Key Points
- Breathing and swallowing processes are closely interrelated in their central control and are highly coordinated.
- Many muscles and structures have dual roles in respiration and swallowing.
- Neural control centers responsible for coordination of breathing and swallowing are contained in the dorsomedial and ventrolateral medullary regions of the brainstem.
- Cortical structures also play an important role in facilitating and modulating the coordination of breathing and swallowing.
- Relationship of the phase of respiration (i.e., Inspiratory, Expiratory, Transition) and duration of the apneic phase associated with swallowing have been extensively investigated.
- Studies of swallowing dynamics and pulmonary function are needed that will investigate the clinical relevance of integrated breathing and swallowing function on the health and nutritional outcomes of dysphagic patients and patients with pulmonary disorders.
Introduction
Respiration and swallowing are physiologic processes that demonstrate specialization in their neural networks and peripheral functions and yet exhibit a finely tuned partnership in the execution of their role in basic survival. It is well established that breathing and swallowing do not occur simultaneously in infant or adult animals and humans. These observations have led clinicians to assume that the functions are mutually exclusive. Recent studies, however, are clarifying this simplistic functional separation and highlight the complementary and overlapping nature of one function with another. The development of animal models and observations from clinical studies cited in this review has laid a foundation for this new thinking regarding the coordination between respiratory and swallowing function.
The term respiration, operationally defined as the process of moving and exchanging oxygen from inhaled air and releasing carbon dioxide via exhalation, and the central regulation of that process, is used synonymously with breathing in this review of the literature. This is done to avoid confusion with the multiple implications of respiration, such as cellular or metabolic respiration. The discussion is restricted to components of respiration that are most pertinent to breathing and swallowing coordination, that is, those necessary to avoid pulmonary contamination via aspiration and to ensure adequate ingestion and swallowing of secretions, liquids, and foods.
Clinical and experimental evidence support the existence of neurophysiologic, structural, and functional interdependence between respiration and swallowing. Health care professionals who treat patients with swallowing disorders are able to modify abnormal swallowing physiology using compensatory techniques that involve both peripheral alterations in breathing and swallowing. Compensatory postures and maneuvers used in the treatment of patients with dysphagia often require voluntary modification of both breathing and swallowing.1, 2 Further, modifications in the volume and texture of swallowed materials are also routinely made by speech-language pathologists because these variables have shown an immediate influence on swallowing behavior observed during videofluoroscopic or endoscopic imaging.3, 4, 5, 6, 7, 8 What is not understood is the impact of behavioral modifications or swallowing treatments, such as exercise, on the overall transfer effect of these strategies directly impacting on the central controllers of breathing and swallowing coordination. Changes in respiration, ventilation, and swallowing occur with normal development and aging, and with multiple disease processes. The arbitrary division of these functions in the majority of the basic and clinical studies, however, has not significantly moved the field along toward improved understanding of the effects of these normal stage-of-life conditions on respiratory and swallowing coordination. This is related in part to the relative difficulty of conducting concurrent studies on the central control of respiration and swallowing in various animal models, and to the limitations of directly applying these models to human species. A few studies have been able to examine perturbations in breathing and swallowing in humans that likely imply modification in the central controllers. Clinical studies of the impact of neurologic,9, 10, 11 pulmonary,12, 13, 14, 15 and oncologic diseases16, 17 on the coordination of breathing and swallowing are also beginning to emerge that are leading basic scientists back to the laboratory to determine what aberrations in central control may be contributing to the functional observations made in the clinic.
Central Control of Respiration and Swallowing
Brainstem Regulation
The current knowledge regarding the central control of breathing and swallowing coordination has been derived from lesion studies and electrophysiologic, neuroanatomic, and pharmacologic data obtained from experimental studies with decerebrate animals. Early investigations demonstrated that swallowing motor activity could be initiated via electrical stimulation of the superior laryngeal nerve (SLN) branch of the vagus nerve [cranial nerve (CN) X] in anesthetized animals. Jean50 pointed out that the stimulated swallows elicited from these animal models relate to "stereotyped basic swallowing movement [as in a reflexive response, rather] than to physiological motor activity" (p. 935). The animal model studies showed that SLN-stimulated swallows are predictable and relatively consistent. In the human swallow, however, there is considerable variability in the early components of swallowing that is not reflected in an SLN-stimulated swallow. This is an important distinction that must be appreciated when attempting to translate the findings from animal studies to human swallowing behavior. Nonetheless, the model has allowed for considerable understanding regarding the potential neural control because the majority of the muscle contractions of human swallows are included in the stimulated swallows in animals.50
It was postulated a century ago by Meltzer51, 52 that swallowing was under the control of a central pattern generator. Doty53 later proposed that the motor patterns involved in swallowing were controlled by a center with three arms: (1) an input arm—peripheral afferents; (2) an organizing arm—commanding interneurons); and (3) an output arm—motoneurons. Doty explained that a control center must have a selective mechanism at its afferent portal that allows the center to be activated by appropriate stimuli. He explained that the swallow center must demonstrate a filtering arrangement to give preferred matching only to those stimuli matching the spatiotemporal code to elicit swallowing. Other simple reflexive synergies, many of which protect the airway, such as coughing and gagging, recruit the same muscles used by swallowing but are filtered out by the center defined by Doty. The synergy of pharyngeal swallowing would be released only by a stimulus pattern specific to it.
