Abstract
Post-traumatic stress disorder (PTSD) is characterized by exaggerated fear expression and impaired fear extinction. The underlying molecular and cellular mechanisms of PTSD are largely unknown. The current pharmacological and non-pharmacological treatments for PTSD are either ineffective or temporary with high relapse rates. Here we report that adiponectin-deficient mice exhibited normal contextual fear conditioning but displayed slower extinction learning. Infusions of adiponectin into the dentate gyrus (DG) of the hippocampus in fear-conditioned mice facilitated extinction of contextual fear. Whole-cell patch-clamp recordings in brain slices revealed that intrinsic excitability of DG granule neurons was enhanced by adiponectin deficiency and suppressed after treatment with the adiponectin mimetic AdipoRon, which were associated with increased input resistance and hyperpolarized resting membrane potential, respectively. Moreover, deletion of AdipoR2, but not AdipoR1 in the DG, resulted in augmented fear expression and reduced extinction, accompanied by intrinsic hyperexcitability of DG granule neurons. Adiponectin and AdipoRon failed to induce facilitation of fear extinction and elicit inhibition of intrinsic excitability of DG neurons in AdipoR2 knockout mice. These results indicated that adiponectin action via AdipoR2 was both necessary and sufficient for extinction of contextual fear and intrinsic excitability of DG granule neurons, implying that enhancing or dampening DG neuronal excitability may cause resistance to or facilitation of extinction. Therefore, our findings provide a functional link between adiponectin/AdipoR2 activation, DG neuronal excitability and contextual fear extinction, and suggest that targeting adiponectin/AdipoR2 may be used to strengthen extinction-based exposure therapies for PTSD.
Similar content being viewed by others
Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition that is characterized by intense feelings of fear and severe flashbacks that follow a traumatic experience. The development of PTSD symptoms could result from the inability to use contextual information to modulate fear expression and extinction.1, 2, 3, 4 The hippocampus is a key brain region for the context specificity of fear and extinction memories.5, 6, 7, 8, 9 Hippocampal lesions selectively impair fear responses to the context.7, 10 Volumetric reductions in the hippocampus, in particular the dentate gyrus (DG), have been reported to be associated with PTSD.11, 12, 13, 14, 15 The DG serves as a gateway for information flow through the hippocampal formation16 and has an essential role in generating contextual fear memories.9, 17, 18, 19, 20, 21 Reactivation of DG neurons activated during fear conditioning is sufficient to induce freezing behavior.21, 22 However, the exact regulatory mechanisms through which the DG influences context-specific fear responses are poorly understood.
The hippocampus is well positioned to sense and respond to changes in peripheral hormonal signals, as it is lying alongside the choroid plexus and adjacent to the cerebral ventricles. Adiponectin is a circulating hormone that is secreted almost exclusively by adipocytes.23, 24 In the plasma, adiponectin exists as a full-length form and a globular form generated by the cleavage of full-length adiponectin. Adiponectin aggregates in multimeric complexes including trimers, hexamers and high-molecular-weight forms.25, 26 It is known that adiponectin can cross the blood–brain barrier from the systemic circulation. Adiponectin can be detected in the cerebrospinal fluid after an intravenous injection in adiponectin knockout mice.27, 28 Low- molecular-weight forms of adiponectin are found in the cerebrospinal fluid of humans and mice.27, 29, 30, 31 The biological effects of adiponectin are mediated mainly through two receptor subtypes, AdipoR1 and AdipoR2, with 67% amino acid identity.32 These receptors contain 7-transmembrane domains such as G-protein-coupled receptors but they are opposite to the membrane topology of all known G-protein-coupled receptors, with their intracellular N terminus and extracellular C terminus.32 AdipoR1 and AdipoR2 have distinct distribution patterns in various tissues and different binding affinity for globular and full-length adiponectin.32 Despite ample evidence for the roles of AdipoR1 and AdipoR2 in metabolic regulation, there are scarce data concerning their involvement in higher brain function. Both AdipoR1 and AdipoR2 are highly expressed in the DG,33 suggesting that this brain region is a major target of adiponectin action in the brain. However, whether adiponectin and its receptors in this brain region contribute to contextual control of fear expression and extinction has not been investigated so far. Here we set out to identify the role of adiponectin by using mice deficient in adiponectin or received intra-DG infusions of adiponectin, to test them in a Pavlovian contextual fear-conditioning and extinction paradigm. This paradigm has been used as a model of fear acquisition relevant to some aspects of PTSD and mimicking extinction-based exposure therapies.34, 35, 36 The involvement of AdipoR1 and AdipoR2 in contextual fear memories was further investigated using specific receptor knockout mice. In order to understand the cellular mechanisms underlying the actions of adiponectin and its receptors, we also examined the intrinsic excitability of DG granule neurons using whole-cell patch-clamp recordings.
Materials and methods
Animals
Adult wild-type (WT) male and female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in groups of five under a 12-h light/dark cycle (lights on at 0900 h) with ad libitum access to food and water. All procedures were carried out in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committees of University of Texas Health Science Center at San Antonio.
Adipo−/− mice
Heterozygous adiponectin gene knockout mice on a C57BL/6J genetic background were generated as previously described and intercrossed to generate adiponectin knockout (Adipo−/−) mice and WT littermates.37 Genotyping was performed by screening for the adiponectin null allele as described previously.37
AdipoR2−/− mice
Heterozygous AdipoR2 gene knockout mice were obtained from Deltagen (San Carlos, CA, USA), which had been backcrossed at least six generations to C57BL/6J mice by the Jackson Laboratory. AdipoR2+/− mice were intercrossed to produce AdipoR2−/− mice and WT littermates. Screening for the AdipoR2-null allele was performed as described previously.38
Generation of AdipoR1-floxed mice (AdipoR1flox/flox)
A targeting vector was designed in which exon 2 of the AdipoR1 gene was flanked by loxP sites. Exon/intron boundary analysis revealed that the deletion of this exon led to a frame shift and the generation of a null mutant of AdipoR1. A 11.5-kb genomic clone derived from C57BL/6 mice containing exons 2–5 of the AdipoR1 gene was obtained and used to generate the AdipoR1 loxP targeting vector (Figure 3a). The targeting construct was generated by inserting a loxP site, a sequence containing a PGK-neo expression unit and two Frt sites into the BamHI site located 232 bp upstream of exon 2 and the other loxP site into the SacI site 295 bp downstream of exon 2. The targeting construct contains 5.3 kb of homologous DNA upstream of the first loxP site and 3.3 kb of homologous DNA downstream of the second loxP site. Bruce4 embryonic stem (ES) cells derived from C57BL/6 mice were microinjected with the targeting vector. Positive cells were selected and screened for homologous recombination and incorporation of the loxP sites. Homologous recombination was confirmed with PCR to detect the WT and AdipoR1-floxed alleles using the primers forward-5′-CCCTGGGGATAGTTCTGGAT-3′ and reverse-5′-TTACTCACTGGGCCCTCTTG-3′, to detect the downstream loxP site and was confirmed using Southern blot analysis. ES cell clones that were found to be AdipoR1flox/+ were injected into B6(Cg)-Tyrc-2J/J-derived blastocysts for generation of chimeras. Chimeras were bred with albino B6(Cg)-Tyrc-2J/J mice to obtain germline transmission. The mice with the floxed AdipoR1 allele were confirmed using Southern blot analysis (Figure 3a). These mice were intercrossed to generate AdipoR1flox/flox mice. Removal of the neomycin selective marker flanked by Frt sites was accomplished by crossing the AdipoR1flox/flox mice with a FLP deleter strain that expresses Flp recombinase (B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ). AdipoR1flox/flox mice used in this study were backcrossed to C57BL/6J for more than ten generations.
