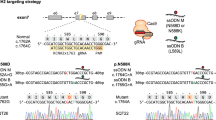
Abstract
We describe a two-stage protocol to generate electrically excitable and actively conducting cell networks with stable and customizable electrophysiological phenotypes. Using this method, we have engineered monoclonally derived excitable tissues as a robust and reproducible platform to investigate how specific ion channels and mutations affect action potential (AP) shape and conduction. In the first stage of the protocol, we combine computational modeling, site-directed mutagenesis, and electrophysiological techniques to derive optimal sets of mammalian and/or prokaryotic ion channels that produce specific AP shape and conduction characteristics. In the second stage of the protocol, selected ion channels are stably expressed in unexcitable human cells by means of viral or nonviral delivery, followed by flow cytometry or antibiotic selection to purify the desired phenotype. This protocol can be used with traditional heterologous expression systems or primary excitable cells, and application of this method to primary fibroblasts may enable an alternative approach to cardiac cell therapy. Compared with existing methods, this protocol generates a well-defined, relatively homogeneous electrophysiological phenotype of excitable cells that facilitates experimental and computational studies of AP conduction and can decrease arrhythmogenic risk upon cell transplantation. Although basic cell culture and molecular biology techniques are sufficient to generate excitable tissues using the described protocol, experience with patch-clamp techniques is required to characterize and optimize derived cell populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sallam, K., Li, Y., Sager, P.T., Houser, S.R. & Wu, J.C. Finding the rhythm of sudden cardiac death: new opportunities using induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 116, 1989–2004 (2015).
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Chong, J.J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014).
Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016).
Yankelson, L. et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation 117, 720–731 (2008).
Klinger, R. & Bursac, N. Cardiac cell therapy in vitro: reproducible assays for comparing the efficacy of different donor cells. IEEE Eng. Med. Biol. Mag. 27, 72–80 (2008).
Gaudesius, G., Miragoli, M., Thomas, S.P. & Rohr, S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 93, 421–428 (2003).
Abraham, M.R. et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ. Res. 97, 159–167 (2005).
Roell, W. et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450, 819–824 (2007).
Iyer, V., Mazhari, R. & Winslow, R.L. A computational model of the human left-ventricular epicardial myocyte. Biophys. J. 87, 1507–1525 (2004).
Kleber, A.G. & Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 84, 431–488 (2004).
Nerbonne, J.M. & Kass, R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 85, 1205–1253 (2005).
Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2, 185–194 (2009).
Noble, D. A modification of the Hodgkin--Huxley equations applicable to Purkinje fibre action and pace-maker potentials. J. Physiol. 160, 317–352 (1962).
Fenton, F. & Karma, A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: filament instability and fibrillation. Chaos 8, 20–47 (1998).
Fenton, F.H., Cherry, E.M., Hastings, H.M. & Evans, S.J. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos 12, 852–892 (2002).
Bueno-Orovio, A., Cherry, E.M. & Fenton, F.H. Minimal model for human ventricular action potentials in tissue. J. Theor. Biol. 253, 544–560 (2008).
Kirkton, R.D. & Bursac, N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat. Commun. 2, 300 (2011).
Kirkton, R.D. & Bursac, N. Genetic engineering of somatic cells to study and improve cardiac function. Europace 14 (Suppl. 5), v40–v49 (2012).
Kirkton, R.D., Badie, N. & Bursac, N. Spatial profiles of electrical mismatch determine vulnerability to conduction failure across a host-donor cell interface. Circ. Arrhythm. Electrophysiol. 6, 1200–1207 (2013).
Gokhale, T.A., Kim, J.M., Kirkton, R.D., Bursac, N. & Henriquez, C.S. Modeling an excitable biosynthetic tissue with inherent variability for paired computational-experimental studies. PLoS Comput. Biol. 13, e1005342 (2017).
Ren, D. et al. A prokaryotic voltage-gated sodium channel. Science 294, 2372–2375 (2001).
Koishi, R. et al. A superfamily of voltage-gated sodium channels in bacteria. J. Biol. Chem. 279, 9532–9538 (2004).
Irie, K. et al. Comparative study of the gating motif and C-type inactivation in prokaryotic voltage-gated sodium channels. J. Biol. Chem. 285, 3685–3694 (2010).
Nguyen, H.X., Kirkton, R.D. & Bursac, N. Engineering prokaryotic channels for control of mammalian tissue excitability. Nat. Commun. 7, 13132 (2016).