Both respiratory and swallowing responses have been elicited in experimental animals via electrical stimulation to the internal branch of the SLN of (CN) X.54, 55, 56, 57, 58, 59, 60, 61 Stimulation of the SLN produced not only the stereotyped swallow pattern but also secretion of mucus in the pharynx, larynx, esophagus, and trachea.62, 63. The type of the elicited response was dependent on the nature of the stimulus (i.e., frequency, intensity, and pattern) that was applied to the SLN.54, 55 These early studies demonstrated that the inhibition of respiration, in the expiratory phase, was easier to elicit with electrical stimulation of the SLN than were swallow responses in various species of experimental animals.54 It was explained that this phenomenon was due to the existence of optimal and limiting frequencies of the electrical stimulation for elicitation of swallowing that varied across species. The optimal frequency was that which resulted in the greatest number of repetitive swallows with the shortest latency at the lowest stimulus intensity. Frequencies above or below the optimal frequency were not as effective in eliciting continuous swallowing. Doty54 demonstrated that respiratory effects were more easily elicited than swallowing at these limiting frequencies. Electrical stimulation to the glossopharyngeal nerve (CN IX) in dogs, rabbits, and cats also resulted in the elicitation of swallowing but only when anesthesia was sufficiently light.54 Stimulation of IX at various frequencies, durations, and intensities could not induce swallowing in anesthetized sheep, but did evoke short latency potentials in the nucleus of the solitary tract (NTS).64 In the same experiment, a conditioning stimulus applied to IX facilitated swallowing elicited by SLN stimulation. The investigators concluded that the afferent fibers running in IX do not have sufficient input on the medullary swallowing neurons to induce the swallowing motor sequence, but likely play a facilitative role. Sinclair65, 66 demonstrated through selective sectioning of IX and the pharyngeal branch of X that IX was the primary afferent pathway of the swallow response initiated from the pharynx in the dog. Additionally, he demonstrated from dissection experiments that sections of IX and SLN anastomose within the pharyngeal plexus. He suggested that the small sensory fibers of IX and the larger fibers of SLN interact centrally to explain the observation that electrical stimulation of SLN elicits swallowing more readily than stimulation to the IX cranial nerve. Clearly, this later model appears to relate best to human function whereby the swallow typically triggers when the sensory afferents in the oropharynx are stimulated. Different branches of these same afferent portals (i.e., IX and X) also carry sensory information from critical sensory end organs for the control of respiratory rhythm, including the pulmonary stretch receptors, carotid and aortic body chemoreceptors, and vascular baroreceptors.67, 68, 69, 70 Anatomic and electrophysiologic studies have demonstrated that afferent inputs from the peripheral swallowing and respiratory regions ascend to the NTS in the medullary region of the brainstem. The NTS is a primary sensory nucleus, lying intimately with the descending fibers of the tractus solitarius, and is the first to receive special and general viscerosensory information from regions of the upper and lower digestive, respiratory, and cardiac systems.71
Ventral and dorsal regions of the medulla have been implicated as including the centers, or central controlling neurons, responsible for programming the swallow motor output and respiratory rhythmogenesis. The exact location of these central command centers varies based on the animal model that has been studied. Early lesion studies in the dog, cat, and monkey reported that the interneurons relevant to pharyngeal and esophageal phases of swallowing resided in different regions of the medulla, and that the central pattern generators or core interneurons for swallowing were located on each side of the brainstem in the medial reticular formation between the posterior pole of the facial nucleus and the rostral pole of the inferior olive.55
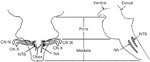
Jean72 also concluded that the controlling regions for different areas of the swallowing tract were located in different regions of the medulla. The location of the areas housing the central controlling neurons in sheep, however, differed considerably from those described in Doty's work. Jean demonstrated that lesions to the dorsomedial NTS in sheep abolished the esophageal components of swallow, whereas lesions placed more rostrally in the NTS eliminated swallows elicited by SLN stimulation. These findings, together with central microelectrode recordings following SLN stimulation in the same species, resulted in the location of two central swallowing regions: (1) a dorsal region that included NTS and adjacent reticular formation, and (2) a ventral region composed of the lateral reticular formation in and around the nucleus ambiguus (NA)56, 57 (Figure 1). The portion of the NTS receiving afferent input from CNs IX and X corresponded to Doty's53 afferent portal, whereas the interneurons in NTS corresponded to the organization arm of the network.73 The ventral region around and including the NA contained interneurons that were described as commanding the motoneurons involved in swallowing. Other early electrophysiologic studies support the anatomic findings of neuronal projections between NTS and NA in various species.67, 70, 74, 75
