-UES but not esophago-UES contractile reflex, suggesting that the afferent limb of the former is mediated by mechanosensitive sensory afferent fibers in the glossopharyngeal nerve (GPN).90 On the other hand, transection of the hyponodal vagus has no effect on pharyngo-UES, but abolishes EUCR, indicating that recurrent laryngeal nerve (RLN) has no function in the former reflex.
Pharyngo-glottal Closure Reflex (PGCR)
Injection of minute amounts of water into the pharynx results in brief closure of the vocal cords92, 93 (Video 3). Gradual entry of liquid into the pharynx induces partial adduction, whereas rapid injection causes complete closure of the cords. An example of laryngeal adductor muscles to pharyngeal water stimulation are shown in Figure 12. It is postulated that this glottal closure reflex is part of a complex mechanisms that protect the airway from retrograde as well as anterograde aspiration. The threshold volume that stimulates this reflex is reported to be significantly smaller than that required to trigger an irrepressible pharyngeal (reflexive) swallow, but similar to that required to induce a PUCR.92, 93 Recent evidence suggests that a significantly larger volume of liquid is needed to trigger a pharyngoglottal reflex in the elderly when compared to the young.92, 93 Similar differences are reported between smokers and nonsmokers.94
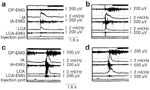
Figure 12: Pharyngoglottal closure reflex: effect of pharyngeal water stimulation on myoelectrical activity.
Example of the effect of pharyngeal water stimulation on myoelectrical activity of the interarytenoid (human equivalent), lateral cricoarytenoid, and cricopharyngeus muscles (CP). Whereas pharyngeal water injection resulted in contraction of the glottal adductor as well as CP muscles (A and B), swallows triggered by pharyngeal water injection resulted in contraction of glottal adductors and relaxation of the CP muscles (C and D). This relaxation, however, was followed by a postdeglutitive contraction. IA, interarytenoid muscle; LCA, lateral cricoarytenoid muscle; EMG, raw electromyographic activity;  , integrated EMG activity. (Source: Shaker R, Medda BK, Ren J, Jaradeh S, Xie P, Lang IM. Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol 1998;275:G521-G525 with permission from the American Physiological Association.)
, integrated EMG activity. (Source: Shaker R, Medda BK, Ren J, Jaradeh S, Xie P, Lang IM. Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol 1998;275:G521-G525 with permission from the American Physiological Association.)
Video 3: Pharyngoglottal closure reflex.

Injection of minute amounts of water into the pharynx results in brief closure of the vocal cords. In this video, the pharyngoglottal closure reflex in a healthy volunteer is stimulated by injection of minute amounts of colored water (for visualization purposes) into the pharynx. Contact of fluid with the posterior pharyngeal wall in the pyriform sinus induces a brief closure of the vocal cords. The event is shown in slow motion for ease of viewing.
Laryngo–Upper Esophageal Sphincter Contractile Reflex (LUCR)
Direct stimulation of the larynx induces a brief adduction of the vocal cords and arytenoids, closing the introitus to the trachea—the vagovagal laryngeal adductors reflex95 (Video 4). Because the UES and larynx both are innervated by the vagus, it is conceivable that stimulation of the larynx may induce contraction of the UES.96 Using an air stimulation technique, recent studies have shown that afferent signals originating from the larynx induce contraction of the UES96 —the LUCR (Figure 13). Frequency elicitation of this reflex is significantly lower in the elderly compared to the young, but the magnitude of UES pressure increase remains unchanged (Figure 14). This indicates a deleterious effect of aging on the afferent arm of this reflex. The LUCR is reported to be altered in some patients with UES dysphagia.96
Figure 13: Upper esophageal sphincter pressure response to laryngeal air stimulation.
An example of the UES pressure response to laryngeal air stimulation of 6 mmHg with 2-second duration in a young volunteer. As seen, UES pressure abruptly increased from about 30 mmHg and reached 60 mmHg after an air stimulation delivered to the interarytenoid area. The poststimulation pressure continued until the subject swallowed by demand. (Source: Kawamura et al.96 with permission from American Gastroenterological Association)
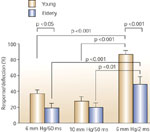
Figure 14: Comparison of response/deflection ratio between young and elderly subjects.