Ashcroft, F.M. From molecule to malady. Nature 440, 440–447 (2006).
Tan, H.L. et al. A sodium-channel mutation causes isolated cardiac conduction disease. Nature 409, 1043–1047 (2001).
Barela, A.J. et al. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J. Neurosci. 26, 2714–2723 (2006).
Zhang, Z.S., Tranquillo, J., Neplioueva, V., Bursac, N. & Grant, A.O. Sodium channel kinetic changes that produce Brugada syndrome or progressive cardiac conduction system disease. Am. J. Physiol. Heart Circ. Physiol. 292, H399–H407 (2007).
Hou, L., Hu, B. & Jalife, J. Genetically engineered excitable cardiac myofibroblasts coupled to cardiomyocytes rescue normal propagation and reduce arrhythmia complexity in heterocellular monolayers. PLoS One 8, e55400 (2013).
Park, J. et al. Screening fluorescent voltage indicators with spontaneously spiking HEK cells. PLoS One 8, e85221 (2013).
McNamara, H.M., Zhang, H., Werley, C.A. & Cohen, A.E. Optically controlled oscillators in an engineered bioelectric tissue. Phys. Rev. X 6, 031001 (2016).
Zhang, H., Reichert, E. & Cohen, A.E. Optical electrophysiology for probingfunction and pharmacology of voltage-gated ion channels. Elife 5, e15202 (2016).
Xie, M. et al. Beta-cell-mimetic designer cells provide closed-loop glycemic control. Science 354, 1296–1301 (2016).
Isom, L.L., De Jongh, K.S. & Catterall, W.A. Auxiliary subunits of voltage-gated ion channels. Neuron 12, 1183–1194 (1994).
Abriel, H. Cardiac sodium channel Na(v)1.5 and interacting proteins: physiology and pathophysiology. J. Mol. Cell Cardiol. 48, 2–11 (2010).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Wang, D. et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum. Gene Ther. 26, 432–442 (2015).
Doroudchi, M.M. et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 19, 1220–1229 (2011).
Han, X. et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62, 191–198 (2009).
Sim, S., Antolin, S., Lin, C.W., Lin, Y. & Lois, C. Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J. Neurosci. 33, 7928–7940 (2013).
Graham, F.L., Smiley, J., Russell, W.C. & Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59–74 (1977).
Shaw, G., Morse, S., Ararat, M. & Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 16, 869–871 (2002).
Varghese, A., Tenbroek, E.M., Coles, J., Jr. & Sigg, D.C. Endogenous channels in HEK cells and potential roles in HCN ionic current measurements. Prog. Biophys. Mol. Biol. 90, 26–37 (2006).
Jiang, B., Sun, X., Cao, K. & Wang, R. Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Mol. Cell Biochem. 238, 69–79 (2002).
Tong, J., Du, G.G., Chen, S.R. & MacLennan, D.H. HEK-293 cells possess a carbachol- and thapsigargin-sensitive intracellular Ca2+ store that is responsive to stop-flow medium changes and insensitive to caffeine and ryanodine. Biochem. J. 343 (Pt 1): 39–44 (1999).
Berjukow, S. et al. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br. J. Pharmacol. 118, 748–754 (1996).
Thomas, P. & Smart, T. G. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51, 187–200 (2005).
Xu, X. et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 29, 735–741 (2011).
Scherer, W.F., Syverton, J.T. & Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 97, 695–710 (1953).
Estacion, M. Characterization of ion channels seen in subconfluent human dermal fibroblasts. J. Physiol. 436, 579–601 (1991).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Lowry, W.E. et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA 105, 2883–2888 (2008).
Vien, T.N. & DeCaen, P.G. Biophysical adaptations of prokaryotic voltage-gated sodium channels. Curr. Top Membr. 78, 39–64 (2016).
Trudeau, M.C., Warmke, J.W., Ganetzky, B. & Robertson, G.A. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 (1995).
Yue, L., Feng, J., Li, G.R. & Nattel, S. Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. J. Physiol. 496 (Pt 3): 647–662 (1996).
Ravens, U. & Wettwer, E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc. Res. 89, 776–785 (2011).
Ivics, Z., Hackett, P.B., Plasterk, R.H. & Izsvak, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510 (1997).
Mates, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 (2009).
