Key Points
-
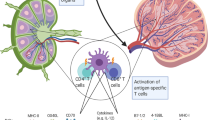
Dendritic cells (DCs) are key regulators of innate and adaptive immune responses. They are used in clinical trials to induce immune responses directed against specific antigens.
-
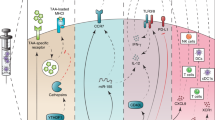
Clinical trials exploring DC-based vaccines mostly involve autologous DCs that are cultured and loaded with antigens ex vivo, which is a costly procedure. Targeting antigens to DC surface receptors in vivo bypasses the necessity for ex vivo DC culturing, allowing large-scale application of DC-based vaccination therapy.
-
Preclinical mouse studies for targeted delivery of antigens to DCs in vivo show successful induction of humoral and cellular responses. Targeting tumour antigens to DC surface receptors has been shown to protect mice from growing tumours and even cure them of existing tumours.
-
Strategies aimed at inducing immunity require means to mature the targeted DCs Without a proper maturation stimulus, targeted delivery of antigen might result in the induction of tolerance.
-
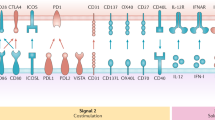
The specific receptor that is targeted may affect the quality of the immune response owing to differences in intracellular receptor routing, signalling pathways and expression patterns. For instance, antigens delivered to receptors preferentially expressed by the mouse CD8+ DC subset predominantly induce cellular responses, whereas antigens delivered to CD8− DC subset mainly induce humoral responses.
-
Advancing knowledge on the intracellular routing of antigen allows for the design of more potent vaccines. The use of powerful DC maturation stimuli, agents that facilitate antigen to escape from endosomes or means to prevent rapid antigen degradation have been shown to enhance humoral responses, cellular responses, or both.
Abstract
The realization that dendritic cells (DCs) orchestrate innate and adaptive immune responses has stimulated research on harnessing DCs to create more effective vaccines. Early clinical trials exploring autologous DCs that were loaded with antigens ex vivo to induce T-cell responses have provided proof of principle. Here, we discuss how direct targeting of antigens to DC surface receptors in vivo might replace laborious and expensive ex vivo culturing, and facilitate large-scale application of DC-based vaccination therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Celis, E. & Chang, T. W. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science 224, 297–299 (1984).
Chang, T. W. Regulation of immune response by antibodies: the importance of antibody and monocyte Fc receptor interaction in T-cell activation. Immunol. Today 6, 245–249 (1985).
Snider, D. P. & Segal, D. M. Targeted antigen presentation using crosslinked antibody heteroaggregates. J. Immunol. 139, 1609–1616 (1987).
Carayanniotis, G. & Barber, B. H. Adjuvant-free IgG responses induced with antigen coupled to antibodies against class II MHC. Nature 327, 59–61 (1987).
Snider, D. P., Kaubisch, A. & Segal, D. M. Enhanced antigen immunogenicity induced by bispecific antibodies. J. Exp. Med. 171, 1957–1963 (1990).
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2, 77–84 (2002).
Keler, T., Ramakrishna, V. & Fanger, M. W. Mannose receptor-targeted vaccines. Expert Opin. Biol. Ther. 4, 1953–1962 (2004).
Karanikas, V. et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J. Clin. Invest. 100, 2783–2792 (1997).
Apostolopoulos, V. et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1. Breast Cancer Res. 8, R27 (2006).
He, L. Z. et al. A novel human cancer vaccine elicits cellular responses to the tumor-associated antigen, human chorionic gonadotropin β. Clin. Cancer Res. 10, 1920–1927 (2004).
Ramakrishna, V. et al. Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules. J. Immunol. 172, 2845–2852 (2004).
He, L. Z. et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J. Immunol. 178, 6259–6267 (2007).
Tarlinton, D. & Lew, A. Antigen to the node: B cells go native. Immunity 26, 388–390 (2007).
Mahnke, K. et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151, 673–684 (2000).
Inaba, K. et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 163, 148–156 (1995).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001). References 17 and 18 show that targeting antigen to DCs without providing a maturation stimulus induces tolerance.
Mahnke, K., Qian, Y. J., Knop, J. & Enk, A. H. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869 (2003).
Bonifaz, L. C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
van Broekhoven, C. L., Parish, C. R., Demangel, C., Britton, W. J. & Altin, J. G. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 64, 4357–4365 (2004).
Boscardin, S. B. et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 203, 599–606 (2006).
Mahnke, K. et al. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 65, 7007–7012 (2005).