Similar to the swallowing research, several ablation, stimulation, and recording techniques have been used to locate the respiratory control areas in the brain. There is general agreement that the production of basic respiratory rhythmicity occurs in the medulla.76 Just as the two regions of swallowing neurons were identified according to their synchronous activity with swallowing muscle contraction, there have been two large concentrations of respiratory neurons identified and characterized by the temporal relationships of their firing activity with phrenic nerve discharge: a dorsal respiratory group (DRG) and a ventral respiratory group (VRG). A region ventrolateral to the solitary tract, DRG is composed of a heavy density of primarily inspiratory neurons.76 Merrill77 divided these cells into three categories: (1) respiratory neurons unaffected by lung inflation; (2) respiratory neurons activated by lung inflation; and (3) pump cells that fired with the pump strokes of a respirator in paralyzed, artificially ventilated animal preparations. The dorsal respiratory region of the NTS receives afferent projections from the larynx, extrathoracic trachea, intrathoracic trachea, main bronchus, and lung.70 Expiratory phase switching neurons have also been found in this region leading to the implication that the NTS alone is capable of generating the respiratory rhythmogenesis.78
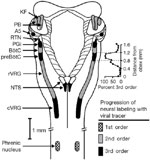
The VRG intermingles with NA motoneurons and has been named the nucleus retroambigualis (NRA). The NRA has not been implicated in the neural control of swallowing, but receives projections from the NTS.67, 70, 75, 77 Beginning in the spinal cord at the level of the first cervical vertebra and extending to the near rostral border of the medulla, Merrill77 distinguished three major subdivisions of the NRA: (1) an area of almost exclusively expiratory neurons, (2) a mainly inspiratory part, and (3) a region near the pontomedullary border composed primarily of expiratory neurons. Previously labeled the retrofacial nucleus, the latter extreme rostral area containing the expiratory neurons is labeled the Botzinger complex.77, 79, 80 More recent studies have supported the subdivision of the VRG into functionally distinct compartments and have identified an area labeled the pre-Botzinger complex, which may contain the central rhythm generating network for breathing.81, 82 Inputs from the pre-Botzinger complex have also been shown to play a critical role in initiating the normal ventilatory response to hypercapnia and hypoxia83 (Figure 2).
Figure 2: Dorsal view of brainstem and cervical spinal cord indicating regions involved in control of breathing and progression of labeling with a viral tracer injected into the phrenic nerve.
KF, Kölliker-Fuse nucleus; PB, parabrachial nuclei; NA, noradrenergic A5 area; RTN, retrotrapezoid nucleus; PGi, paragigantocellular reticular nucleus; BötC, Bötzinger Complex; preBotC, preBötzinger Complex; rVRG, rostral ventral respiratory group; cVRG, caudal ventral respiratory group. (Source: Annual Review of Physiology, Volume 60, with permission. ©1998 by Annual Reviews. www.annualreviews.org.)
Doty's53 final criterion for a control center of swallow muscular activity is that of an efferent portal. He reported that the center must have an exceptionally effective access to motoneurons, and must be able to inhibit competing centers. Evidence for the existence of this third criterion has been illustrated from neurophysiologic studies of central respiratory and swallowing activity.
The swallow-related motor output to musculature of the mouth, pharynx, and larynx is transmitted by axons whose cell bodies reside in the brainstem. These include the trigeminal motor nucleus located toward the cranial part of the brainstem near the level of the mid-pons, the facial motor nucleus located at the level of the caudal pons, the NA running rostrocaudally in the medulla, and the hypoglossal motor nucleus. The NA consists of not only interneurons (i.e., premotor commanding neurons) but also large motor neurons that eventually distribute to the striated musculature innervated by CNs IX and X. Fibers that emerge from its upper end join IX, and those that emerge at the lower level join fibers of X and the cranial part of the accessory CN (XI). The NA gives rise to fibers of X that are eventually distributed to the constrictor muscles of the pharynx and the intrinsic muscles of the larynx. A group of fibers in NA also give rise to IX fibers that innervate the stylopharyngeus muscle. From this anatomic description, it appears that the organizational arm of the swallow, as defined by Doty, does have direct access to the motor nuclei that precisely initiate and time the movements of the muscles that they supply.
