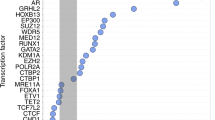
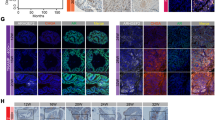
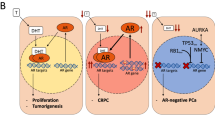
Abstract
Prostate cancer treatment is dominated by strategies to control androgen receptor (AR) activity. AR has an impact on prostate cancer development through the regulation of not only transcription networks but also genomic stability and DNA repair, as manifest in the emergence of gene fusions. Whole-genome maps of AR binding sites and transcript profiling have shown changes in the recruitment and regulatory effect of AR on transcription as prostate cancer progresses. Defining other factors that are involved in this reprogramming of AR function gives various opportunities for cancer detection and therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
20 March 2014
In the original version of this article, several references were incorrect. References 13, 29, 34, 41, 88, 89, 90 and 91 were incorrect and have now been replaced with the correct citations. In addition, reference 59 was incorrectly cited in the first sentence of the "Speckle-type POZ protein (SPOP)" subsection at the top of page 195, and this has now been removed. All of these corrections have been made online.
References
Huggins, C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann. Surg. 115, 1192–1200 (1942).
Mulder, E., Vrij, A. A. & Brinkmann, A. O. DNA and ribonucleotide binding characteristics of two forms of the androgen receptor from rat prostates. Biochem. Biophys. Res. Commun. 114, 1147–1153 (1983).
McEwan, I. J. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr.-Related Cancer 11, 281–293 (2004).
Cutress, M. L., Whitaker, H. C., Mills, I. G., Stewart, M. & Neal, D. E. Structural basis for the nuclear import of the human androgen receptor. J. Cell Sci. 121, 957–968 (2008).
Yuan, X., Cai, C., Chen, S., Yu, Z. & Balk, S. P. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene http://dx.doi.org/10.1038/onc.2013.235 (2013).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl. J. Med. 368, 138–148 (2013).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl. J. Med. 367, 1187–1197 (2012).
Visakorpi, T. et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nature Genet. 9, 401–406 (1995).
Li, Y. et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 73, 483–489 (2013).
Balbas, M. D. et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2, e00499 (2013).
Urbanucci, A. et al. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene 31, 2153–2163 (2012).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 (2009).
Kim, J., Yu, J. Interrogating genomic and epigenomic data to understand prostate cancer. Biochim. Biophys. Acta 1825, 186–196 (2012).
Massie, C. E. & Mills, I. G. Mapping protein-DNA interactions using ChIP-sequencing. Methods Mol. Biol. 809, 157–173 (2012).
Lin, B. et al. Integrated expression profiling and ChIP-seq analyses of the growth inhibition response program of the androgen receptor. PLoS ONE 4, e6589 (2009).
Yu, J. et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 (2010).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Massie, C. E. et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 (2011).
Costello, L. C. & Franklin, R. B. Testosterone and prolactin regulation of metabolic genes and citrate metabolism of prostate epithelial cells. Hormone Metabol. Res. 34, 417–424 (2002).
Haffner, M. C. et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nature Genet. 42, 668–675 (2010).
Ju, B. G. et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006).
Haffner, M. C., De Marzo, A. M., Meeker, A. K., Nelson, W. G. & Yegnasubramanian, S. Transcription-induced DNA double strand breaks: both oncogenic force and potential therapeutic target? Clin. Cancer Res. 17, 3858–3864 (2011).
Langelier, M. F. & Pascal, J. M. PARP-1 mechanism for coupling DNA damage detection to poly(ADP-ribose) synthesis. Curr. Opin. Struct. Biol. 23, 134–143 (2013).
Schiewer, M. J. et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2, 1134–1149 (2012).
Brenner, J. C. et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 19, 664–678 (2011).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Mani, R. S. et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science 326, 1230 (2009).
Weischenfeldt, J. et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell 23, 159–170 (2013).
Hessels, D. & Schalken, J. A. Recurrent gene fusions in prostate cancer: their clinical implications and uses. Curr. Urol. Rep. 14, 214–222 (2013).