Bozzacco, L. et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl Acad. Sci. USA 104, 1289–1294 (2007).
Kato, M. et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int. Immunol. 18, 857–869 (2006).
Geijtenbeek, T. B. H. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000).
Soilleux, E. J. et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71, 445–457 (2002).
Gramberg, T., Caminschi, I., Wegele, A., Hofmann, H. & Pohlmann, S. Evidence that multiple defects in murine DC-SIGN inhibit a functional interaction with pathogens. Virology 345, 482–491 (2005).
Caminschi, I. et al. Functional comparison of mouse CIRE/mouse DC-SIGN and human DC-SIGN. Int. Immunol. 18, 741–753 (2006).
Tacken, P. J. et al. Effective induction of naive and recall T cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 106, 1278–1285 (2005).
Granelli-Piperno, A. et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175, 4265–4273 (2005).
Shortman, K. & Liu, Y. J. Mouse and human dendritic cell subtypes. Nature Rev. Immunol. 2, 151–161 (2002).
den Haan, J. M. M., Lehar, S. M. & Bevan, M. J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Caminschi, I. et al. Molecular cloning of F4/80-like-receptor, a seven-span membrane protein expressed differentially by dendritic cell and monocyte-macrophage subpopulations. J. Immunol. 167, 3570–3576 (2001).
Caminschi, I. et al. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 38, 365–373 (2001).
Vremec, D. & Shortman, K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 159, 565–573 (1997).
McLellan, A. D. et al. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood 99, 2084–2093 (2002).
Corbett, A. J. et al. Antigen delivery via two molecules on the CD8− dendritic cell subset induces humoral immunity in the absence of conventional 'danger'. Eur. J Immunol. 35, 2815–2825 (2005).
Carter, R. W., Thompson, C., Reid, D. M., Wong, S. Y. C. & Tough, D. F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 177, 2276–2284 (2006). Together with references 34 and 39 , this study demonstrates that the targeting of receptors preferentially expressed on two different DC subsets can result in disparate immune responses.
Dakappagari, N. et al. Internalizing antibodies to the C-type lectins, L-SIGN and DC-SIGN, inhibit viral glycoprotein binding and deliver antigen to human dendritic cells for the induction of T cell responses. J. Immunol. 176, 426–440 (2006).
Pasquier, B. et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity 22, 31–42 (2005).
Herre, J. et al. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104, 4038–4045 (2004).
Woof, J. M. & Burton, D. R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nature Rev. Immunol. 4, 89–99 (2004).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors: old friends and new family members. Immunity 24, 19–28 (2006).
Kanazawa, N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J. Dermatol. Sci. 45, 77–86 (2007).
Underhill, D. M., Rossnagle, E., Lowell, C. A. & Simmons, R. M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 (2005).
Rogers, N. C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Gantner, B. N., Simmons, R. M., Canavera, S. J., Akira, S. & Underhill, D. M. Collaborative induction of inflammatory responses by Dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Geijtenbeek, T. B. H. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
de Vries, I. J. et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin. Cancer Res. 9, 5091–5100 (2003).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Fujii, S. i., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R. M. Activation of natural killer T cells by α-Galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
van Duin, D., Medzhitov, R. & Shaw, A. C. Triggering TLR signaling in vaccination. Trends Immunol. 27, 49–55 (2006).
Datta, S. K. et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 170, 4102–4110 (2003).
Wilson, N. S. et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nature Immunol. 7, 165–172 (2006). This paper shows that systemic exposure to TLR ligands prior to vaccination downmodulates cross-presentation.
Butler, M. et al. Altered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205–DCL-1 fusion protein upon dendritic cell maturation. Immunology 120, 362–371 (2006).
Jackson, D. C. et al. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl Acad. Sci. USA 101, 15440–15445 (2004).
Zhang, L. et al. An adenoviral vector cancer vaccine that delivers a tumor-associated antigen/CD40-ligand fusion protein to dendritic cells. Proc. Natl Acad. Sci. USA 100, 15101–15106 (2003).
Delneste, Y. et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17, 353–362 (2002).
Wille-Reece, U., Wu, C. y., Flynn, B. J., Kedl, R. M. & Seder, R. A. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J. Immunol. 174, 7676–7683 (2005).
Cho, H. J. et al. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nature Biotech. 18, 509–514 (2000).
Yarovinsky, F., Kanzler, H., Hieny, S., Coffman, R. L. & Sher, A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity 25, 655–664 (2006).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006). An elegant study demonstrating that TLR ligands need to be physically linked to the antigen to be efficiently presented by MHC class II.