Studies have supported the early observations that the NA contains the premotor neurons that control swallowing. In sheep it has been demonstrated that only the swallowing neurons in the ventral group (region surrounding NA) could be antidromically activated by stimulation of the trigeminal motor nucleus, whereas none of the neurons in the dorsal region exhibited this antidromic activation.84 Anatomic studies also showed that the trigeminal motor nucleus received axon terminals from medullary neurons in the reticular formation surrounding NA.85 The ventral medullary region around NA was also found to receive projections from the homologous contralateral medullary region, and from the bilateral facial (CN VII), X, and XII motor nuclei that are all involved in swallow related motor activity. These data showed that the ventral region contains the premotor neurons (i.e., motor command area), which have precise access to the muscles involved in deglutition. Neurons in the ventral medullary region have also been found to play a critical role in the control of respiratory muscle activity.
Intracellular recordings from intercostal motor neurons in the ventral horn of the spinal cord reveal the presence of descending excitatory and inhibitory activity. Projections from brainstem respiratory regions, VRG and DRG, to the spinal cord have been identified.76, 77, 86 Respiratory impulses from the brainstem descend to various segments of the spinal cord where they are integrated with intrasegmental and intersegmental information. This descending neural activity alternatively depolarizes and hyperpolarizes the motor cells, leading to rhythmic increases and decreases in their excitability.22 The proposed central controllers of respiration, such as those for swallowing, have direct access to the spinal motor neurons supplying the muscles of respiration. The major muscle of inspiration, the diaphragm, is supplied by the phrenic nerve arising from cervical levels four through six. The external and internal intercostals muscles that assist in inspiration and expiration, respectively, are controlled by descending axons that synapse at the levels of the cervical and thoracic spinal motoneurons in the ventral horn of the spinal cord.
It is generally accepted that the neural control centers responsible for breathing and swallowing coordination are contained in the dorsomedial and ventrolateral medullary regions of the brainstem. Given the complexity of the intramedullary control of swallow initiation, execution, respiratory rhythm, and ventilation, it is not surprising that few neurophysiologic studies exist that simultaneously examine the central control centers. An early investigation of single neurons in the medulla of cats demonstrated that pouring water into the pharynx usually, but not consistently, caused swallowing movement, and always inhibited the discharge of inspiratory neurons.87 The swallow evoked a brief burst of impulses in inspiratory neurons followed by a period of inhibition when the swallowing act was elicited by water pouring. When the inhibitory effect of the swallowing neurons on the inspiratory cells was prolonged, the succeeding discharge volley of the inspiratory neuron was longer in duration and higher in frequency. The expiratory neurons that intermingled with the inspiratory neurons began to discharge instantaneously as the inspiratory neurons stopped their discharge, and were inhibited only transitorily when the swallowing act occurred.87
Sumi88 showed that motor fibers from NA and XII demonstrated a momentary dominance by the swallowing pathway during swallow; however, this dominance was lost during occlusion of the intratracheal tube. During asphyxia, as the oxygen partial pressure decreased and the partial pressure of carbon dioxide increased, the motor fibers that are normally silent during a swallow began to discharge rhythmically during either inspiration or expiration. With continued asphyxia, the swallow fibers no longer demonstrated motor discharge to stimulation of the pharyngeal or laryngeal mucosa or to electrical stimulation of the SLN. The two physiologic events reversed in importance, with a patent airway becoming the priority of the motor nuclei and their muscles.71, 89, 90 Neurons firing during inspiration and expiration were identified and characterized into different types depending on their discharge pattern when water was poured on the pharyngeal mucosa. Sumi's findings indicated that two types of interneurons were active in respiration: those inhibited by swallowing input and those that continue their activity and may serve functions of both swallowing and respiration.88
Recent studies have built upon Sumi's88 early notion that central neurons may play dual roles. Experiments have demonstrated not only that the central pattern generation of breathing and swallowing may anatomically overlap, but that certain groupings of medullary interneurons may have more than one function.50, 58, 91, 92, 93 Interneurons in the dorsal and ventral regions of the swallowing central pattern generator (CPG) have been found to discharge during different behaviors such as swallowing, respiration, mastication. and vocalization.91, 94, 95, 96, 97, 98, 99, 100, 101 Jean50 reports:
Common motoneurons might therefore be triggered by common pools of interneurons. These results indicate that in mammals, the neurons liable to be involved in pattern generation can belong to different CPGs. Multifunctional neurons of this kind would make for great functional flexibility. (p. 959)
If the concept of multifunctional neurons holds true in humans, there are implications regarding plasticity of brainstem structures that may contribute to recovery of functional swallowing and breathing coordination in patients recovering from brainstem stroke.