When comparing the response/deflection ratio among the three tested stimulation intensities, responses to air pulses of 6 mmHg pressure with 2-second duration were significantly higher than air pulses of shorter duration in both groups. For both 6 mm Hg/2-second duration and 6 mm Hg/50-msec duration stimuli, the response/deflection ratio in elderly subjects was significantly lower than that in young subjects. The differences for 10 mm Hg/50-msec stimulation did not reach statistical significance. (Source: Kawamura et al.96 with permission from American Gastroenterological Association)
Video 4: Laryngeal abductor reflex.
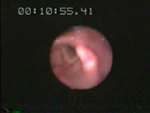
This video demonstrates the stimulation of the laryngeal abductor reflex by air injection as seen in the injection of a short burst of air into the area of the arytenoid, which results in median abduction of this structure for a brief period of time.
The afferent arm of this reflex in humans includes the laryngeal mechanoreceptor and internal division of the superior laryngeal nerve,97 a branch of the vagus nerve. The efferent arm is undoubtedly the vagus nerve,98, 99 including the superior laryngeal nerve and recurrent laryngeal nerve, although the glossopharyngeal nerve cannot be excluded because it serves branches into the pharyngeal plexus. The central control for this reflex is probably different from those of swallowing because the contractile response to the stimulation of this reflex is the opposite of the relaxation response of the UES to a volitional, subconscious, and reflexive pharyngeal swallow.
Laryngo-UES reflex is different from the PUCR that is triggered by stimulation of mechanoreceptors in the posterior pharyngeal wall.47, 85 Although the effector organ and efferent arc are the same for both reflexes, the sensory field and the afferent arc are different, in that the PUCR is mediated via the glossopharyngeal47 nerve with possible contribution from the superior laryngeal nerve.
Air stimulation of the larynx has an inhibitory effect on the LES.100 It is conceivable that activation of the LUCR may counteract the possible consequences of the inhibitory effect of laryngeal stimulation on the LES, namely, pharyngeal reflux of gastric content? by increasing the UES pressure. Although this is highly speculative, this reflex may be activated during pharyngeal reflux events by contact of regurgitated material with the laryngeal mucosa and by inducing an augmentation of UES pressure, possibly preventing further entry of refluxate into the pharynx and larynx. It is possible that deterioration of this reflex in the elderly may negatively affect airway protection against aspiration in this age group, especially during nighttime reflux events in the supine position when the airway is most vulnerable.
In summary, there are, to date, at least nine reflex mechanisms identified that either augment the UES pressure barrier, close the airway, or clear the pharynx and esophagus from gas, liquid, or solid volumes.
These reflexes may be activated by pressure events associated with antegrade or retrograde transit of material through the pharyngoesophageal axis. Available evidence indicates that one or several of these reflexes may be stimulated in response to single stimuli.
As an example, stimulation of the PUCR and PGCR occur owing to stimulation of the pharyngeal mucosa or stimulation of secondary peristalsis, EUCR and EGCR occur in response to esophageal distention. Aging appears to adversely affect these reflexes. Emerging evidence suggests the impairment of these reflexes in some disorders involving the aerodigestive tract, such as posterior acid laryngitis or dysphagia with pre-deglutitive aspiration.
Airway Protection During Belching
Belching is defined as audible voiding of gas from the stomach and esophagus through the mouth. However, it is known that distention of the esophagus by air may also initiate an esophageal belching that does not involve gas reflux from the stomach. Ventilation of gastric or esophageal gas across the UES into the pharynx may be accompanied by entry of food particles, acid mist, etc. into the hypopharynx, and predispose the airway to aspiration.
The glottis is actively involved in the belch reflex by activation of its closure mechanism,4 resulting in full closure of the introitus to the trachea, which begins before UES relaxation and its subsequent opening during belching. This indicates the existence of close coordination between the UES and glottal function during belching (Figure 15). The existence of a similar coordination during regurgitation and vomiting has been speculated but not yet studied. Glottal function during belching consists of the following sequence: (1) vocal cord adduction concurrent with adduction of arytenoids, resulting in full closure of the introitus to the trachea before the UES relaxes or opens; (2) anterior/caudad movement of the glottis; and (3) opening of the UES that is either slit-like or triangular (4) (Video 5).
Figure 15: Coordination between the UES and glottal function during belching.