Generation of AdipoR1POMC-Cre mice
AdipoR1flox/flox mice were crossed with mice expressing Cre recombinase under the control of the proopiomelanocortin promoter (POMC-Cre),39 to generate AdipoR1flox/flox, POMC-Cre mice. POMC-Cre mice were bred with the Ai14-tdTomato reporter mice to confirm the Cre-mediated excision in the DG. Subsequently, male POMC-Cre/+ mice were crossed to female AdipoR1flox/flox mice, to yield AdipoR1flox/flox, POMC-Cre/+ (termed AdipoR1POMC-Cre) mice and AdipoR1flox/flox ‘WT’ littermate controls (termed fWT). Animals from generation F4–6 were used in this study. The PCR primers used for genotyping were as follows: POMC-Cre, forward-5′-GCGGTCTGGCAGTAAAACTATC-3′, reverse-5′-GTGAAACAGCATTGCTGTCACTT-3′ and AdipoR1 WT and flox, forward-5′-CCCTGGGGATAGTTCTGGAT-3′, reverse-TTACTCACTGGGCCCTGCTTG-3′.
RNA extraction and reverse transcriptase-PCR
Mice were decapitated and their brains were removed immediately and placed in a Petri dish containing pre-chilled phosphate-buffered saline. The DG was isolated under a stereomicroscope using a dissection procedure, as previously described.40 The isolated DG was homogenized and total RNA was extracted with PureLink RNA Mini Kit (Life Technologies, Grand Island, NY, USA). SuperScript first-strand synthesis system (Life Technologies) was used to generate cDNA using the oligo (dT) 25 as the template primer. The resulting cDNA was used for PCR amplification of exon 2 of AdipoR1 and 18s ribosomal RNA with Accuprime pfx Supermix (Life Technologies). The primers used to amplify each product are as follows: AdipoR1 exon 2, forward-5′-CCCGTATCCACCAGACACCGG-3′, reverse-5′-GGCAATGGGGCTCCTTCTGG-3′ and 18s, forward-5′-CACGGACAGGATTGACAGAT-3′, reverse-5′-CAAATCGCTCCACCAACTAA-3′.
Adult (8–12 weeks old) male mice of different genotypes were used for all behavioral and electrophysiological experiments.
Drugs
Recombinant mouse adiponectin (R&D Systems, Minneapolis, MN, USA) was dissolved in artificial cerebrospinal fluid (137 mM NaCl, 2.7 mM KCl, 0.5 mM MgCl2·6H2O, 0.9 mM CaCl2·2H2O, 1.5 mM KH2PO4 and 8.1 mM Na2HPO4). AdipoRon, 2-(4-benzoylphenoxy)-N-[1-(phenylmethyl)-4 piperidinyl]acetamide (AdipoGen, San Diego, CA, USA), was dissolved in dimethyl sulfoxide.
Behavioral tests
Contextual fear conditioning and extinction
Fear conditioning was performed in a mouse fear-conditioning chamber equipped with the Coulbourn Freezeframe system and positioned inside a sound-attenuating cabinet (Coulbourn Instruments, Whitehall, PA, USA). The rear wall of the cabinet was covered with black and white vertical striped vinyl wallpaper. The chamber (18 × 18 × 30 cm) had two white aluminum side walls and Plexiglas rear and front walls with a hinged front door. The floor of the chamber consisted of 16 stainless steel rods (5-mm diameter), which were evenly spaced 1-cm apart (center-to-center) and connected to a shock generator and a scrambler for the delivery of foot shock. A houselight was located on one side wall of the chamber and served to illuminate the chamber. A digital camera was mounted on the top of the chamber for monitoring and recording animal behaviors. A stainless steel pan was placed beneath the grid floor and contained a sheet of paper sprayed with 20% isopropyl alcohol. Mice were trained in a contextual fear-conditioning paradigm.41 Mice were first acclimated to the conditioning chamber for 3 min. Next, a shock (0.8 mA, 2 s duration) was delivered four times with a 60-s inter-shock interval. Mice were removed from the conditioning chamber 60 s following the last shock. During extinction training, mice were repeatedly re-exposed to the conditioning chamber for 5 min without shock delivery once daily for 4 or 6 days. Freezing responses during the pre-conditioning phase, conditioning phase, extinction and retrieval testing were recorded and measured as an index of fear memory with the Freezeframe3 software. The chamber was cleaned and dried using 20% isopropyl alcohol between each trial.
Elevated plus maze test
The maze consisted of two white acrylic open arms (30 × 5 cm) and two closed arms (30 × 5 × 15 cm) that form the shape of a ‘plus’ sign. The apparatus was elevated to a height of 70 cm from the floor. Mice were placed in the central square (5 × 5 cm) facing the corner between a closed arm and an open arm, and allowed to explore the elevated plus maze for 5 min. The percentage of open arm entries and the percentage of time spent in the open arms were quantified as a measure of anxiety. Total arm entries were analyzed as an index of exploratory activity.
Hot-plate test
Pain sensitivity was assessed by testing reflexes in response to a thermal stimulus. The surface of the hot plate was heated to a constant temperature of 55 °C. Mice were placed on the hot plate for a maximum duration of 90 s. The latency to respond with hind paw lick or jump was recorded. After the mouse responded, it was immediately removed from the hot plate and returned to its home cage. If the mouse did not respond, the test was terminated at the end of 90 s.
Visual cliff test
A clear acrylic box with half of the inner surface covered with checkerboard paper was used for the mouse visual cliff test. The box was placed ~70 cm above the floor. A sheet of clear Plexiglas provided a solid horizontal surface in spite of the visual appearance of a cliff. Each mouse was placed at the midline of the box and the direction in which the mouse stepped was recorded on 10 consecutive trials.