Spans, L. et al. The genomic landscape of prostate cancer. Int. J. Mol. Sci. 14, 10822–10851 (2013).
Baena, E. et al. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev. 27, 683–698 (2013).
Carver, B. S. et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nature Genet. 41, 619–624 (2009).
Massie, C. E. et al. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 8, 871–878 (2007).
Tomlins, S. A. et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10, 177–188 (2008).
Paulo, P. et al. Molecular subtyping of primary prostate cancer reveals specific and shared target genes of different ETS rearrangements. Neoplasia 14, 600–611 (2012).
Yuan, W. et al. H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J. Biol. Chem. 286, 7983–7989 (2011).
Cha, T. L. et al. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 310, 306–310 (2005).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell. Metabolism 7, 11–20 (2008).
Eichholz, A., Ferraldeschi, R., Attard, G. & de Bono, J. S. Putting the brakes on continued androgen receptor signaling in castration-resistant prostate cancer. Mol. Cell. Endocrinol. 360, 68–75 (2012).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Kaestner, K. H. The FoxA factors in organogenesis and differentiation. Curr. Opin. Genet. Dev. 20, 527–532 (2010).
Shah, S., Prasad, S. & Knudsen, K. E. Targeting pioneering factor and hormone receptor cooperative pathways to suppress tumor progression. Cancer Res. 72, 1248–1259 (2012).
Carroll, J. S. et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 (2005).
Hurtado, A., Holmes, K. A., Ross-Innes, C. S., Schmidt, D. & Carroll, J. S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nature Genet. 43, 27–33 (2011).
Sahu, B. et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011).
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Wang, Q. et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27, 380–392 (2007).
Bohm, M., Locke, W. J., Sutherland, R. L., Kench, J. G. & Henshall, S. M. A role for GATA-2 in transition to an aggressive phenotype in prostate cancer through modulation of key androgen-regulated genes. Oncogene 28, 3847–3856 (2009).
Andreu-Vieyra, C. et al. Dynamic nucleosome-depleted regions at androgen receptor enhancers in the absence of ligand in prostate cancer cells. Mol. Cell. Biol. 31, 4648–4662 (2011).
Clinckemalie, L. et al. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol. Endocrinol. http://dx.doi.org/10.1210/me.2013-1098 (2013).
Bambury, R. M. & Gallagher, D. J. Prostate cancer: germline prediction for a commonly variable malignancy. BJU Int. 110, E809–E818 (2012).
Karlsson, R. et al. A population-based assessment of germline HOXB13 G84E mutation and prostate cancer risk. Eur. Urol. 65, 169–176 (2014).
Laitinen, V. H. et al. HOXB13 G84E mutation in Finland: population-based analysis of prostate, breast, and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 22, 452–460 (2013).
Norris, J. D. et al. The homeodomain protein HOXB13 regulates the cellular response to androgens. Mol. Cell 36, 405–416 (2009).
De Vita, F. et al. Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J. Interferon Cytokine Res. 21, 45–52 (2001).
Uehara, H. et al. Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate 64, 40–49 (2005).
Araki, S. et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 67, 6854–6862 (2007).
Suh, J. & Rabson, A. B. NF-κB activation in human prostate cancer: important mediator or epiphenomenon? J. Cell. Biochem. 91, 100–117 (2004).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Nadiminty, N. et al. Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res. 70, 3309–3319 (2010).
McCall, P. et al. NFκB signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br. J. Cancer 107, 1554–1563 (2012).
Wilson, C., Wilson, T., Johnston, P. G., Longley, D. B. & Waugh, D. J. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol. Cancer Ther. 7, 2649–2661 (2008).
Maxwell, P. J. et al. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur. Urol. 64, 177–188 (2013).
Fang, L. Y. et al. Infiltrating macrophages promote prostate tumorigenesis via modulating androgen receptor-mediated CCL4-STAT3 signaling. Cancer Res. 73, 5633–5646 (2013).
Tan, S. H. et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 68, 236–248 (2008).