Suprabulbar Influence
Even though electrically stimulated swallows and respiratory rhymicity persist in experimental animals after removal of suprabulbar structures, there is increasing evidence that cortical structures play a role in facilitating and modulating the coordination of breathing and swallowing in adult humans. Certainly the employment of various compensatory maneuvers directed toward improving airway protection in patients with dysphagia must involve cortical centers. Patients are often trained to "think swallow or swallow on cue," "hold your breath, and then swallow," etc. These instructions must involve recruitment of premotor and motor cortex for effective initiation.
Cortical stimulation to the frontal orbital gyrus and lateral precentral gyrus has elicited swallowing or facilitated swallowing elicted by other means.102 It was further demonstrated that stimulation to the motor cortex did not evoke swallowing in monkeys, yet swallowing was readily elicited via electrical stimulation to the precentral area.103 Lesion studies in animals and observations of humans following cortical damage lend support for the cortical influence on swallowing. Experimental lesions made in the lateral precentral region of monkeys resulted in significant impairment in the initiation and progression of the oral aspects of swallowing.104 It has also been shown that destruction to Brodmann's area 6 in monkeys resulted in persistent difficulty in making the transition between chewing and swallowing.105 Neurophysiologic studies have identified projections from cortical regions to the medullary swallowing CPG in sheep. Electrical stimulation to the frontal orbital cortex resulted in an antidromic response in the NTS region at the entrance of the peripheral swallowing afferents (i.e., the SLN)106. It was also shown that swallowing could be elicited upon stimulation to the frontal orbital region, but this cortically induced swallowing was absent after destruction of the NTS.107 It was further demonstrated that initial excitatory activity could be elicited in the NTS dorsal swallowing center with a single pulse to the frontal cortex, but could only be elicited in the ventral NA region after tetanization of the cortex. These findings seem to suggest that the cortical swallowing regions have an influence on the initiation of the swallow program or on the early stages of swallow, but do not contain the central controlling neurons responsible for the swallow program.108
Anatomic studies have supported these neurophysiologic findings. Cortical projections have been found from the lateral precentral cortical regions to the NTS in humans, monkeys, and chimps,109, 110 and to the NA in humans.109 Other anatomic studies in laboratory animals have traced connections between the NTS in several forebrain and infralimbic structures, such as the hypothalamus, amygdala, and insular regions.111, 112 These findings were varied, and may have represented the central mediation of feeding and prey behaviors. Subcortical sites have also been associated with swallowing activities in neurophysiologic studies.113, 114, 115, 116, 117 More recent studies support this early work regarding a role of the cortex in swallowing activity. Two studies have shown that neurons in the primary motor cortex are active during swallowing.118, 119 Innovative technologies such as cortical evoked potentials,120 transcranial magnetic stimulation,121 functional magnetic resonance imaging (fMRI).122 and positron emission tomography (PET)123 are pointing to multiple cerebral regions (sensorimotor cortex, insula, temporal cortex, and cerebellum) involved in the central modulation of swallowing. Though the exact influence of these cortical and subcortical structures to the swallowing CPG remain unclear,50 the multiple cortical and subcortical regions that have been implicated in their swallowing roles may be reflected in the complex swallowing problems seen in patients with focal lesions, as in the case of stroke, or in dysphagic patients with progressive neurologic diseases.