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunol. 6, 769–776 (2005).
Ramakrishna, V. et al. Toll-like receptor activation enhances cell-mediated immunity induced by an antibody vaccine targeting human dendritic cells. J. Transl. Med. 5, 5 (2007).
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Carbone, F. R. & Bevan, M. J. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J. Exp. Med. 171, 377–387 (1990).
Reis e Sousa, C. & Germain, R. N. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J. Exp. Med. 182, 841–851 (1995).
Brossart, P. & Bevan, M. J. Presentation of exogenous protein antigens on major histocompatability complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 90, 1594–1599 (1997).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Ackerman, A. L., Kyritsis, C., Tampe, R. & Cresswell, P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nature Immunol. 6, 107–113 (2005).
Yewdell, J. W., Bennink, J. R. & Hosaka, Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science 239, 637–640 (1988).
Moore, M. W., Carbone, F. R. & Bevan, M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54, 777–785 (1988).
Melikov, K. & Chernomordik, L. V. Arginine-rich cell penetrating peptides: from endosomal uptake to nuclear delivery. Cell. Mol. Life Sci. 62, 2739–2749 (2005).
Batchu, R. B. et al. Protein transduction of dendritic cells for NY-ESO-1-based immunotherapy of myeloma. Cancer Res. 65, 10041–10049 (2005).
Kim, D. T. et al. Introduction of soluble proteins into the MHC class I pathway by conjugation to an HIV tat peptide. J. Immunol. 159, 1666–1668 (1997).
Shibagaki, N. & Udey, M. C. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J. Immunol. 168, 2393–2401 (2002).
Kielian, M. & Rey, F. A. Virus membrane-fusion proteins: more than one way to make a hairpin. Nature Rev. Microbiol. 4, 67–76 (2006).
Mastrobattista, E. et al. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J. Biol. Chem. 277, 27135–27143 (2002).
Plank, C., Oberhauser, B., Mechtler, K., Koch, C. & Wagner, E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J. Biol. Chem. 269, 12918–12924 (1994).
Wadia, J. S., Stan, R. V. & Dowdy, S. F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Med. 10, 310–315 (2004).
Laus, R., Graddis, T. J., Hakim, I. & Vidovic, D. Enhanced major histocompatibility complex class I-dependent presentation of antigens modified with cationic and fusogenic peptides. Nature Biotechnol. 18, 1269–1272 (2000).
Schwarze, S. R., Ho, A., Vocero-Akbani, A. & Dowdy, S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Lisziewicz, J. et al. DermaVir: a novel topical vaccine for HIV/AIDS. J. Invest. Dermatol. 124, 160–169 (2005).
Lisziewicz, J. et al. Control of viral rebound through therapeutic immunization with DermaVir. AIDS 19, 35–43 (2005).
O'Connor, T. P. & Crystal, R. G. Genetic medicines: treatment strategies for hereditary disorders. Nature Rev. Genet. 7, 261–276 (2006).
Walsh, G. Biopharmaceutical benchmarks 2006. Nature Biotechnol. 24, 769–776 (2006).
Tillman, B. W. et al. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J. Immunol. 162, 6378–6383 (1999).
Korokhov, N. et al. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biol. Ther. 4, 289–294 (2005).
Hedley, S. J. et al. An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther. 13, 88–94 (2005).
Lennon-Dumenil, A. M. et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196, 529–540 (2002).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Delamarre, L., Couture, R., Mellman, I. & Trombetta, E. S. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J. Exp. Med. 203, 2049–2055 (2006). This report indicates that the immunogenicity of antigens can be enhanced by preventing rapid degradation.
Waeckerle-Men, Y. & Groettrup, M. PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv. Drug Deliv. Rev. 57, 475–482 (2005).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Accapezzato, D. et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202, 817–828 (2005). This study shows that the cross-presentation of antigens is enhanced by a relatively simple trick: preventing endosomal acidification by co-administering the antimalarial drug chloroquine.
Kloetzel, P. M. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nature Immunol. 5, 661–669 (2004).
Toes, R. E. M. et al. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 194, 1–12 (2001).
Gaczynska, M., Rock, K. L. & Goldberg, A. L. γ-Interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 365, 264–267 (1993).
Van Kaer, L. et al. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1, 533–541 (1994).
Basler, M., Moebius, J., Elenich, L., Groettrup, M. & Monaco, J. J. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 176, 6665–6672 (2006).
Chapatte, L. et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 66, 5461–5468 (2006). An elegant example of how T cells recognizing peptide epitopes that are not generated by the immunoproteasome can be primed by DCs expressing the peptide, but not the protein, antigen.