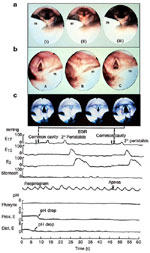
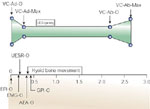
Figure shows temporal relationship among the function of the glottis, UES, and hyoid bone and pressure phenomena of esophagus and stomach during belching induced by intraesophageal injection of 40 mL of room air. Figure is constructed using videoendoscopic, videofluoroscopic, manometric, and electromyographic (EMG) data obtained concurrently. Belch event begins with vocal cord adduction and ends with return to resting position. All other events, including UES opening and closure, occur while cords are fully adducted and thereby the introitus to the trachea is closed. Fluoroscopic UES opening begins 0.35  0.08 sec after onset of hyoid bone movement. VC-Ad-O, onset of vocal cord adduction; VC-Ad-Max, maximum onset of vocal cord adduction; VC-Ab-O, onset of vocal cords opening; VC-Ab-Max, return of vocal cords to resting position; EPI-O, onset of increase in intraesophageal pressure; AEA-O, onset of approximation of arytenoids toward base of epiglottis; GPI-O, onset of increase in intragastric pressure; EMG-O, onset of EMG signal recorded from geniohyoid, mylohyoid muscle groups; UESR-O, onset of UES relaxation recorded manometrically. (Source: Shaker et al.4 with permission from the American Physiological Association)
0.08 sec after onset of hyoid bone movement. VC-Ad-O, onset of vocal cord adduction; VC-Ad-Max, maximum onset of vocal cord adduction; VC-Ab-O, onset of vocal cords opening; VC-Ab-Max, return of vocal cords to resting position; EPI-O, onset of increase in intraesophageal pressure; AEA-O, onset of approximation of arytenoids toward base of epiglottis; GPI-O, onset of increase in intragastric pressure; EMG-O, onset of EMG signal recorded from geniohyoid, mylohyoid muscle groups; UESR-O, onset of UES relaxation recorded manometrically. (Source: Shaker et al.4 with permission from the American Physiological Association)
Video 5: Glottal function during belching.
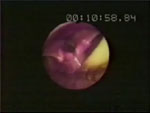
Videoendoscopic demonstration of belching triggered by intraesophageal air injection through a catheter seen at the 6 o'clock position.
Glottal function during belching consists of the following sequences: (1) vocal cord adduction concurrent with adduction of arytenoids, resulting in full closure of the introitus to the trachea before the UES relaxes or opens; (2) anterior/caudad movement of the glottis; and (3) opening of the UES that is either slit-like or triangular.
During some belches, the adducted arytenoids approximate the base of the epiglottis, entailing closing the supraglottic portion of the laryngeal vestibule. Supraglottic closure of the laryngeal vestibule, when present, is associated with an increase in intragastric pressure.4 Because food particles, acid mist, and gastric content may be regurgitated into the pharynx during belching and could be aspirated into the trachea, glottal closure and its precedence to UES relaxation and opening during belching is an important protective function against aspiration. Glottal closure during belching in humans is in sharp contrast to the glottal function in ruminants, in which the glottis remains open during belching, resulting in tracheal reflux of gastric gas.101
The time interval between the onset of vocal cord adduction and the cords' complete closure, as well as the time interval between the onset of their abduction to their return to resting open position during belching, is fixed, indicating the existence of a stereotyped neural program. On the other hand, the duration of complete cord closure during belching is modified according to the volume of belched air.4 It has been suggested that the neural pathway during belching includes both those pathways controlling the larynx and the UES functions.4 The neural pathway for glottal closure during belching begins with the vagus nerve and the stretch receptors in the esophageal wall that carries the signal to the brainstem. The efferent vagal fibers, through the recurrent laryngeal nerve, carry the signal to the glottal adductor muscles—interarytenoid, lateral cricoarytenoid, and thyroarytenoid.
Coupled with control of UES and glottis, the signals carried by the vagus to the brainstem from the esophageal wall stretch receptors stimulate supra- and infrahyoid muscles through the fifth, tenth, and 12th nerves and ansae cervicalis, resulting in UES opening by the anterior displacement of the laryngeal and cricoid cartilages induced by constriction of the supra- and infrahyoid muscle groups.
A comparison of the functions of the various elements involved in belching and swallowing is shown in the Table 2.
Pharyngeal, Laryngeal, and Esophageal Inhibitory Reflexes
In addition to stimulatory reflexes emanating from the pharynx, larynx, and esophagus as described above, pharyngeal, laryngeal, and esophageal stimulations may exert an inhibitory effect on esophageal peristalsis as well as the LES or the gastric fundus.
