Abstract
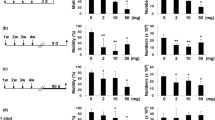
Soluble carbon nanotubes show promise as materials for in vivo delivery and imaging applications. Several reports have described the in vivo toxicity of carbon nanotubes, but their effects on male reproduction have not been examined. Here, we show that repeated intravenous injections of water-soluble multiwalled carbon nanotubes into male mice can cause reversible testis damage without affecting fertility. Nanotubes accumulated in the testes, generated oxidative stress and decreased the thickness of the seminiferous epithelium in the testis at day 15, but the damage was repaired at 60 and 90 days. The quantity, quality and integrity of the sperm and the levels of three major sex hormones were not significantly affected throughout the 90-day period. The fertility of treated male mice was unaffected; the pregnancy rate and delivery success of female mice that mated with the treated male mice did not differ from those that mated with untreated male mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lacerda, L., Bianco, A., Prato, M. & Kostarelos, K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv. Drug Deliv. Rev. 58, 1460–1470 (2006).
Liu, Z. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotech. 2, 47–52 (2007).
Usui, Y. et al. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small 4, 240–246 (2008).
Chen, J. Y. et al. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc. 130, 16778–16785 (2008).
Singh, R. et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl Acad. Sci. USA 103, 3357–3362 (2006).
Yang, S. T. et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 181, 182–189 (2008).
Manna, S. K. et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappa B in human keratinocytes. Nano Lett. 5, 1676–1684 (2005).
Shvedova, A. A. et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, 552–565 (2008).
Ravichandran, P. et al. Induction of apoptosis in rat lung epithelial cells by multiwalled carbon nanotubes. J. Biochem. Mol. Toxicol. 23, 333–344 (2009).
Bottini, M. et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 160, 121–126 (2006).
Warheit, D. B. et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 77, 117–125 (2004).
Li, Z. et al. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ. Health Perspect. 115, 377–382 (2007).
Shvedova, A. A. et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, 698–708 (2005).
Ma-Hock, L. et al. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 112, 468–481 (2009).
Sakamoto, Y. et al. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J. Toxicol. Sci. 34, 65–76 (2009).
Miyawaki, J., Yudasaka, M., Azami, T., Kubo, Y. & Iijima, S. Toxicity of single-walled carbon nanohorns. ACS Nano 2, 213–226 (2008).
Bonde, J. P. Male reproductive organs are at risk from environmental hazards. Asian J. Androl. 12, 152–156 (2010).
Yoshida, S. et al. Effect of nanoparticles on the male reproductive system of mice. Int. J. Androl. 32, 337–342 (2009).
Yoshida, S. et al. Effects of fetal exposure to carbon nanoparticles on reproductive function in male offspring. Fertil. Steril. 93, 1695–1699 (2010).
Purvis, K. & Christiansen, E. Male infertility: current concepts. Ann. Med. 24, 259–272 (1992).
Howards, S. S. Treatment of male infertility. N. Engl. J. Med. 332, 312–317 (1995).
Saleh, R. A. & Agarwal, A. Oxidative stress and male infertility: from research bench to clinical practice. J. Androl. 23, 737–752 (2002).
Tremellen, K. Oxidative stress and male infertility—a clinical perspective. Hum. Reprod. Update 14, 243–258 (2008).
Xia, T. et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6, 1794–1807 (2006).
Liu, Z. et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 68, 6652–6660 (2008).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Kwon, J. T. et al. Body distribution of inhaled fluorescent magnetic nanoparticles in the mice. J. Occup. Health 50, 1–6 (2008).
Li, M. W. M., Mruk, D. D., Lee, W. M. & Cheng, C. Y. Cytokines and junction restructuring events during spermatogenesis in the testis: an emerging concept of regulation. Cytokine Growth Factor Rev. 20, 329–338 (2009).
Cheng, C. Y. & Mruk, D. D. Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 (2002).
Meistrich, M. L. et al. Rapid recovery of spermatogenesis after mitoxantrone, vincristine, vinblastine and prednisone chemotherapy for Hodgkin's disease. J. Clin. Oncol. 15, 3488–3495 (1997).