Translation to Human Function
A body of literature reporting results from studies of breathing and swallowing coordination in humans has grown over the past 20 years. These studies exhibit improved sophistication of simultaneous recording methods and analysis. However, the instrumentation used in many of these studies was assembled in, and was unique to, a particular laboratory, and generally small sample sizes were employed. Investigators have attempted to compensate for the small number of subjects by including the recording and analysis of several swallows per subject. The problem with this method, however, is that it does not account for the known variability in swallowing behavior between individuals. Further, the majority of the studies implemented indirect methods, such as submental EMG (sEMG) or records of swallow sounds from a stethoscope placed on the lateral neck9, 10, 11, 124, 125, 126, 127, 128, 129, 130, 131 to mark swallowing activity without visual confirmation. The signals were synchronized using a timer counter, and judgments were made regarding the phase of swallowing activity that was associated with preswallow or postswallow activity. These issues have limited the ability to generalize the findings to the population of adult humans. A number of studies have confirmed the swallowing onset and physiologic events related to swallowing and respiration via simultaneous recordings of visual images with either videoendoscopy or videofluoroscopy16, 17, 132, 133, 134, 135, 136, 137, 138, 139 and a respiratory signal, adding greater external validity and reliability to the collected data. The studies of breathing and swallowing coordination located by this author in Medline have been summarized chronologically in Table 1, which demonstrates the different methodologies (i.e., recording, bolus texture and volume, swallowing tasks, and study end points).140
Most investigations of breathing and swallowing relationships in adult humans targeted two primary end points: (1) phase(s) of respiration (i.e., inspiratory, expiratory, transition) that were associated with oropharyngeal swallowing activity, and (2) duration of the apneic interval. The methodologies used in these clinical experiments, as viewed in Table 1, varied in study sample characteristics, bolus characteristics, mode of oral intake, swallow task, and instrumentation. Respiratory bands, nasal or oral masks with airflow, or temperature-sensitive transducers were typically employed to record the respiratory activity while the subject was administered some type of liquid or food bolus. The respiratory signal was displayed on a monitor and recorded for later demarcation and temporal analysis. Many of these studies involved indirect marking of the swallowing signal using the noninvasive techniques described above.
Phase Relationships and Swallow Apnea
Respiratory Phase
The results from the studies listed in Table 1 confirm that the expiratory phase of respiration is the favored limb of the respiratory cycle surrounding the oropharyngeal swallow, regardless of the variation in study method. Solid food tends to differ from liquids and viscous materials in terms of the regularity of respiration, yet the cycle of breathing interrupted by the swallow (i.e., the apneic interval) continues to occur at some point in the expiratory phase of respiration.137, 141 The predominant respiratory pattern surrounding swallowing activity in healthy adults reported in the majority of these studies is the EX/EX pattern (expiration before the swallow and expiration after the swallow), followed next in order of frequency of occurrence by IN/EX (inspiration before the swallow and expiration after the swallow), and rarely by the EX/IN (expiration before the swallow and inhalation after the swallow) or IN/IN (inhalation before the swallow and inhalation after the swallow) patterns. McFarland et al.130 showed, however, that the segment of the expiratory limb during which swallowing occurs can be influenced by the changes in the whole body posture of the individual in adult humans. This study discovered that when human adults attempted to swallow with the hands and knees planted on the ground (i.e., "on all fours"), the swallow occurred during the early part of the respiratory cycle and when standing, swallow occurred late in the expiratory cycle. It should be mentioned that the pattern preference of expiration preceding swallowing is reversed in most animal models. Swallowing has been shown typically to occur during the inspiratory limb of respiration in unanesthetized141, 142 and anesthetized47, 143 animals. In infant humans, the production of spontaneous swallows reportedly is equally distributed between the expiratory and inspiratory phases of respiration.144
The differences among animal, human infant, and human adult swallows may be related to the differences in neural structures, neural development and maturation, and the overall head and neck anatomy. In animals and human infants, the larynx is seated high in the neck and the epiglottis opposes the base of the tongue and soft palate. This anatomic position is a favorable feeding, breathing, and swallowing arrangement that allows bolus entry into the pharynx around the side channels of the larynx formed by the aryepiglottic folds. Nasal airflow during breathing can be maintained during the sucking and feeding process, yet all of the animal studies mentioned have observed a clearly distinguishable apneic interval that follows a sucking/feeding sequence. In contrast to animals and infant humans, the larynx of adult humans descends with development and reaches its final position around the time of puberty. The lowered laryngeal position provides a unique resonating chamber for human voice and speech production, yet comprises the once anatomically protected airway from liquid and food entry during swallowing. This optimal anatomic configuration for a resonant voice requires that the hyoid and larynx be lifted and pulled forward to prevent aspiration of a flowing bolus through the pharynx.
A small group of investigators have taken the study of the respiratory phases and cycles surrounding swallowing activity a step further toward understanding the influence of the swallow CPG on the respiratory CPG. Surely this information will assist scientists in the translation of the peripheral coordination of these basic physiologic behaviors to the operation of their central controllers.125, 131, 134, 137, 145 These studies included an analysis of the timing in the respiratory phase when swallowing occurred, and calculated the duration of the preswallow, swallow, and postswallow breaths. In unanesthetized humans,125 both spontaneous and water-induced swallows during expiration increased expiratory time and total time of the swallow breath. The tidal volume of the postswallow breath immediately after the swallow was increased. Martin132 also found increased expiratory time in pre- and postswallow breaths when compared to basal respiration. McFarland and Lund131 also reported that swallowing occurred during the expiratory phase and the duration of that phase was prolonged. Charbonneau et al.145 also demonstrated that swallowing significantly increased the duration of respiratory cycles of the swallow breath and in subsequent respiratory cycles. This notion was carried a step further in the classic study by Paydarfar et al.134 that demonstrated that swallows produced a "true" resetting of the respiratory rhythm.
