Abstract
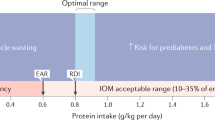
This paper evaluates the safety for humans with regard to consumption of protein hydrolysates and fractions thereof, including bioactive peptides. The available literature on the safety of protein, protein hydrolysates, fractions thereof and free amino acids on relevant food legislation is reviewed and evaluated. A new concept for the safety assessment of protein hydrolysates and fractions thereof is developed. Benchmarks for the evaluation are safety of total protein intake, safety of free amino acid intake, documented history of safe use, outcome of questionnaires in efficacy studies and safety studies. Similar to the intake of intact proteins with a history of safe use, the intake of hydrolysates made from them, does not raise concern about safety, provided the applied proteolytic enzymes are food grade and thus of suitable quality. The safety of hydrolysates and of fractions thereof, including the so-called bioactive peptides, should always be assessed by the company before market introduction (company safety assessment). Only when a novel protein source is used or a novel production process is applied, which results in significant changes in nutritional value, metabolic effect or increased level of undesirable substances, that products might fall under novel food regulations. This means that company safety assessment should be reviewed and approved by external independent experts (external safety evaluation) and the novel protein hydrolysate (fraction) is authorized by competent authorities before market introduction. It is argued that good judgement on the safety of hydrolysates and the fractions thereof can be obtained by comparing the anticipated intake of amino acids by these products with those levels to be reasonably expected to be ingested under normal conditions of consumption of a balanced and varied diet. The paper shows a decision tree that can be used for safety assessment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Affertsholt T (2007). 3A Business Consulting, Aarhus, Denmark, personal communication.
Bilsborough S, Mann N (2006). A review of issues of dietary protein intake in humans. Int J Sport Nutr Exerc Metab 16, 129–152.
Bütikofer U, Meyer J, Sieber R, Wechsler D (2007). Quantification of the angiotensin-converting enzyme-inhibiting tripeptides Val-Pro-Pro and Ile-Pro-Pro in hard, semi-hard and soft cheeses. Int Dairy J 17, 968–975.
Codex Alimantarius Commission (1992). Inventory of processing aids. Codex Alimantarius 1A, 1–39.
Commission of the European Community (1992). Scientific Committee for Food of the European Communities, twenty seventh series, Guidelines for the presentation of data on food enzymes, opinion expressed on 11 April 1991. Luxembourg, office for official publications of the European Communities pp 13–21.
Commission of the European Communities (1997). Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 Januari 1997 concerning novel foods and novel food ingredients. Official Journal of the European Communities No L 43/1.
Commission of the European Communities (2001). Commission directive 2001/15/EC of 14 February 2001 on substances that may be added for specific nutritional purposes in foods for particular nutritional uses. Official Journal of the European Communities No. L 52/19.
Commission of the European Communities (2002). Regulation EC No 178/2002 of the European Parliament and of the Council of 28 January 2002, laying down the general principles and requirements of food law, establishing the European Food Safety Authority laying down procedures in matters of food safety. Official Journal of the European Communities No. L31/1.
Commission of the European Communities (2006a). Proposal for a regulation of the European Parliament and of the Council. Establishing a common authorisation procedure for food additives, food enzymes and food flavourings, Brussels 28-07-2006 COM (2006)423 final, 2006/0143 COD.
Commission of the European Communities (2006b). Regulation (EC) No 1925/2006 of the Eropean Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Official Journal of the European Union No. L 404/27.
Commission of the European Communities (2008). Proposal for a regulation of the European Parliament and of the Council on novel foods and amending Regulation (EC) No xxx/xxx. Brussels 14 01 2008, COM (2007) 872, final.
Committee on Nutrition of the American Academy of Pediatrics (1989). Hypoallergenic infant formulas. Pediatrics 83, 1068–1069.
Decsi T, Veitl V, Szasz M, Pinter Z, Mehes K (1996). Plasma amino acid concentrations in health, full-term infants fed hydrolysate infant formula. J Pediatr Gastroenterol Nutr 22, 62–67.
Di Pasquale MG (1997). Amino acids and proteins for the athlete; The anabolic edge. CRC Press: Boca Raton, Florida.
Dziuba M, Darewicz M (2007). Food proteins as precursors of bioactive peptides—classification into families. Food Sci Technol Inter 13, 393–404.
Eisenstein J, Roberts SB, Dallal G, Salzman E (2002). High-protein weight loss diets: are they safe and do they work/ A review of the experimental and epidemiological data. Nutr Rev 60, 189–200.
Erlandsen H, Patch MG, Gamez A, Straub M, Stevens RC (2003). Structural studies on phenylalanine hydroxylase and implications toward understanding and treating phenylketonuria. Pediatrics 112, 1557–1565.
European Food Information Council (2006). Food allergy and food intolerance. The Basics nr. 06. http://www.eufic.org/article/en/page/BARCHIVE/expid/basics-food-allergy-intolerance.
FDA (2001). Partial list of enzyme preparations that are used in foods. US Food and Drug Administration. Centre of Food Safety and Applied Nutrition, Office of Food Additive Safety.
FDA (2003). Code of Federal Regulations: 21 CFR 184, Direct food substances affirmed as generally recognized as safe, 1551. http://www.cfsan.fda.gov/~lrd/FCF184html.