Stereotaxic surgery
Stereotaxic surgery was performed as previously described.42, 43 Mice were anesthetized with an intramuscular injection of a cocktail containing ketamine 60 mg ml−1, acepromazine 1 mg ml−1 and xylazine 8 mg ml−1 (0.1 ml kg−1, intramuscular) and mounted onto a stereotaxic frame. For intra-DG microinjections, WT mice received bilateral guide cannula (23-gauge; Plastics One, Roanoke, VA, USA) targeting the DG (coordinates: anteroposterior (AP)=−2.1 mm, mediolateral (ML)=±1.5 mm, dorsoventral (DV)=−1.2 mm from the bregma) as previously described.42 Following surgery, mice were individually housed, handled daily and allowed to recover for 7 days. Intra-DG microinjections were performed on conscious, unrestrained and freely moving mice in their home cage. On the experimental day, a 33-G stainless-steel injector connected to a 5-μl syringe was inserted into the guide cannula and extended 1 mm beyond the tip. Adiponectin (0.5 μg μl−1) or artificial cerebrospinal fluid was infused bilaterally in a volume of 0.5 μl (0.25 μl per side) over 2 min. The injector tips were held in place for additional 5 min after the end of infusion to avoid backflow.
For intra-DG microinjection of AAV-DJ vectors containing the genes for Cre recombinase (AAV-Cre-GFP) or GFP alone (Vector Biolabs, Malvern, PA, USA), a total volume of 1.0 μl AAV vectors (0.5 μl per side) were injected bilaterally into the DG (coordinates: AP=−2.1 mm, ML=±1.5 mm, DV=−2.3 mm from the bregma) of adult AdipoR1flox/flox mice, at a rate of 0.10 μl min−1 with a 33-gauge stainless steel injector. Additional 5 min were allowed for diffusion and prevention of backflow. Behavioral experiments were conducted 21 days after AAV injection.
Whole-cell patch-clamp recordings
Adult (8–10 weeks old) male mice were anesthetized with isoflurane and then perfused through the left ventricle with ice-cold perfusion solution containing 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 25 mM NaHCO3, 11 mM glucose and 1.25 mM Kynurenic acid. The brains were quickly transferred to an ice-cold cutting solution containing 254 mM sucrose, 3 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 1.25 mM NaH2PO4, 10 mM D-glucose and 24 mM NaHCO3. Coronal slices (300 μm) containing the DG were prepared with a Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL, USA) and allowed to recover at 30 °C for at least 1 h in an oxygenated (95% O2/5% CO2) extracellular solution containing 124 mM NaCl, 2 mM KCl, 2 mM MgSO4, 1.25 mM NaH2PO4, 2 mM CaCl2, 26 mM NaHCO3, 10 mM D-dextrose and 0.4 mM vitamin C. Brain slices were constantly perfused with the extracellular bath solution during whole-cell patch-clamp recordings. Whole-cell recording pipettes with tip resistances between 4 and 7 MΩ were pulled from thin-walled borosilicate glass capillaries (TW-150 F, World Precision Instruments, Sarasota, FL, USA) using the Sutter P-87 (Sutter Instrument, Novato, CA, USA). Pipettes were filled with a potassium gluconate-based internal solution containing 120 mM potassium gluconate, 20 mM KCl, 2 mM MgCl2, 10 mM HEPES, 2 mM ATP, 0.25 mM GTP and 0.1 mM EGTA, with pH adjusted to 7.4 and osmolarity of 295 mOsm. Granule cells were identified using infrared microscopy and a charge-coupled device camera. Cells in the granule cell layer of the DG were clamped in the whole-cell mode using the whole-cell mode of a HEKA EPC10 feedback amplifier and Patchmaster software (HEKA Instruments, Lambrecht/Pfalz, Germany). Recordings were considered acceptable if cells had a resting membrane potential of −70 mV or more negative and input resistance of 300 MΩ or larger. Data were collected with a hardware filter of 3 kHz. To investigate the firing properties of neurons, current-clamp recordings were made from a −80 mV holding current and incremental stepwise current injections (15 pA) for a 1-s duration. The number of action potentials was counted for each current injection during the first 300 ms with steady firing rates. Analysis of passive membrane properties of cells was made at the resting membrane potential. Input resistance was measured from a hyperpolarizing current injection of −20 pA from a −80 mV holding current. The fast afterhyperpolarization (fAHP) size was measured as the difference between the spike threshold and voltage minimum after the action potential peak. The spike amplitude, half-width and spike threshold were measured from the first spike during a 90-pA current injection.
Statistical analysis
Statistical significance was assessed by one-way analysis of variance, two-way repeated-measures analysis of variance or two-tailed, unpaired and paired t-tests, where appropriate. Significant effects in analysis of variances were followed up with Bonferroni post-hoc tests. Results were considered significantly different when P<0.05. All data were presented as means±s.e.m.
Results
Normal acquisition of contextual fear but impaired extinction learning in adiponectin knockout mice
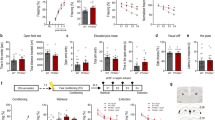
Adiponectin knockout (Adipo−/−) mice and WT littermates were trained to associate an unconditioned stimulus (4 foot shocks; 0.8 mA, 2 s duration, 60 s inter-shock interval) with a context (the shock chamber). During contextual fear-conditioning training, there was no difference between genotypes in the level of postshock freezing (Figure 1a), suggesting that adiponectin is not required for the acquisition of contextual fear. At 24 h after conditioning, extinction training was performed on Adipo−/− mice and WT littermates by repeatedly re-exposing the mice to the conditioning context daily without foot shock for 4 consecutive days. It has been suggested that extinction learning may be triggered by memory retrieval.44 To examine whether initial retrieval of fear memory after conditioning is affected in Adipo−/− mice, freezing responses during the first 3 min of extinction training session E1 was analyzed. Mice of both genotypes displayed high levels of freezing responses on E1 and showed no genotype difference (Figure 1a). Adipo−/− mice exhibited a significantly slower time course of contextual fear extinction, as compared with WT littermates (Figure 1a). These data indicate that adiponectin deficiency resulted in impaired extinction of contextual fear. Innate anxiety was assessed in the elevated plus-maze test and showed no effect of genotype (Supplementary Figure 1a). Adipo−/− mice also displayed normal nociceptive responses, as measured by the hot-plate test (Supplementary Figure 1b) and normal visual acuity assessed in the visual cliff test (Supplementary Figure 1c).
Adiponectin regulates contextual fear extinction. (a) Mice were subjected to fear conditioning (FC) followed by extinction training for 4 days (E1–E4). Adipo−/− mice showed normal contextual fear acquisition (left; genotype: F(1,24)=1.842, P=0.187; shock: F(4,96)=208.6, P<0.001; genotype × shock interaction: F(4,96)=1.354, P=0.256) and retrieval (middle; t(24)=0.225, P=0.824) but displayed slower extinction (right, genotype: F(1,24)=8.017, P=0.009; day: F(3,72)=101.6, P<0.001; genotype × day interaction: F(3,72)=2.739, P=0.0496). Adipo−/−, n=14; wild type (WT), n=12. *P<0.05 and **P<0.01 compared with WT littermate controls. (b) After contextual FC, dentate gyrus (DG)-cannulated WT mice received intra-DG infusions of adiponectin or vehicle (artificial cerebrospinal fluid (aCSF)) for 3 days, followed by extinction training for 4 days (E1–E4). Intra-DG infusion was given daily 30 min before re-exposure to the context during extinction training. In fear-conditioned mice (left), intra-DG infusions of adiponectin did not affect fear retrieval (t(19)=0.238, P=0.815; middle left) but facilitated fear extinction (middle right; treatment: F(1,19)=3.422, P=0.08; day: F(3,57)=17.6, P<0.001; treatment × day interaction F(3,57)=2.892, P=0.043). Right, microinjection sites in the DG (open circles for vehicle-treated mice and closed circles for adiponectin-treated mice). Intra-DG vehicle (Veh), n=11; intra-DG adiponectin (Adipo), n=10. **P<0.01, #P=0.057 compared with the vehicle-treated group. Error bars represent s.e.m.