Mandal, T. K. & Das, N. S. Testicular toxicity in cannabis extract treated mice: association with oxidative stress and role of antioxidant enzyme systems. Toxicol. Ind. Health 26, 11–23 (2010).
Lacerda, L. et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine 3, 149–161 (2008).
Safe, S. H. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 24, 87–149 (1994).
Rodamilans, M. et al. Lead toxicity on endocrine testicular function in an occupationally exposed population. Hum. Toxicol. 7, 125–128 (1988).
Li, C. M. et al. Effects of inhaled nanoparticle-rich diesel exhaust on regulation of testicular function in adult male rats. Inhal. Toxicol. 21, 803–811 (2009).
Ramdhan, D. H. et al. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol. Lett. 191, 103–108 (2009).
Davies, K. J. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 61, 1–31 (1995).
Kazzaz, J. A. et al. Cellular oxygen toxicity: oxidant injury without apoptosis. J. Biol. Chem. 271, 15182–15186 (1996).
Alvarez, J. G., Touchstone, J. C., Blasco, L. & Storey, B. T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 8, 338–348 (1987).
Sergerie, M., Laforest, G., Bujan, L., Bissonnette, F. & Bleau, G. Sperm DNA fragmentation: threshold value in male fertility. Hum. Reprod. 20, 3446–3451 (2005).
Calvo, L., Dennison-Lagos, L., Banks, S. M. & Sherins, R. J. Characterization and frequency distribution of sperm acrosome reaction among normal and infertile men. Hum. Reprod. 9, 1875–1879 (1994).
Goyal, H. O. et al. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol. Reprod. 68, 2081–2091 (2003).
Zhou, H. Y. et al. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity and immune response. Nano Lett. 8, 859–865 (2008).
Watanabe, T. & Endo, A. Effects of selenium deficiency on sperm morphology and spermatocyte chromosomes in mice. Mutat. Res. 262, 93–99 (1991).
Santos, J. R. et al. Probing the structure and function of mammalian sperm using optical and fluorescence microscopy. Mod. Res. Educ. Topics Microsc. 21, 394–402 (2007).
Ozaki, T., Takahashi, K., Kanasaki, H. & Miyazaki, K. Evaluation of acrosome reaction and viability of human sperm with two fluorescent dyes. Arch. Gynecol. Obstet. 266, 114–117 (2002).
Acknowledgements
The authors would like to thank Q. Jia, J. Han, Q. Wang, Y. Liu, T. Powell, D. Broughton, A. L. Vavere and L. Mann for technical assistance, and B. Ma for stimulating discussions. This work was supported by the National Basic Research Program of China (973 Program 2010CB933504), the National Cancer Institute (P30CA027165), the American Lebanese Syrian Associated Charities and St Jude Children's Research Hospital.
Author information
Authors and Affiliations
Contributions
B.Y. and Y.B. conceived and designed the experiments. Y.B., Y.Z., J.Z., Q.M., W.Z., E.R.B. and S.E.S. performed the experiments. B.Y., Y.B. and Y.Z. analysed the data. Y.B., Q.M., W.Z. and S.E.S. contributed materials and analysis tools. B.Y. and Y.B. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 562 kb)
Rights and permissions
About this article
Cite this article
Bai, Y., Zhang, Y., Zhang, J. et al. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nature Nanotech 5, 683–689 (2010). https://doi.org/10.1038/nnano.2010.153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.153
This article is cited by
-
Assessment of reproductive toxicity of gold nanoparticles and its reversibility in male albino rats
Toxicological Research (2024)
-
Silica Nanoparticle–Induced Reproductive Toxicity in Male Albino Rats via Testicular Apoptosis and Oxidative Stress
Biological Trace Element Research (2023)
-
Switching to nanonutrients for sustaining agroecosystems and environment: the challenges and benefits in moving up from ionic to particle feeding
Journal of Nanobiotechnology (2022)
-
Epicatechin Surface Coating in Combating Toxicity of Silver Nanoparticle in Mice Male Reproductive System
BioNanoScience (2022)
-
Polyamine metabolism links gut microbiota and testicular dysfunction
Microbiome (2021)