Federation of American Societies for Experimental Biology (1992) In: Anderson SA, Raiten DJ (eds). Safety of Amino Acids Used as Dietary Supplements. Federation of American Societies for Experimental Biology: Bethesda, USA.
Garlick PJ (2001). Assessment of the safety of glutamine and other amino acids. J Nutr 132, 2556S–2561S.
Health Council in the Netherlands (1999). Committee on amino acid supplementation Safety of amino acid supplementation. Health Council of the Netherlands, publication No 1999/06: The Hague.
Health Council in the Netherlands (2001). Dietary reference intakes; energy proteins, fats and digestible carbohydrates. Health Council of the Netherlands, publication No 2001/19E: the Hague.
Hernell O, Lönnerdal B (2003). Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, haematology, and trace elements. Am J Clin Nutr 78, 296–301.
Host H, Halken S (2004). Hypoallergenic formulas—when, to whom and how long: after more than 15 years we know the indication!. Allergy 59, 45–52.
Institute of Medicine (2002). Institute of Medicine in the US. Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). The National Academies Press: Washington, DC, 2005.
Kajimoto Y, Aihara K, Hirata H, Takahashi R, Nakamura J (2001). Safety evaluation of excessive intake of the tablets containing Lactotripeptides (VPP, IPP) on healthy volunteers. J Nutritional Food 4, 37–46.
Kluifhooft JD (2005). An introduction to the unlimited world of enzymes VMT symposium additives and processing aids in foods. Ede, The Netherlands 13 April 2005.
Korhonen H, Pihlanto A (2006). Bioactive peptides: production and functionality. Int Dairy J 16, 945–960.
Lessof MH (1994). Food allergy and other adverse reactions to food International Life Sciences Institute Europe Concise Monograph Series. ILSI Europe: Brussels, Belgium.
Madden D (1995). Food biotechnology, an introduction International Life Sciences Institute Europe Concise Monograph Series. ILSI Europe: Brussels, Belgium.
Manninen AH (2004). Protein hydrolysates in sports and exercise: a brief review. J Sports Sci Med 3, 60–63.
Meredith C (2005). Allergenic potential of novel foods. Proc Nutr Soc 64, 487–490.
Nolles JA (2006). Postprandial fate of amino acids: adaptation to molecular forms Thesis. Wageningen University and Research Center: Wageningen, The Netherlands.
Ponstein-Simarro Doorten AY, van der Wiel JAG, Jonker DD (2009). Safety evaluation of an IPP tripeptide-containing milk protein hydrolysate. Food Chem Toxicol 47, 55–61.
Rigo J, Senterre J (1994). Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica Suppl 405, 98–104.
Sarwar G, Peace RW (1994). The protein quality of some enteral products is inferior to that of casein as assessed by rat growth methods and digestibility-corrected amino acid scores. J Nutr 124, 2223–2232.
Rodrigues ICC (2008). The European Novel Food Regulation, where do we stand after 10 years. An evaluation comprising genesis, present and future. MSc thesis Law and Governance, Wageningen University and Research Center, Department of law and Governance: Wageningen, The Netherlands. pp 60–72.
Szajewska H, Albrecht P, Stoinska B, Prochowska A, Gawecka A, Laskowska-Klita T (2001). Extensive and partial protein hydrolysate perterm formulas: The effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. J Pediatr Gastroenterol Nutr 32, 303–309.
US Patent (2003). US patent 6620778-Cysteine/glycine rich peptides. http://www.patentstorm.us/patents/6620778/descriptionhtml.
US Patent (2004). US patent 6692933-Method for producing a gluten-free peptide preparation and preparation this obtained. http://www.patentstorm.us/patents/6692933/descriptionhtml.
World Intellectual Property Organization (2004). Use of tryptophane rich peptides. International Application No: PCT/NL 2003/000084.http://www.wipo.int/pctdb/en/wo.jsp?wo=2004069265.
Vanden plas Y, Hauser B, Blecker U, Suys B, Peeters S, Keymolen K, Loeb H (1993). The nutritional value of a whey hydrolysate formula compared with a whey-predominant formula in healthy infants. J Pediatr Gastroenterol Nutr 17, 92–96.
Acknowledgements
The critical comments on the paper by Dr Jeanine van der Wiel (DSM), Dr Armand Janssen (DMV International) and Mrs M van der Lugt (Friesland Foods) are gratefully acknowledged. This review was financially supported by DSM, Delft, The Netherlands, Friesland Foods, Meppel, The Netherlands and DMV International, Veghel, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur J Clin Nutr 63, 1161–1168 (2009). https://doi.org/10.1038/ejcn.2009.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2009.56
Keywords
This article is cited by
-
Advancing the impact of plant biostimulants to sustainable agriculture through nanotechnologies
Chemical and Biological Technologies in Agriculture (2023)
-
Identification and characterization of bioactive peptides from marine crustacean crabs: a possible drug candidate for Alzheimer’s disease
Aquaculture International (2023)
-
Vegetal protein hydrolysates reduce the yield losses in off-season crops under combined heat and drought stress
Physiology and Molecular Biology of Plants (2023)
-
Sustainable production through biostimulants under fruit orchards
CABI Agriculture and Bioscience (2022)
-
Influence of chicken feather waste derived protein hydrolysate on the growth of tea plants under different application methods and fertilizer rates
Environmental Science and Pollution Research (2022)