Facilitation of contextual fear extinction by intra-DG infusions of adiponectin
Given the abundant expression of AdipoR1 and AdipoR2 in the DG of the hippocampus33 and the critical role of this brain region in contextual fear extinction,18, 19, 20 we reasoned that the DG could be a site mediating the effects of adiponectin on contextual fear extinction. To test this possibility, mice were bilaterally cannulated in the DG and subjected to contextual fear conditioning (Figure 1b). Fear-conditioned mice received an intra-DG infusion (0.25 μg) per day for 3 days before extinction training. Over the course of 4-day extinction training, adiponectin was infused into the DG 30 min before each extinction session (Figure 1b). Initial fear retrieval during the first 3 min of extinction session E1 showed no difference between treatment groups (Figure 1b). Mice that received intra-DG infusions of adiponectin showed accelerated fear-extinction learning, spending less time in freezing during extinction sessions 2 and 3 than vehicle-treated mice (Figure 1b). These results suggest that adiponectin in the DG facilitates extinction of contextual fear.
Modulation of intrinsic excitability of DG granule neurons by adiponectin
To explore the cellular mechanisms of adiponectin modulation of contextual fear extinction, we examined whether adiponectin regulates excitability of DG granule neurons. First, whole-cell current clamp recordings were performed on DG granule neurons to examine the effects of adiponectin deficiency on their intrinsic excitability properties in response to depolarizing current injection (300 ms). DG granule neurons from Adipo−/− mice were hyperexcitable (Figure 2a), exhibiting a greater number of action potentials in response to current injections ranging from 45 to 90 pA (Figure 2a1), lower rheobase current (Figure 2a2) and higher input resistance (Figure 2a4) than DG neurons from WT littermates. Other membrane properties including resting membrane potential, action potential threshold and fAHP amplitude, half-width and amplitude of action potentials did not significantly differ between genotypes (Figures 2a3 and 2a5, and Supplementary Table 1).
Adiponectin modulates intrinsic excitability of dentate gyrus (DG) granule neurons. (a) Increased excitability of DG neurons in Adipo−/− mice. (a1) Left, action potential (AP) trains induced by injecting a 90-pA current. The shaded area indicating the first 300 ms. Right, number of APs (genotype: F(1,19)=5.484, P=0.03; current: F(6,114)=77.18, P<0.001; genotype × current interaction: F(6,114)=3.113, P=0.007); (a2) rheobase current (t(19)=1.873, P=0.076); (a3) resting membrane potential (t(19)=0.175, P=0.863); (a4) input resistance (t(19)=2.632, P=0.0164); (a5) AP threshold (t(19)=0.475, P=0.640). n=10 from 3 wild-type (WT) mice; n=11 from 3 Adipo−/− mice. *P<0.05, #P=0.076, **P<0.01 and ***P<0.001 compared with WT littermates. (b) Decreased excitability of DG neurons in WT mice after bath application of 15 μM AdipoRon. (b1) Left, action potential trains elicited by injecting a 90-pA current. Right, number of AP (treatment: F(1,9)=8.757, P=0.016; current: F(6,54)=34.61, P<0.001; treatment × current interaction: F(6,54)=7.338, P<0.001); (b2) rheobase current (t(9)=2.449, P=0.0368); (b3) resting membrane potential (t(9)=3.496, P=0.0068); (b4) input resistance (t(9)=1.533, P=0.159); (b5) AP threshold (t(9)=0.235, P=0.819). n=10 from 4 mice. *P<0.05, **P<0.01 and ***P<0.001 compared with artificial cerebrospinal fluid (aCSF) treatment. (c) Decreased excitability of DG neurons in Adipo−/− mice by bath application of 15 μM AdipoRon. (c1) Left, action potential trains induced by injecting a 90-pA current. Right, number of APs (treatment: F(1,8)=8.475, P=0.02; current: F(6,48)=24.30, P<0.001; treatment × current interaction: F(6,48)=5.846, P<0.001); (c2) rheobase current (t(8)=3.500, P=0.0081); (c3) resting membrane potential (t(8)=5.487, P=0.0006); (c4) input resistance (t(8)=1.688, P=0.130); (c5) AP threshold (t(8)=1.294, P=0.232). n=9 from 4 mice. **P<0.01 and ***P<0.001 compared with aCSF treatment. Error bars represent s.e.m.
Next, we tested whether adiponectin acutely modulates intrinsic excitability of DG granule neurons. A baseline recording of DG granule cells from WT mouse brain slices was first made followed by bath application of 15 μM AdipoRon, an AdipoR agonist that binds to both AdipoR1 and AdipoR2 with comparable high affinities.45 In response to depolarizing current injections (300 ms), AdipoRon treatment decreased the excitability of DG granule neurons, causing reduced number of action potentials elicited by current injections (60–90 pA) (Figure 2b1), increased rheobase current (Figure 2b2) and a more negative resting membrane potential (Figure 2b3) without changing other membrane properties including input resistance, action potential threshold, fAHP and action potential waveforms (Figures 2b4 and Supplementary Table 2). To determine whether hyperexcitability of DG neurons in Adipo−/− mice can be reversed by AdipoRon, we examined the effects of bath application of AdipoRon. AdipoRon significantly decreased the number of action potentials (Figure 2c1), increased rheobase (Figure 2c2) and caused the resting membrane potential to be more negative (Figure 2c3) without changing other membrane properties (Figures 2c4 and Supplementary Table 2), similar to those observed in WT mice. These data suggest that AdipoRon acutely suppress the excitability of DG granule neurons.
Normal contextual fear conditioning and extinction in mice lacking AdipoR1 in the DG
To determine what role AdipoR1 has in the formation and extinction of contextual fear, we generated AdipoR1 floxed mice with conditional alleles carrying loxP sites inserted using a gene-targeting strategy (Figure 3a). The AdipoR1flox/flox mice were cross-bred with POMC-Cre mice to generate AdipoR1POMC-Cre mice.39 POMC-Cre-mediated excision was detected primarily in the DG in Ai14 tdTomato reporter mice (Figure 3b). Deletion of AdipoR1 exon 2 was confirmed by reverse transcriptase-PCR in dissected DG tissue from AdipoR1POMC-Cre mice (Figure 3b). The deletion of exon 2 led to a frame shift and the generation of a null mutant of AdipoR1. AdipoR1POMC-Cre mice were similar to WT littermate controls in freezing responses during contextual fear conditioning. There was no difference in freezing responses during the first 3 min of the initial extinction training E1 and the freezing levels over the 4-day extinction training (E1–4) were comparable between AdipoR1POMC-Cre mice and WT littermate controls (Figure 3b). Mice were then given two additional days of extinction training, in order to determine whether a greater number of training sessions might reveal some effects of AdipoR1 conditional knockout. However, no significant difference in freezing responses was observed between the two genotypes following extended training sessions (E5–6) (Figure 3b).