In addition to the phase relationship, and influence of swallow on the duration of the respiratory cycle, the glottic position associated with the phases of respiration bracketing swallow has also been explored. Simultaneous recordings of videoendoscopy and respiratory air flow showed that the glottis assumes a paramedian position at the onset of laryngeal elevation (i.e., after the onset of apnea) in the majority of the tested swallows.132, 133 That is, the undulating glottic movement characteristic of rest breathing ceased, medialized, and became fixed in the intermediate or paramedian exhalatory position just before (0.03 s) the onset of laryngeal elevation. Shaker and colleagues146 similarly found early vocal fold medialization at the onset of the swallow prior to the onset of laryngeal elevation, but the association of this posturing with respiration was not studied in their work. Finally, the glottic configuration was also described on the last video frame during which final laryngeal descent occurred. The majority of swallows were again characterized by a paramedian, expiratory glottic configuration at the time of final laryngeal descent.132, 133 This study demonstrated that the glottic configuration associated with expiration, and the cycle of respiration bracketing the majority of liquid swallow activity (3 mL, 10 mL, 20 mL), may account for the priority given to the expiratory cycle in and around the time of swallow in young, healthy individuals. That is, there appears to be a built-in, reliable, physiologic protection mechanism of a partially occluded glottic airway during the cycle associated with early and late oropharyngeal swallowing activity.125, 132, 133, 134, 136, 139, 145 It has also been postulated that swallowing during the expiratory phase of respiration may also facilitate elevation of the larynx because the muscles and forces responsible for the elevation have less resistance to upward movement when the diaphragm is relaxed.130, 145
It has been explained that the order of preference for respiratory-swallowing patterns in most studies of healthy humans is EX/EX, IN/EX, EX/IN, and IN/IN, respectively. A growing body of evidence is demonstrating, however, a reversal in the order of occurrence of these respiratory patterns in patients with advanced age and diseases often associated with aging. It can be seen from Table 1 that in patients with neurologic disease,9, 10 chronic obstructive pulmonary disease,147 and those treated for head and neck cancer,16, 17 the inspiratory phase of the respiratory cycle appears to increase its relationship to swallowing. The switch of expiration to inspiration preference, however, did not relate to the occurrence of aspiration pneumonia in patients with stroke10 but did relate to higher (e.g., worsened) Penetration-Aspiration Scale148, 149 scores during videofluoroscopic exams.16, 17 The clinical significance of these findings remains uncertain until clinical trials that include larger numbers of like-patient cohorts are conducted. The inhalatory gesture surrounding the onset of the swallow, and characterized by an open laryngeal airway, would seem to pose an aspiration threat particularly in patients with compromised swallowing function related to focal or generalized weakness, missing structures following surgical ablation, or radiation fibrosis. A few studies126, 127, 129, 139, 147 have shown trends that aging may also disrupt the normal patterning between breathing and swallowing with a greater occurrence of inspiration surrounding swallowing. Older individuals are also those who frequently suffer from the diseases and conditions mentioned above. Although these minor pattern changes may not pose significant functional concern to the healthy, old individual, occurrence of stroke, pulmonary condition, or head and neck cancer may pose an increased threat of swallow-related aspiration.
Swallow Apnea
The second primary aspect of breathing and swallowing coordination that has been studied in the clinical arena relates to the issue of the presence and duration of the apneic interval. All adult human studies that have combined and synchronized videofluorographic and respiratory recordings have shown that an apneic interval consistently occurs with swallowing and is triggered at some point either before or after initial bolus transit through the oral cavity.132, 133, 134, 136, 138, 139 The onset of apnea has been found to be highly variable during cup drinking tasks136, 139 and during solid food mastication131, 137, 145 followed by swallowing. The onset of apnea, however, has been found to be much more predictable and consistent in the onset of its occurrence during syringe swallow tasks.132, 133, 134, 138 Apnea duration has been found to increase with liquid bolus volume in a few small studies,129, 150 but other studies have not supported this notion and instead find no significant differences in apnea with bolus increments between 5- and 25-mL liquid swallows.132, 133, 138, 151 These varied findings, and the known influence of bolus volume on swallowing physiology, indicate that further studies are warranted to examine the potential influence of bolus volume on apnea duration. If increasing volumes of liquid up to 25 mL, a bolus size found to be the average liquid intake in healthy adults,152 does indeed result in longer apnea, this challenge may surpass the respiratory capability of dysphagic patients with pulmonary disease and interfere with the extent and duration of airway closure that is required for the prevention of aspiration.