Curiel, T. J. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 117, 1167–1174 (2007).
Ratzinger, G. et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J. Immunol. 173, 2780–2791 (2004).
Benitez-Ribas, D. et al. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after FcγRII-mediated uptake. J. Exp. Med. 203, 1629–1635 (2006).
Zhang, J. et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 107, 3600–3608 (2006).
Hsu, F. J. et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med. 2, 52–58 (1996).
Adema, G. J., de Vries, I. J., Punt, C. J. & Figdor, C. G. Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr. Opin. Immunol. 17, 170–174 (2005).
Mignot, G., Roux, S., Thery, C., Segura, E. & Zitvogel, L. Prospects for exosomes in immunotherapy of cancer. J. Cell. Mol. Med. 10, 376–388 (2006).
Engering, A. et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168, 2118–2126 (2002).
Heijnen, I. A. et al. Antigen targeting to myeloid-specific human FcγRI/CD64 triggers enhanced antibody responses in transgenic mice. J. Clin. Invest. 97, 331–338 (1996).
Liu, Y. et al. Regulated expression of FcγR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J. Immunol. 177, 8440–8447 (2006).
Mende, I. et al. Highly efficient antigen targeting to M-DC8+ dendritic cells via FcγRIII/CD16-specific antibody conjugates. Int. Immunol. 17, 539–547 (2005).
Otten, M. A., Groenveld, I., van De Winkel, J. G. & van Egmond, M. Inefficient antigen presentation via the IgA Fc receptor (FcαRI) on dendritic cells. Immunobiol. 211, 503–510 (2006).
Fayolle, C., Sebo, P., Ladant, D., Ullmann, A. & Leclerc, C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J. Immunol. 156, 4697–4706 (1996).
Fayolle, C. et al. Delivery of multiple epitopes by recombinant detoxified adenylate cyclase of Bordetella pertussis induces protective antiviral immunity. J. Virol. 75, 7330–7338 (2001).
Saron, M. F. et al. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl Acad. Sci. USA 94, 3314–3319 (1997).
Sebo, P. et al. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying multiple copies of a viral CD8+ T-cell epitope. FEMS Immunol. Med. Microbiol. 26, 167–173 (1999).
Romani, N. et al. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180, 83–93 (1994).
Nestle, F. O. et al. Vaccination of melanoma patients with peptide- or tumorlysate-pulsed dendritic cells. Nature Med. 4, 328–332 (1998).
Holtl, L. et al. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J. Urol. 161, 777–782 (1999).
Holtl, L. et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin. Cancer Res. 8, 3369–3376 (2002).
Thurner, B. et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 190, 1669–1678 (1999).
Dhodapkar, M. V. et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Invest. 104, 173–180 (1999).
Mackensen, A. et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Int. J Cancer 86, 385–392 (2000).
Banchereau, J. et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001).
Fong, L. et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl Acad. Sci. USA 98, 8809–8814 (2001).
Fong, L., Brockstedt, D., Benike, C., Wu, L. & Engleman, E. G. Dendritic cells injected via different routes induce immunity in cancer patients. J. Immunol. 166, 4254–4259 (2001).
Mullins, D. W. et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198, 1023–1034 (2003).
Dhodapkar, M. V. & Steinman, R. M. Antigen-bearing immature dendritic cells induce peptide-specific CD8+ regulatory T cells in vivo in humans. Blood 100, 174–177 (2002).
Jonuleit, H. et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int. J. Cancer 93, 243–251 (2001).
Lau, R. et al. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J. Immunother. 24, 66–78 (2001).
Fong, L. et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J. Immunol. 167, 7150–7156 (2001).
Kikuchi, T. et al. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol. Immunother. 50, 337–344 (2001).
Schuler-Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195, 1279–1288 (2002).
Su, Z. et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 63, 2127–2133 (2003).
Nair, S. K. et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann. Surg. 235, 540–549 (2002).
Nair, S. K. et al. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int. J. Cancer 82, 121–124 (1999).
Heiser, A. et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest. 109, 409–417 (2002).
Pecher, G., Haring, A., Kaiser, L. & Thiel, E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol. Immunother. 51, 669–673 (2002).
Banchereau, J. et al. Immune and clinical outcomes in patients with stage IV melanoma vaccinated with peptide-pulsed dendritic cells derived from CD34+ progenitors and activated with type I interferon. J. Immunother. 28, 505–516 (2005).