Deletion of AdipoR1 in the dentate gyrus (DG) does not affect the formation or extinction of contextual fear memory. (a) Generation of AdipoR1-floxed and conditional knockout mice. Left, schematic drawing of the wild-type (WT) allele, targeting vector, the floxed AdipoR1 allele, Frt recombination and Cre recombination. Coding exons (white boxes) and the loxP sites (red arrowheads) are indicated. The targeting vector contains homologous DNA fragments to the AdipoR1 WT allele (red dashed lines), to facilitate homologous recombination. The positions of diagnostic Southern blot probes (red bars) and the sizes of the restriction fragments are indicated. Right, Southern blot showing the WT and AdipoR1 floxed alleles. (b) AdipoR1 deletion induced by Cre recombinase under the control of the proopiomelanocortin promoter (POMC-Cre). Left top panel, POMC-Cre-mediated excision in a Ai14-tdTomato reporter mouse. Left bottom panel, POMC-Cre-mediated deletion of AdipoR1 exon 2 (AdipoR1POMC-Cre) in the DG confirmed by reverse transcriptase-PCR. AdipoR1POMC-Cre mice displayed normal contextual fear acquisition (genotype: F(1,14)=0.176, P=0.681; shock: F(4,56)=150.7, P<0.001; genotype × shock interaction: F(4,56)=1.846, P=0.133), retrieval (t(14)=0.203, P=0.842) and extinction (genotype: F(1,14)=0.387, P=0.544; day: F(5,70)=27.92, P<0.001; genotype × day interaction: F(5,70)=0.153, P=0.979). fWT, n=9; AdipoR1POMC-Cre, n=7. (c) AAV-Cre-mediated deletion of AdipoR1 in adult DG. Left, stereotactic injection of AAV-Cre-GFP in the DG. GCL, granule cell layer. Mice received intra-DG injection of AAV-Cre-GFP (n=9) and AAV-GFP (n=7) showed comparable levels of freezing during contextual fear acquisition (treatment: F(1,14)=0.695, P=0.419; shock: F(4,56)=145.4, P<0.001; treatment × shock interaction: F(4,56)=0.472, P=0.756), retrieval (t(14)=0.7824, P=0.447) and extinction learning (treatment: F(1,14)=0.102, P=0.754; day: F(5,70)=30.54, P<0.001; treatment × day interaction: F(5,70)=1.318, P=0.267). Error bars represent s.e.m.
The possibility of compensatory changes during development could mask a phenotype and produce false-negative results. To rule out this possibility, we used AAV-Cre-GFP to induce deletion of AdipoR1 in the DG in adult mice. AdipoR1flox/flox mice received bilateral intra-DG injection of AAV-Cre-GFP or AAV-GFP. Three weeks after AAV injection in the DG, mice were subjected to contextual fear conditioning followed by extinction training for 6 days. There were no detectable differences in the acquisition, retrieval and extinction between AAV-Cre-GFP- and AAV-GFP-injected mice (Figure 3c). AAV-Cre-mediated knockdown of AdipoR1 in the DG was confirmed using western blotting 3 weeks after virus injection in AdipoR1flox/flox mice (Supplementary Figure 2).
AdipoR2 knockout mice exhibit abnormal contextual fear responses
To study the role of AdipoR2 in contextual fear memory, AdipoR2 knockout (AdipoR2−/−) mice and WT littermates received contextual fear-conditioning training followed by 4 days of extinction training (one session per day). AdipoR2−/− mice exhibited normal freezing responses during contextual fear conditioning but exaggerated freezing responses to the conditioning context during the first 3 min of extinction training session E1 (Figure 4a). Increased freezing levels were sustained across the extinction training sessions (Figure 4a). Owing to higher freezing responses in the first session of extinction (E1) in AdipoR2−/− mice compared with WT littermate controls, freezing levels were normalized to that of extinction session E1 for each group. AdipoR2−/− mice showed a slower rate of contextual fear extinction over the 4-day course of extinction training (Figure 4a). These results indicate that AdipoR2 knockout leads to enhanced and sustained expression of contextual fear but impaired extinction learning. These behavioral changes were not associated with increased anxiety measured in the elevated plus maze test (Supplementary Figure 3a) or caused by abnormal nociceptive responses and visual function (Supplementary Figure 3b,c).
AdipoR2 knockout causes abnormal contextual fear responses and resistance to the facilitating effect of intra-dentate gyrus (DG) adiponectin on contextual fear extinction. (a) AdipoR2−/− mice showed normal contextual fear acquisition (genotype: F(1,20)=1.292, P=0.269; shock: F(4,80)=159.1, P<0.001; genotype × shock interaction: F(4,80)=0.679, P=0.609), but elevated levels of freezing during fear retrieval (t(20)=3.648 P=0.0016) and fear extinction (Freezing time; genotype: F(1,20)=38.16, P<0.001; day: F(3,60)=130.0, P<0.001; genotype × day interaction: F(3,60)=2.043, P=0.118) and slower extinction rate (% of initial freezing; genotype: F(1,20)=5.895, P=0.0247; day: F(3,60)=126.9, P<0.001; genotype × day interaction: F(3,60)=3.102, P=0.033). Wild type (WT), n=11; AdipoR2−/−, n=11. *P<0.05, **P<0.01 and ***P<0.001 compared with WT. (b) AdipoR2−/− mice and WT mice bilaterally cannulated in the DG showed similar levels of freezing during contextual fear conditioning (genotype: F(1,19)=1.626, P=0.218; shock: F(4,76)=95.7, P<0.001; genotype × shock interaction: F(4,76)=2.218, P=0.075). Subsequently, the WT mice received intra-DG infusions of vehicle (WT-Veh, n=7) and AdipoR2−/− mice received intra-DG infusions of vehicle (AdipoR2−/−-Veh, n=6) or adiponectin (AdipoR2−/−-Adipo, n=8) for 3 days, followed by 4 days of extinction training (E1–E4) with daily intra-DG infusion 30 min before re-exposure to the context. AdipoR2−/− mice displayed increased levels of freezing during contextual fear retrieval (F(2,18)=4.940, P=0.0195) and extinction (Freezing time; treatment: F(2,18)=12.083, P<0.001; day: F(3,54)=55.34, P<0.001; treatment × day interaction: F(6,54)=1.358, P=0.248). There was no difference in extinction rates between three treatment groups (% initial freezing; treatment: F(2,18)=3.344, P=0.058; day: F(3,54)=53.36, P<0.001; treatment × day interaction: F(6,54)=1.831, P=0.110). *P<0.05 and **P<0.01 compared with the WT-Veh group. *P<0.05 and **P<0.01, AdipoR2−/−-Veh versus WT-Veh; #P<0.05, ###P<0.001, AdipoR2−/−-Adipo versus WT-Veh. Error bars represent s.e.m.