Though the time of apnea onset varies with the swallowing tasks, it is obligatory at the initiation of the pharyngeal swallow (i.e., as marked by the onset of hyolaryngeal excursion).133, 134, 136, 138, 139 The reported apnea interval duration ranges from 0.5 to 3.5 s. Table 1 shows that the reported average apneic interval is typically between 1.0 to 1.5 s in most healthy adults. Further, it has been shown that apnea offset is not always a postswallow phenomenon (i.e., occurs after complete descent of the larynx). In many individuals, apnea ceases during descent of the larynx at the later stages of swallow and is marked by the brief exhalation that occurs during this time.132, 133, 136, 139 Even though the results from the majority of studies demonstrate a predominant pattern of breathing and swallowing coordination, age- and disease-related aberrations in the duration of the apneic interval have been reported in healthy, old individuals129, 139 and in patient groups when compared to young, healthy individuals or age-matched cohorts.127
The definition of "old" varies in these studies and often mean age is reported. In general, healthy, older adults and those with neurologic, pulmonary, and treated oncologic head and neck cancer have longer apnea durations in the few studies that have addressed these state of life and health issues on apnea duration. In the case of aging and disease, the literature seems to indicate less stability in the neural respiratory-swallow phasing and patterning. The clinical implications of these findings should become clearer when breathing and swallowing patterns in these groups are found to be true aberrations from "normal" and when the relationship of these aberrations to the overall health outcome of the patients becomes known.
Conclusion
Breathing and swallowing processes are highly complex and intricately related in their central control and functional coordination. This review provides only a glimpse at the vast and proliferating body of investigations that seek to determine the nature of this coordination in the brainstem, cortex, and periphery. The majority of the work that has examined these integrated processes at the neural level has been conducted in the animal model. Functional imaging studies are emerging, however, that are beginning to assist basic and clinical scientists in their understanding of the applicability of these models to human eating and drinking behavior. Though much is yet to be understood in this regard, the recent work in animals that implicates the presence of multifunctional neurons common to both respiratory and swallowing CPGs may have relevance for improved understanding of the role of central nervous system (CNS) plasticity. On one hand, if there were injury to the swallowing CPG, the neurons common to both respiration and swallowing may reduce the overall impact of the injury on swallowing function or vice versa. On the other hand, if one CPG contains neurons common to both functions, this may place the individual at a functional disadvantage—and at greater risk—for both processes to be affected in the case of an isolated injury. These notions are all speculative based on the current evidence, yet they create a pathway for the development of questions to be addressed in future studies that investigate the central control of breathing and swallowing.
The seemingly artificial separation of breathing and swallowing as functionally discrete behaviors is not only being questioned as to the CNS, but also with regard to peripheral function. It has been shown that breathing can continue even after the onset of oral bolus transport at the beginning of the swallow and often resumes prior to the completion of the pharyngeal swallow.139 This finding underscores the provocative notion mentioned in the introductory section of this review that questioned whether breathing and swallowing could occur simultaneously. The two basic physiologic processes appear to be highly integrated at a functional level and not mutually exclusive, that is, an all-or-none physiologic phenomenon.
New recording technologies, methods, and analyses have improved in accuracy, reliability, and commercial availability. These factors afford investigators opportunities both to build on the current body of knowledge regarding central and peripheral breathing and swallowing coordination and serve to better position laboratories for implementation of human behavioral and translational studies that can be compared across study sites. Though the phenomenon of breathing and swallowing coordination, or the respective disruption in this coordination, would appear to have substantial influence on an individual's ability to protect the airway during swallowing, this hypothesis has not been adequately tested. Studies are warranted that will determine the relationship of breathing and swallowing coordination on the overall swallowing impairment of various patient populations with dysphagia. In addition to the study of breathing-swallowing phase relationships, temporal characteristics of swallow apnea, and changes in respiratory cycle durations surrounding the swallow, studies are on the horizon that will investigate other potentially relevant aspects of swallowing dynamics and pulmonary function on the health and nutritional outcomes of swallowing-impaired individuals. Evidence-based swallowing treatments, such as compensatory posturing, swallowing maneuvers, and exercises on the coordination of breathing and swallowing are also under study. It is believed that these investigations will help reveal whether deviations from dominant trends in breathing and swallowing coordination will have an influence on the success of these treatments.