Pharyngoesophageal Inhibitory Reflex
Depending on the type of stimulus, there are two kinds of esophageal peristalsis: primary and secondary. Primary peristalsis is generally induced by swallow, and secondary peristalsis is initiated in response to local esophageal stimuli such as distention.102, 103 Secondary esophageal peristalsis plays a pivotal role in the clearance of residual ingested materials and refluxate from the stomach. This function is particularly important in preventing acid and other gastric contents from reaching the airway. In humans91, 104, 105, 106, 107 as well as experimental animals,108 it has been shown that pharyngeal mechanical stimulation by air or water injection produces a general inhibition of both primary (Figure 16) and secondary peristalsis—the pharyngoesophageal inhibitory reflex (PEIR).
Figure 16: Inhibition of progressing primary esophageal peristalsis.
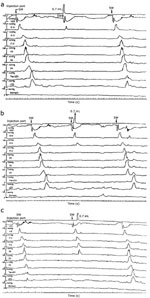
Examples of inhibition of progressing primary esophageal peristalsis in the proximal striated (A,B) and distal (C) smooth muscle esophagus by pharyngeal water stimulation. A: Rapid injection of 0.7 mL of room-temperature water into the pharynx immediately after UES relaxation and arrival of peristaltic wave at the site 18 cm above lower esophageal sphincter (LES) inhibited the progression of peristalsis to the sites below. B: Similar injection when the peristaltic wave had reached the site 15 cm above the LES inhibited its progression to the more distal sites. C: Rapid pulse injection of 0.7 ml of room-temperature water into the pharynx when the peristaltic wave was 9 cm above the LES resulted in its inhibition in the smooth muscle portion in the distal 6 cm of the esophagus. Note that these inhibitions were not followed by another peristaltic pressure wave. Each inhibition trial is preceded and followed by a normal peristaltic pressure wave induced by a dry swallow. SW, swallow. (Source: Trifan et al.104 with permission from American Gatroenterological Association)
Inhibition of the progression of primary esophageal peristalsis105 by sensory impulses initiated form the pharynx by water injection in turn inhibits esophageal bolus transit. This inhibitory effect is capable of overcoming the facilitating effect that the presence of a bolus induces on the swallowing apparatus. The threshold volume required to inhibit the progression of peristalsis induced by swallowing liquid boluses is significantly higher than that induced by the dry swallows; however, this threshold is not dependent on bolus volume or consistency.105 Inhibition of esophageal peristalsis by PEIR is not followed by generation of a new peristatic pressure wave. This phenomenon is different from inhibition of an ongoing peristaltic wave by a closely timed second swallow, which results in inhibition of peristalsis due to the first swallow and generation of peristalsis by the second.104, 105, 106, 107, 108 The PEIR occurs in both the striated and smooth muscle portion of the esophagus, but its effect is relatively stronger in the striated muscle compared to smooth muscle.105, 108 This difference could be attributed to the nature of innervation of these two types of esophageal muscles.
In the cat, PEIR is intensity-dependent: the stronger the pharyngeal stimulation, the greater the inhibition of the secondary peristalsis.108 In addition, the PEIR is very specific to hypopharyngeal stimulation, because the stimulation of the nasopharyngeal area does not produce any inhibition of peristalsis. Transection of GPN markedly, but not completely, attenuates PEIR. Transection of the superior laryngeal nerve (SLN) reduces the number of pharyngeal stimulation-induced inhibitions of esophageal peristalsis, indicating that the afferent pathways of PEIR include both in the GPN and SLN, although it is predominantly via GPN.108 Studies in humans and cats have shown that stimulation of PEIR is triggered by pharyngeal mucosal mechanoreceptors activation, which can be readily blocked by applying lidocaine to pharyngeal mucosa.104, 108
Pharyngo–Lower Esophageal Sphincter Relaxation Reflex (PLESRR)
The LES is the first barrier for preventing the entry of gastric contents into the esophagus and beyond. This barrier is maintained by a high resting tone in the LES muscle. There are several peripheral factors that alter the LES pressure. The LES reflex relaxation can occur orthogradely by stimulation of the pharynx (pharyngo-LES relaxation reflex), larynx (laryngo-LES relaxation reflex), and the esophagus (esophago-LES relaxation reflex), and retrogradely by gastric distention. In human109 and animal studies,110 it has been shown that intrapharyngeal instillation of minute amounts of water produces a prolonged LES relaxation. The threshold volume of water when injected rapidly is significantly less than slow continuous infusion.109 The PLESRR is more prevalent in elderly when compared with younger subjects.111
Minute amounts of liquid injected either abruptly or slowly into the pharynx induce LES relaxation.109 In the postprandial period, occurrence of PLESRR associated with acid reflux is less (6%) in younger volunteers compared with a significantly higher incidence (44%) in the elderly subjects.111 The duration and magnitude of LES relaxation induced by pharyngeal water injection are different from those of the normal swallow. Neither the duration nor the magnitude of this relaxation is volume dependent.109 In a substantial minority of young and elderly volunteers, the postprandial period111 pharyngeal water stimulation results in gastroesophageal acid reflux by inducing LES relaxation.