Intra-DG adiponectin fails to facilitate contextual fear extinction in AdipoR2 knockout mice
To address whether AdipoR2 mediates the effects of intra-DG adiponectin on contextual fear extinction, AdipoR2−/− mice were bilaterally cannulated in the DG and subjected to the same contextual fear-conditioning and extinction-training paradigms as did in the study of intra-DG adiponectin injection in WT mice. In addition, the WT littermates of AdipoR2−/− mice were included as controls for stereotaxic cannulation and microinjection procedures. Chronically cannulated AdipoR2−/− mice and their WT littermate controls exhibited similar freezing levels during contextual fear conditioning (Figure 4b). Subsequently, fear-conditioned mice received intra-DG infusions of adiponectin or vehicle for 3 days. AdipoR2−/− mice that received either intra-DG adiponectin or intra-DG vehicle showed higher freezing levels in response to the conditioning context during the first 3 min of extinction training session E1, as compared with vehicle-infused WT littermate controls (Figure 4b). AdipoR2−/− mice exhibited higher freezing levels than WT controls during extinction training and showed no difference between adiponectin- and vehicle-treated groups (Figure 4b). Furthermore, freezing levels were normalized to that of the first extinction session E1 for each group. The rate of extinction of adiponectin-infused AdipoR2−/− mice was comparable to that of vehicle-infused AdipoR2−/− mice (Figure 4b). These results indicate that AdipoR2−/− mice are resistant to the effects of intra-DG adiponectin on contextual fear extinction.
AdipoR2 knockout mice display hyperexcitability of DG granule neurons
Next, we determined whether AdipoR2 knockout affects intrinsic excitability of DG granule neurons. As observed in Adipo−/− mice, DG granule neurons from AdipoR2−/− mice were hyperexcitable in response to depolarizing current injections (300 ms) (Figure 5a), displaying a greater number of action potentials (Figure 5a1), lower rheobase (Figure 5a2) and higher input resistance (Figure 5a4) than DG neurons from WT littermates. In addition, AdipoR2−/− DG neurons showed a lower action potential threshold (Figure 5a5). Resting membrane potential (Figure 5a3), fAHP and the half-width and amplitude of action potentials did not differ significantly between the genotypes (Supplementary Table 1).
AdipoR2 deletion enhances intrinsic excitability of dentate gyrus (DG) granule neurons and abolishes the inhibitory effect of AdipRon. (a) Enhanced intrinsic excitability of DG granule neurons in AdipoR2−/− mice. (a1) Left, representative action potential trains induced by injecting a 90-pA current. The shaded area indicating the first 300 ms. Right, a greater number of action potentials was elicited by depolarizing current injection in AdipoR2−/− mice (genotype: F(1,27)=7.257, P=0.012; current: F(6,162)=45.11, P<0.001; genotype × current interaction: F(6,162)=5.23, P<0.001); (a2) rheobase current (t(27)=2.631, P=0.0139); (a3) resting membrane potential (t(27)=1.790, P=0.0846); (a4) input resistance (t(27)=2.307, P=0.029); (a5) action potential threshold (t(22)=2.653, P=0.0145). n=14 from 4 wild-type (WT) mice; n=15 from 4 AdipoR2−/− mice. *P<0.05, **P<0.01 and ***P<0.001 compared with WT. (b) Bath application of 15 μM AdipoRon failed to decrease excitability of DG granule neurons (n=9 from 3 mice) in AdipoR2−/− mice. (b1) Left, action potential trains induced by injecting a 90-pA current. Right, number of action potentials (treatment: F(1,8)=0.217, P=0.654; current: F(6,48)=40.26, P<0.001; treatment × current interaction: F(6,48)=0.545, P=0.771); (b2) rheobase (t(8)=0.359, P=0.729); (b3) resting membrane potential (t(8)=1.852, P=0.101); (b4) input resistance (t(8)=2.037, P=0.076); (b5) action potential threshold (t(8)=0.8162, P=0.438). Error bars represent s.e.m.
AdipoRon fails to modulate intrinsic excitability of DG granule neurons in AdipoR2 knockout mice
To determine whether AdipoR2 mediates the inhibitory effect of adiponectin on intrinsic excitability of DG granule neurons, DG granule cells from AdipoR2−/− mouse brain slices was first recorded under baseline, followed by bath application of 15 μM AdipoRon. AdipoRon failed to induce any significant change in intrinsic excitability of DG granule neurons (Figure 5b1). Intrinsic membrane properties including rheobase, resting membrane potential, input resistance and action potential threshold (Figures 5b2), as well as fAHP and action potential waveforms were not significantly altered by AdipoRon (Supplementary Table 2). Taken together, these data indicate that AdipoR2 is required for adiponectin actions on DG granule neuron excitability.
Discussion
Adipose tissue is now considered as an active and important endocrine organ, secreting a number of protein hormones (adipokines) into the circulation.46 Adiponectin is the most abundant adipokine in the circulation and can cross the blood–brain barrier to target neurons directly,27, 28, 33, 47 thus presumably participating in various brain functions under physiological and pathological states. In this study, we report the identification of a novel role for adiponectin in fear memories. Adiponectin deficiency did not affect acquisition of contextual fear but impaired extinction learning. By contrast, intra-DG infusions of adiponectin facilitated contextual fear extinction. Deletion of AdipoR2, but not AdipoR1, enhanced fear expression and resulted in resistance to intra-DG adiponectin-induced facilitation of fear extinction. Furthermore, the cellular mechanisms underlying behavioral changes may involve modulation of intrinsic excitability of DG granule neurons. We believe these findings provide the first evidence that adiponectin and AdipoR2 have important roles in the regulation of fear memories and DG neuronal excitability.