Horinaga, M. et al. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology 66, 671–675 (2005).
Thomas, C. et al. Transcription factor Stat5 knockdown enhances androgen receptor degradation and delays castration-resistant prostate cancer progression in vivo. Mol. Cancer Ther. 10, 347–359 (2011).
Liao, Z. & Nevalainen, M. T. Targeting transcription factor Stat5a/b as a therapeutic strategy for prostate cancer. Am. J. Translat. Res. 3, 133–138 (2011).
Ueda, T., Bruchovsky, N. & Sadar, M. D. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J. Biol. Chem. 277, 7076–7085 (2002).
Ribeiro, F. R., Henrique, R., Martins, A. T., Jeronimo, C. & Teixeira, M. R. Relative copy number gain of MYC in diagnostic needle biopsies is an independent prognostic factor for prostate cancer patients. Eur. Urol. 52, 116–125 (2007).
Antonarakis, E. S. et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 118, 6063–6071 (2012).
Lin, C. Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151, 56–67 (2012).
Zou, J. X. et al. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 69, 3339–3346 (2009).
Ciro, M. et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 69, 8491–8498 (2009).
Jang, M. K. et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 (2005).
Chase, A. & Cross, N. C. Aberrations of EZH2 in cancer. Clin. Cancer Res. 17, 2613–2618 (2011).
Varambally, S. et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 (2008).
Xu, K. et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 338, 1465–1469 (2012).
Crea, F. et al. EZH2 inhibition: targeting the crossroad of tumor invasion and angiogenesis. Cancer Metastasis Rev. 31, 753–761 (2012).
Kuo, A. J. et al. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol. Cell 44, 609–620 (2011).
Asangani, I. A. et al. Characterization of the EZH2-MMSET histone methyltransferase regulatory axis in cancer. Mol. Cell 49, 80–93 (2013).
Qi, J. et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 23, 332–346 (2013).
Qi, J. et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell 18, 23–38 (2010).
Ahmad, I., Sansom, O. J. & Leung, H. Y. The role of murine models of prostate cancer in drug target discovery and validation. Expert Opin. Drug Discov. 4, 879–888 (2009).
Qi, J., Pellecchia, M. & Ronai, Z. A. The Siah2-HIF-FoxA2 axis in prostate cancer — new markers and therapeutic opportunities. Oncotarget 1, 379–385 (2010).
Li, C. et al. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene 30, 4350–4364 (2011).
Zhou, H. J. et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 65, 7976–7983 (2005).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature Genet. 44, 685–689 (2012).
Geng, C. et al. Prostate cancer-associated mutations in speckle-type POZ protein (SPOP) regulate steroid receptor coactivator 3 protein turnover. Proc. Natl Acad. Sci. USA 110, 6997–7002 (2013).
Denmeade, S. R. & Isaacs, J. T. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 70, 1600–1607 (2010).
McDermott, J. E., Costa, M., Janszen, D., Singhal, M. & Tilton, S. C. Separating the drivers from the driven: Integrative network and pathway approaches aid identification of disease biomarkers from high-throughput data. Dis. Markers 28, 253–266 (2010).
Schmid, E. M. & McMahon, H. T. Integrating molecular and network biology to decode endocytosis. Nature 448, 883–888 (2007).
Rhee, H. S. & Pugh, B. F. ChIP-exo method for identifying genomic location of DNA-binding proteins with near-single-nucleotide accuracy. In Current Protocols in Molecular Biology Ch. 21, Unit 21.24 (eds Frederick M. A. et al.) (Wiley, 2012).
Shankaranarayanan, P. et al. Single-tube linear DNA amplification (LinDA) for robust ChIP-seq. Nature Methods 8, 565–567 (2011).
Zwart, W. et al. A carrier-assisted ChIP-seq method for estrogen receptor-chromatin interactions from breast cancer core needle biopsy samples. BMC Genom. 14, 232 (2013).
Mohammed, H. et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 3, 342–349 (2013).