Trakatelli, M. et al. A new dendritic cell vaccine generated with interleukin-3 and interferon-β induces CD8+ T cell responses against NA17-A2 tumor peptide in melanoma patients. Cancer Immunol. Immunother. 55, 469–474 (2006).
Escudier, B. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med. 3, 10 (2005).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
Holtl, L. et al. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol. Immunother. 54, 663–670 (2005).
Spisek, R. et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 109, 4839–4845 (2007).
Hildenbrand, B. et al. Immunotherapy of patients with hormone-refractory prostate carcinoma pre-treated with interferon-γ and vaccinated with autologous PSA-peptide loaded dendritic cells — a pilot study. Prostate 67, 500–508 (2007).
Marten, A. et al. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum. Gene Ther. 14, 483–494 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Adjuvant
-
An agent that does not have any specific antigenic effect in itself, but stimulates the immune system to increase the response to antigens.
- Cross-presentation
-
The mechanism by which certain antigen-presenting cells take up, process and present extracellular antigens on MHC class I molecules to stimulate CD8+ T cells.
- Immunoproteasome
-
The standard proteasome is composed of 14 α and β subunits, of which three, β1, β2 and β5, are involved in peptide-bond cleavage. Interferon-γ induces the expression of the immunosubunits β1i, β2i and β5i that can replace the catalytic subunits of the standard proteasome to generate the immunoproteasome, which has distinct cleavage-site preferences.
- C-type lectin receptor
-
A receptor belonging to the family of Ca+-dependent lectins that share primary structural homology in their carbohydrate-recognition domains.
- Regulatory T cell
-
(TReg cell). A specialized subpopulation of CD4+ T cells that suppresses immune responses to maintain tolerance to (self) antigens.
- B16 melanoma model
-
A well-characterized model to study tumour growth in C57BL/6 mice. There are many sublines of the B16 mouse melanoma cell line, each with its own characteristics.
- Hypervariable domains
-
Three regions within the immunoglobulin variable region that are highly divergent. Together they form a surface that is complementary to the antigen.
- Framework regions
-
Regions adjoining the hypervariable domains, located at the N terminus of the immunoglobulin.
- Humanized antibody
-
Genetically engineered antibody in which the hypervariable domains of a non-human antibody have been transplanted onto a human antibody.
- ITAM
-
(Immunoreceptor tyrosine-based activation motif). A structural motif containing a tyrosine residue that is found in the cytoplasmic tails of several signalling molecules. The consensus sequence consists of Tyr-Xaa-Xaa-Leu/Ile, and the tyrosine is a target for phosphorylation by Src tyrosine kinases and subsequent binding of proteins containing SRC homologue 2 domains.
- ITIM
-
(Immunoreceptor tyrosine-based inhibitory motif). A structural motif found in the cytoplasmic domains of many receptors that negatively regulates intracellular signalling complexes. The consensus sequence consists of Ile/Val-Xaa-Tyr-Xaa-Xaa-Leu/Val.
- Toll-like receptors
-
(TLRs). A family of membrane-spanning proteins that recognize structurally conserved molecules that are shared by various microorganisms. Signalling through TLRs generally results in immune activation.
- Single chain antibody
-
An antibody consisting of only one heavy and one light chain.
- Poly (D,L-lactide-co-glycolide) microspheres
-
Biodegradable microparticles suitable for drug or antigen delivery, consisting of a polymeric ester of lactic and glycolic acid that is approved for application in humans.
- Lysosomotropic
-
Having affinity for, and thus accumulating in, lysosomes. Lysosomotropic weak bases that are capable of crossing biological membranes selectively accumulate in acidic compartments by protonation, thereby affecting organelle pH and function.
Rights and permissions
About this article
Cite this article
Tacken, P., de Vries, I., Torensma, R. et al. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol 7, 790–802 (2007). https://doi.org/10.1038/nri2173
Issue Date:
DOI: https://doi.org/10.1038/nri2173
This article is cited by
-
Differentiation, maturation, and collection of THP-1-derived dendritic cells based on a PEG hydrogel culture platform
Biotechnology Letters (2024)
-
Current status of vaccine immunotherapy for gastrointestinal cancers
Surgery Today (2023)
-
ADP-ribosylating adjuvant reveals plasticity in cDC1 cells that drive mucosal Th17 cell development and protection against influenza virus infection
Mucosal Immunology (2022)
-
Upconversion nanoparticle platform for efficient dendritic cell antigen delivery and simultaneous tracking
Microchimica Acta (2022)
-
Sulfavant A as the first synthetic TREM2 ligand discloses a homeostatic response of dendritic cells after receptor engagement
Cellular and Molecular Life Sciences (2022)