Our previous studies have shown that adiponectin insufficiency increases susceptibility to stress-induced depressive behaviors, including social defeat-induced social avoidance.33 The avoidance is also a common feature of PTSD. In this study, we used a classical Pavlovian fear-conditioning model, in which animals learn to associate a particular context with footshock. Extinction occurs when the animal is repeatedly exposed to the context without footshock after conditioning.48, 49, 50 Extinction is thought to be a new learning process to form ‘inhibitory association’ between the context and footshock.49, 50, 51 Using this paradigm, we found that mice deficient for adiponectin exhibited normal contextual fear conditioning but impaired extinction learning. These data suggest that adiponectin is necessary for extinction but not for acquisition of contextual fear memory. By contrast, infusions of adiponectin directly into the DG before extinction training facilitated extinction of contextual fear, suggesting that increasing adiponectin levels in the DG is sufficient to accelerate fear extinction. Because of high levels of expression of both AdipoR1 and AdipoR2 in the DG, the question was then raised as to which adiponectin receptor subtype(s) mediate the effects of adiponectin on contextual fear memories. AdipoR1 is widely distributed in the brain;33 therefore, we generated AdipoR1 floxed mice in order to achieve neuron- and/or region-specific gene deletion. We found that deletion of AdipoR1 from DG neurons had little or no effect on acquisition, retrieval and extinction of contextual fear. This is unlikely due to developmental compensation/adaptation, as similar results were obtained from mice with AAV-Cre-mediated deletion of AdipoR1 in adult DG. In contrast to AdipoR1, expression of AdipoR2 is restricted largely to the DG with weak-to-moderate levels in a few other brain regions such as the hypothalamus.27, 33 AdipoR2 knockout mice exhibited normal acquisition but increased retrieval/expression of contextual fear memories and slowed fear extinction rate. These behavioral phenotypes overlapped with, but were not identical to, those observed in mice deficient in adiponectin. Disruption of both AdipoR1 and AdipoR2 abolishes adiponectin binding, suggesting that they are the major receptors for adiponectin in vivo.52 As AdipoR1 conditional knockout mice showed no detectable phenotype, one would expect mice deficient in adiponectin or AdipoR2 to have similar phenotypes. In vitro studies have reported that AdipoR1 and AdipoR2 are capable of forming homomers and heteromers, and have distinct adiponectin binding affinities and functional signaling preferences.32, 52, 53, 54 Although AdipoR1 activates AMP-activated protein kinase, AdipoR2 induces peroxisome proliferator-activated receptor-α signaling.32, 38, 53 Interestingly, the presence of AdipoR1/AdipoR2 heteromers provokes a delay in the onset of adiponectin-induced AMP-activated protein kinase activation.53 Presumably, deletion of AdipoR2 would increase AdipoR1 homodimerization and improve AdipoR1 signaling on adiponectin stimulation. However, the lack of behavioral responses of AdipoR2 knockout mice to intra-DG adiponectin argues against a possible role of AdipoR1 in this region in mediating the effects of adiponectin, but supports the essential role of AdipoR2. One alternative explanation for the enhanced retrieval/expression of contextual fear in AdipoR2 knockout mice but not in adiponectin-deficient mice could be due to adiponectin action via AdipoR1 outside the DG counteracting its effect via AdipoR2 within the DG.
Fear conditioning and extinction have been shown to differentially modulate intrinsic neuronal excitability in several brain structures including the hippocampus.55, 56, 57, 58, 59, 60 Pharmacological and genetic modifications of intrinsic excitability are associated with the strength of new learning.61, 62, 63, 64 The intrinsic excitability of a neuron determines the output of that neuron, thus endowing the cell with a unique functional identity.65 The DG functions as a gate to filter out aberrant or excessive excitatory input to the hippocampus66, 67 and the unique intrinsic membrane properties of granule neurons participate in governing resistance to excessive excitation.68 In order to understand the cellular mechanisms underlying adiponectin action on extinction learning and fear memories, we investigated the intrinsic membrane excitability of DG granule neurons in brain slices. We found that mice deficient in adiponectin displayed enhanced intrinsic excitability of DG granule neurons. Similar changes were also seen in DG granule neurons from AdipoR2 knockout mice. This intrinsic hyperexcitability in both mutant mouse lines was reflected by an increased number of action potentials evoked by the depolarizing intracellular current injection and was attributed to an increase in input resistance. A lower action potential threshold could also contribute to AdipoR2 knockout-induced intrinsic hyperexcitability. In contrast, acute bath application of AdipoRon, a small-molecule adiponectin mimetic45, decreased intrinsic excitability of DG granule neurons. However, such effect was not caused by a decrease in input resistance but by a more negative resting membrane potential. Together, these results suggest that the opposing effects of loss of endogenous adiponectin/AdipoR2 and acute AdipoR agonist treatment on DG neuronal excitability are mediated via modulating distinct intrinsic membrane properties. Although the underlying molecular mechanisms remain to be investigated, the increase in input resistance observed in adiponectin and AdipoR2 knockout mice could be the result of a decrease in total membrane surface area. This notion is supported by our recent study, demonstrating a reduction in dendritic branching and length in DG granule neurons of adiponectin-deficient mice (D. Zhang, X. Wang and X.Y. Lu, unpublished observations) and the evidence for a functional link between dendritic structural degeneration and neuronal hyperexcitability.69 However, a change in total membrane surface area of DG neurons is unlikely to occur following acute AdipoRon application in minutes. This might explain the unaltered input resistance in response to AdipoRon. Instead, AdipoRon was found to induce hyperpolarization of the resting membrane potential, consequently rendering the neuron less excitable. The possible mechanisms may involve modulating voltage-independent ‘leak’ potassium channels, which are essential for establishing the resting membrane potential.70 Supporting evidence comes from the report showing that adiponectin hyperpolarizes a subset of hypothalamic neurons through the activation of voltage-independent potassium current.71 Although AdipoR2 knockout and AdipoRon treatment affected excitability of DG granule neurons via different modifications of intrinsic membrane properties, AdipoR2 knockout abolished the effect of AdipoRon. This is consistent with the absence of behavioral responses of AdipoR2 knockout mice to intra-DG infusions of adiponectin. The modulation of intrinsic excitability may explain the behavioral alterations observed in mice deficient in adiponectin or AdipoR2 and following intra-DG administration of adiponectin after fear conditioning. Although it is currently unknown whether fear conditioning and extinction modulate intrinsic excitability of DG granule neurons, studies have shown that DG neurons are activated during contextual fear conditioning and on re-exposure to the conditioning context.41 Optogenetic reactivation of DG neurons that are activated during contextual fear conditioning is sufficient to drive the recall of fear memory.21, 22 Based on our data, we hypothesize that enhancing or dampening DG neuronal activation evoked by a fear-inducing context would cause resistance to or acceleration of fear extinction. However, this hypothesis requires further investigation in future studies.
In summary, our results support a functional link between adiponectin/AdipoR2 activation, intrinsic excitability of DG granule neurons and extinction of contextual fear. Exposure-based therapy for PTSD is thought to rely on extinction mechanisms.72, 73 However, this therapy produces only temporary PTSD remission. Enhancing the formation and retention of extinction memory will therefore be critical to solving this challenge. Our current findings suggest that increasing adiponectin production or activating AdipoR2 receptors might be useful for facilitating extinction-based exposure treatments for PTSD and other trauma- and stress-related disorders.
References
Milad MR, Orr SP, Pitman RK, Rauch SL . Context modulation of memory for fear extinction in humans. Psychophysiology 2005; 42: 456–464.
Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther 2011; 17: 227–236.
Maren S, Phan KL, Liberzon I . The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14: 417–428.
Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci 2014; 34: 13435–13443.
Ji J, Maren S . Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 2007; 17: 749–758.
Goosens KA . Hippocampal regulation of aversive memories. Curr Opin Neurobiol 2011; 21: 460–466.
Phillips RG, LeDoux JE . Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106: 274–285.
Corcoran KA, Maren S . Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 2001; 21: 1720–1726.
Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 2013; 77: 955–968.
Kim JJ, Fanselow MS . Modality-specific retrograde amnesia of fear. Science 1992; 256: 675–677.
Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry 2010; 67: 296–303.
Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 2002; 52: 119–125.
Smith ME . Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus 2005; 15: 798–807.
Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003; 160: 924–932.
Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40: 1091–1099.
Hsu D . The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res 2007; 163: 601–613.
Sekeres MJ, Mercaldo V, Richards B, Sargin D, Mahadevan V, Woodin MA et al. Increasing CRTC1 function in the dentate gyrus during memory formation or reactivation increases memory strength without compromising memory quality. J Neurosci 2012; 32: 17857–17868.
Deng W, Saxe MD, Gallina IS, Gage FH . Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 2009; 29: 13532–13542.
Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J 2011; 30: 4071–4083.
Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci 2007; 10: 1012–1019.
Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 2012; 484: 381–385.
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL et al. Creating a false memory in the hippocampus. Science 2013; 341: 387–391.
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF . A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270: 26746–26749.
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K . cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 1996; 221: 286–289.
Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 2003; 278: 9073–9085.
Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 2003; 278: 40352–40363.
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 2007; 6: 55–68.
Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA 2014; 111: 15810–15815.
Kusminski CM, McTernan PG, Schraw T, Kos K, O'Hare JP, Ahima R et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 2007; 50: 634–642.
Neumeier M, Weigert J, Buettner R, Wanninger J, Schaffler A, Muller AM et al. Detection of adiponectin in cerebrospinal fluid in humans. Am J Physiol Endocrinol Metab 2007; 293: E965–E969.
Ebinuma H, Miida T, Yamauchi T, Hada Y, Hara K, Kubota N et al. Improved ELISA for selective measurement of adiponectin multimers and identification of adiponectin in human cerebrospinal fluid. Clin Chem 2007; 53: 1541–1544.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762–769.
Liu J, Guo M, Zhang D, Cheng SY, Liu M, Ding J et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc Natl Acad Sci USA 2012; 109: 12248–12253.
Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M et al. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology 2010; 35: 846–857.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082.
Rothbaum BO, Davis M . Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 2003; 1008: 112–121.
Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006; 281: 2654–2660.
Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 2007; 56: 583–593.
McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 2007; 317: 94–99.
Hagihara H, Toyama K, Yamasaki N, Miyakawa T . Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp 2009 33: 1543.
Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 2013; 83: 189–201.
Wang X, Zhang D, Lu XY . Dentate gyrus-CA3 glutamate release/NMDA transmission mediates behavioral despair and antidepressant-like responses to leptin. Mol Psychiatry 2015; 20: 509–519.
Guo M, Huang TY, Garza JC, Chua SC, Lu XY . Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol 2013; 16: 857–867.
Ouyang M, Thomas SA . A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA 2005; 102: 9347–9352.
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013; 503: 493–499.
Kershaw EE, Flier JS . Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556.
Zhang D, Guo M, Zhang W, Lu XY . Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem 2011; 286: 44913–44920.
Maren S . Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron 2010; 70: 830–845.
Myers KM, Davis M . Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120–150.
Bouton ME . Context and behavioral processes in extinction. Learn Mem 2004; 11: 485–494.
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A . Neuronal circuits of fear extinction. Eur J Neurosci 2010; 31: 599–612.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007; 13: 332–339.
Almabouada F, Diaz-Ruiz A, Rabanal-Ruiz Y, Peinado JR, Vazquez-Martinez R, Malagon MM . Adiponectin receptors form homomers and heteromers exhibiting distinct ligand binding and intracellular signaling properties. J Biol Chem 2013; 288: 3112–3125.
Kosel D, Heiker JT, Juhl C, Wottawah CM, Bluher M, Morl K et al. Dimerization of adiponectin receptor 1 is inhibited by adiponectin. J Cell Sci 2010; 123 (Pt 8): 1320–1328.
Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E . A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex 2006; 16: 460–468.
Moyer JR Jr., Thompson LT, Disterhoft JF . Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 1996; 16: 5536–5546.
Santini E, Quirk GJ, Porter JT . Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 2008; 28: 4028–4036.
Kaczorowski CC, Disterhoft JF . Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem 2009; 16: 362–366.
McKay BM, Matthews EA, Oliveira FA, Disterhoft JF . Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol 2009; 102: 2763–2770.
Song C, Detert JA, Sehgal M, Moyer JR Jr . Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol 2012; 107: 3397–3408.
Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA et al. Neuronal competition and selection during memory formation. Science 2007; 316: 457–460.
Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J et al. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 2009; 12: 1438–1443.
Santini E, Porter JT . M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci 2010; 30: 12379–12386.
Santini E, Sepulveda-Orengo M, Porter JT . Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 2012; 37: 2047–2056.
Schulz DJ . Plasticity and stability in neuronal output via changes in intrinsic excitability: it's what's inside that counts. J Exp Biol 2006; 209: 4821–4827.
Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL . The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl 1992; 7: 273–280.
Lothman EW, Stringer JL, Bertram EH . The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl 1992; 7: 301–313.
Fricke RA, Prince DA . Electrophysiology of dentate gyrus granule cells. J Neurophysiol 1984; 51: 195–209.
Siskova Z, Justus D, Kaneko H, Friedrichs D, Henneberg N, Beutel T et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron 2014; 84: 1023–1033.
Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M . Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 2001; 409: 88–92.
Hoyda TD, Ferguson AV . Adiponectin modulates excitability of rat paraventricular nucleus neurons by differential modulation of potassium currents. Endocrinology 2010; 151: 3154–3162.
McNally RJ . Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev 2007; 27: 750–759.
Hermans D, Craske MG, Mineka S, Lovibond PF . Extinction in human fear conditioning. Biol Psychiatry 2006; 60: 361–368.
Acknowledgements
This work was supported by NIH grants MH100583 and MH076929 (X-YL) and a NARSAD Independent Investigator Award (X-YL). JCG was supported by a NRSA predoctoral fellowship MH083442, and R.B. was supported by NSF grant 1456862. A patent application has been filed by The University of Texas Health Science Center at San Antonio.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, D., Wang, X., Wang, B. et al. Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Mol Psychiatry 22, 1044–1055 (2017). https://doi.org/10.1038/mp.2016.58
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.58
This article is cited by
-
Liver-specific adiponectin gene therapy suppresses microglial NLRP3-inflammasome activation for treating Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Peroxisome proliferator-activated receptor-α activation facilitates contextual fear extinction and modulates intrinsic excitability of dentate gyrus neurons
Translational Psychiatry (2023)
-
Increased intrinsic and synaptic excitability of hypothalamic POMC neurons underlies chronic stress-induced behavioral deficits
Molecular Psychiatry (2023)
-
Protective effects of intracerebroventricular adiponectin against olfactory impairments in an amyloid β1–42 rat model
BMC Neuroscience (2021)
-
Modulation of depression-related behaviors by adiponectin AdipoR1 receptors in 5-HT neurons
Molecular Psychiatry (2021)