Kowarz, E., Loscher, D. & Marschalek, R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 10, 647–653 (2015).
Hu, T., Fu, Q., Chen, P., Zhang, K. & Guo, D. Generation of a stable mammalian cell line for simultaneous expression of multiple genes by using 2A peptide-based lentiviral vector. Biotechnol. Lett. 31, 353–359 (2009).
Kim, J.H. et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556 (2011).
Badie, N. & Bursac, N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 96, 3873–3885 (2009).
Scull, J.A., McSpadden, L.C., Himel, H.D.t., Badie, N. & Bursac, N. Single-detector simultaneous optical mapping of V(m) and [Ca(2+)](i) in cardiac monolayers. Ann. Biomed. Eng. 40, 1006–1017 (2012).
Badie, N., Scull, J.A., Klinger, R.Y., Krol, A. & Bursac, N. Conduction block in micropatterned cardiomyocyte cultures replicating the structure of ventricular cross-sections. Cardiovasc. Res. 93, 263–271 (2012).
Nguyen, H., Badie, N., McSpadden, L., Pedrotty, D. & Bursac, N. in Cardiac tissue engineering. Vol. 1181 Methods in Molecular Biology (eds. Radisic, M. & Black Iii, L.D.) Ch. 21, 249–262 (Springer, 2014).
Davie, J.T. et al. Dendritic patch-clamp recording. Nat. Protoc. 1, 1235–1247 (2006).
Mortensen, M. & Smart, T.G. Single-channel recording of ligand-gated ion channels. Nat. Protoc. 2, 2826–2841 (2007).
de Boer, T.P. et al. Inhibition of cardiomyocyte automaticity by electrotonic application of inward rectifier current from Kir2.1 expressing cells. Med. Biol. Eng. Comput. 44, 537–542 (2006).
Nygren, A. et al. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ. Res. 82, 63–81 (1998).
Hodgkin, A.L. & Huxley, A.F. Propagation of electrical signals along giant nerve fibers. Proc. R Soc. Lond. B Biol. Sci. 140, 177–183 (1952).
Hodgkin, A.L. & Rushton, W.A. The electrical constants of a crustacean nerve fibre. Proc. R Soc. Med. 134, 444–479 (1946).
Van Rijen, H.V., Wilders, R., Rook, M.B. & Jongsma, H.J. Dual patch clamp. Methods Mol. Biol. 154, 269–292 (2001).
Veenstra, R.D. Voltage clamp limitations of dual whole-cell gap junction current and voltage recordings. I. Conductance measurements. Biophys. J. 80, 2231–2247 (2001).
ten Tusscher, K.H., Noble, D., Noble, P.J. & Panfilov, A.V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 286, H1573–H1589 (2004).
Nguyen, H., Badie, N., McSpadden, L., Pedrotty, D. & Bursac, N. Quantifying electrical interactions between cardiomyocytes and other cells in micropatterned cell pairs. Methods Mol. Biol. 1181, 249–262 (2014).
Acknowledgements
We thank M.R. Abraham (John Hopkins University) for Kir2.1 cDNA, A.O. Grant (Duke University) for Nav1.5 cDNA, and K. Irie (Kyoto University) for BacNav cDNA. This work was supported by predoctoral fellowships from the National Science Foundation and the American Heart Association to R.D.K. and H.X.N., respectively, as well as by research grants to N.B. from the American Heart Association (AHA 0530256N) and National Institutes of Health (HL106203, HL104326, HL083342, HL095069, HL104326, HL132389, HL126524, and HL126193).
Author information
Authors and Affiliations
Contributions
H.X.N., R.D.K., and N.B. jointly developed the protocol; H.X.N. and R.D.K. performed the experiments and generated the figures; and H.X.N. and N.B. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data
AP simulation. (ZIP 1386 kb)
Rights and permissions
About this article
Cite this article
Nguyen, H., Kirkton, R. & Bursac, N. Generation and customization of biosynthetic excitable tissues for electrophysiological studies and cell-based therapies. Nat Protoc 13, 927–945 (2018). https://doi.org/10.1038/nprot.2018.016
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.016
This article is cited by
-
Engineered bacterial voltage-gated sodium channel platform for cardiac gene therapy
Nature Communications (2022)
-
Generation of ring-shaped human iPSC-derived functional heart microtissues in a Möbius strip configuration
Bio-Design and Manufacturing (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.