Rubin, M. A. & Chinnaiyan, A. M. Bioinformatics approach leads to the discovery of the TMPRSS2:ETS gene fusion in prostate cancer. Lab. Invest. 86, 1099–1102 (2006).
Liu, X., Wang, J. & Chen, L. Whole-exome sequencing reveals recurrent somatic mutation networks in cancer. Cancer Lett. 340, 270–276 (2012).
Chugh, P. & Dittmer, D. P. Potential pitfalls in microRNA profiling. Wiley Interdiscip. Rev. RNA 3, 601–616 (2012).
Zhou, X. et al. Progress in concurrent analysis of loss of heterozygosity and comparative genomic hybridization utilizing high density single nucleotide polymorphism arrays. Cancer Genet. Cytogenet. 159, 53–57 (2005).
Costa, J. L., Meijer, G., Ylstra, B. & Caldas, C. Array comparative genomic hybridization copy number profiling: a new tool for translational research in solid malignancies. Semin. Radiat. Oncol. 18, 98–104 (2008).
Ni, M. et al. Amplitude modulation of androgen signaling by c-MYC. Genes Dev. 27, 734–748 (2013).
Gao, L. et al. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLoS One 8, e63563 (2013).
Cao, Q. et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell 20, 187–199 (2011).
Ezponda, T. et al. The histone methyltransferase MMSET/WHSC1 activates TWIST1 to promote an epithelial-mesenchymal transition and invasive properties of prostate cancer. Oncogene 32, 2882–2890 (2013).
Yang, P. et al. Histone methyltransferase NSD2/MMSET mediates constitutive NF-κB signaling for cancer cell proliferation, survival, and tumor growth via a feed-forward loop. Mol. Cell. Biol. 32, 3121–3131 (2012).
Jin, H. J., Zhao, J. C., Ogden, I., Bergan, R. C. & Yu, J. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res. 73, 3725–3736 (2013).
Naderi, A., Meyer, M. & Dowhan, D. H. Cross-regulation between FOXA1 and ErbB2 signaling in estrogen receptor-negative breast cancer. Neoplasia 14, 283–296 (2012).
Naderi, A. & Hughes-Davies, L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia 10, 542–548 (2008).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature Genet. 44, 685–689 (2012).
Kim, Y. R. et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol. Cancer 9, 124 (2010).
Zhang, L. et al. NF-κB regulates androgen receptor expression and prostate cancer growth. Am. J. Pathol. 175, 489–499 (2009).
Lessard, L., Begin, L. R., Gleave, M. E., Mes-Masson, A. M. & Saad, F. Nuclear localisation of nuclear factor-κB transcription factors in prostate cancer: an immunohistochemical study. Br. J. Cancer 93, 1019–1023 (2005).
Acknowledgements
The author would like to thank members of the Mills laboratory for their critical reading of the manuscript. The manuscript has evolved from discussions with many close colleagues within the field, particularly D. Neal, N. Sharma, C. Massie, H. Itkonen, S. Barfeld, A. Urbanucci, P. Rennie and G. Coetzee, and many more. Research within the Mills laboratory is funded by the Movember Foundation, the US National Institutes of Health, Helse Sør-Øst, Norway, the Norwegian Research Council, the Norwegian Cancer Society, the Finnish Cultural Society and the European Union. The author would like to apologize to all colleagues whose work he was unable to cite in this Opinion article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
DATABASES
Rights and permissions
About this article
Cite this article
Mills, I. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nat Rev Cancer 14, 187–198 (2014). https://doi.org/10.1038/nrc3678
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3678
This article is cited by
-
USP54 is a potential therapeutic target in castration-resistant prostate cancer
BMC Urology (2024)
-
Suppression of bone metastatic castration-resistant prostate cancer cell growth by a suicide gene delivered by JC polyomavirus-like particles
Gene Therapy (2023)
-
Castration-resistant prostate cancer cells are dependent on the high activity of CDK7
Journal of Cancer Research and Clinical Oncology (2023)
-
HP1α promotes the progression of prostate cancer
Molecular Biology Reports (2023)
-
H7N9 avian influenza virus infection in men is associated with testosterone depletion
Nature Communications (2022)