Laryngo–Lower Esophageal Sphincter Relaxation Reflex (LLESRR)
Laryngeal stimulation by air pulse induces LES relaxation, which is independent of swallowing and esophageal peristalsis.112 The stimulation of the epiglottis and arytenoids produces a higher incidence of LES relaxation compared to the base of the tongue.
The magnitude of LES relaxation differs significantly depending on the three anatomic sites, with greater relaxation occurring at the epiglottis and arytenoids compared with the base of the tongue.112 It is believed that stimulation of the laryngopharyngeal mechanoreceptors mediate this type of LES relaxation.
Esophago–Lower Esophageal Sphincter Relaxation Reflex (ELESRR)
Esophageal distention in humans and experimental animals induces isolated LES relaxation.110, 113, 114 It is widely accepted that ELESRR is a vagovagal reflex phenomenon.115, 116 The afferent limb of this reflex is undoubtedly vagal sensory afferent fibers projecting to the nucleus tractus solitarius (NTS). In the rat it is more specifically in the region of the NTS centralis.115, 116 Esophageal distention-sensitive muscle afferent fibers play a major role in the activation of ELESR. In achalasia patients, esophageal balloon distention does not produce LES relaxation but the phasic contraction of the esophagus proximal to balloon distention exhibits a normal response, suggesting that extrinsic innervation of the esophagus is preserved in achalasia despite loss of intrinsic inhibitory neurons in the LES.113
Laryngogastric Reflex Relaxation (LGRR)
The stomach actively relaxes before entry of food. This phenomenon is known as receptive relaxation.117 Studies have reported that administration of water into the larynx and epiglottis or electrical stimulation of the central cut end of the SLN in rats inhibits gastric motility.118 Transection of cervical vagi, but not the spinal cord (T3-T4), prevents development of this inhibition. This finding suggests that the afferent limb of laryngogastric inhibitory reflex is exclusively vagally mediated. The laryngeal afferent stimulation possibly inhibits the preganglionic vagal motor neurons to inhibit the gastric motility.118 Kobashi et al.119 documented that electrical stimulation of the central cut end of the SLN inhibits the tonic firing of the neurons in the intermediate dorsal motor nucleus of the vagus of the rat. Systemic application of atropine blocks inhibition of gastric motility induced by water stimulation, suggesting the involvement of cholinergic pathways.
Pharyngogastric Reflex Relaxation (PGRR)
Pharyngeal distention in anesthetized dogs produces relaxation of the fundus and corpus.120, 121 However, the motility of antrum remains unaffected. Anesthesia of the pharynx by 2% Xylocaine or bilateral cervical vagotomy abolishes relaxation of the proximal stomach.
Esophagogastric Reflex Relaxation (EGRR)
It has been shown that esophageal distention induces reflex relaxation of the proximal stomach.122, 123, 124, 125, 126 This reflex requires an intact vagovagal connection among the esophagus, brainstem, and stomach124 for its provocation. The majority of the vagal afferent fibers from the esophagus project to the central part of the NTS which acts as a relay station for EGRR. It has been shown that the bilateral electrolytic lesion of NTS abolishes esophageal distention-induced gastric relaxation.125
It is believed that activation of esophageal vagal afferent fibers inhibits the tonically active preganglionic motor neurons in the dorsal motor nucleus of the vagus in order to produce relaxation of the proximal stomach. Noradrenergic, but not neurergic, neurons in the NTS contribute in the modulation of EGRR. Microinjection of norepinephrine into the dorsal motor nucleus of the vagus can mimic this reflex.125
Conclusion
There are a large number of anterograde and retrograde vagovagal reflexes that provide a means for a close interaction between the upper gut and the aerodigestive tract. Although most of these reflexes are well defined and extensively studied under different conditions, a few have been reported recently and await either confirmation or better delineation. Some of these reflexes seem to work counter to normal physiologic functions such as LES relaxation reflexes induced by pharyngeal and esophageal stimulation. Others enhance or regulate physiologic functions. Over- or underresponsiveness of any of these reflexes can potentially contribute to abnormalities of coordination of the upper gastrointestinal and the aerodigestive tracts.