Abstract
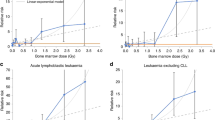
Risks of acute myeloid leukemia (AML) and/or myelodysplastic syndromes (MDS) are known to increase after cancer treatments. Their rise-and-fall dynamics and their associations with radiation have, however, not been fully characterized. To improve risk definition we developed SEERaBomb R software for Surveillance, Epidemiology and End Results second cancer analyses. Resulting high-resolution relative risk (RR) time courses were compared, where possible, to results of A-bomb survivor analyses. We found: (1) persons with prostate cancer receiving radiation therapy have increased RR of AML and MDS that peak in 1.5–2.5 years; (2) persons with non-Hodgkin lymphoma (NHL), lung and breast first cancers have the highest RR for AML and MDS over the next 1–12 years. These increased RR are radiation specific for lung and breast cancer but not for NHL; (3) AML latencies were brief compared to those of A-bomb survivors; and (4) there was a marked excess risk of acute promyelocytic leukemia in persons receiving radiation therapy. Knowing the type of first cancer, if it was treated with radiation, the interval from first cancer diagnosis to developing AML or MDS, and the type of AML, can improve estimates of whether AML or MDS cases developing in this setting are due to background versus other processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gale RP, Bennett JM, Hoffman FO, Therapy-related AML . a slip of the lip can sink a ship. Leuk Res 2014; 38: 418–420.
SEER. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973–2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch http://www.seer.cancer.gov, released April 2015, based on the November 2014 submission. 2015.
Mukherjee S, Reddy CA, Ciezki JP, Abdel-Wahab M, Tiu RV, Copelan E et al. Risk for developing myelodysplastic syndromes in prostate cancer patients definitively treated with radiation. J Natl Cancer Inst 2014; 106: djt462.
Armitage JO, Carbone PP, Connors JM, Levine A, Bennett JM, Kroll S . Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol 2003; 21: 897–906.
Kaplan HG, Malmgren JA, Atwood MK . Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer 2011; 11: 260.
Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 2011; 12: 353–360.
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood 2013; 121: 2996–3004.
Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res 2013; 179: 361–382.
Iwanaga M, Hsu WL, Soda M, Takasaki Y, Tawara M, Joh T et al. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors. J Clin Oncol 2011; 29: 428–434.
Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A et al. Cancer incidence in atomic bomb survivors Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res 1994; 2/1994 137: S68–S97.
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014; 28: 241–247.
Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074.
Hastie T, Tibshirani R . Generalized additive models for medical research. Stat Methods Med Res 1995; 4: 187–196.
Ihaka R, Gentleman R . R: a language for data analysis and graphics. J Comput Graph Stat 1996; 5: 299–314.
Garwood F . Fiducial limits for the Poisson distribution. Biometrika 1936; 28: 437–442.
Matsuo T, Tomonaga M, Bennett JM, Kuriyama K, Imanaka F, Kuramoto A et al. Reclassification of leukemia among A-bomb survivors in Nagasaki using French-American-British (FAB) classification for acute leukemia. Jpn J Clin Oncol 1988; 18: 91–96.
Radivoyevitch T, Ramsey MJ, Tucker JD . Estimation of the target stem-cell population size in chronic myeloid leukemogenesis. Radiat Environ Biophys 1999; 38: 201–206.
Tomonaga M . Leukaemia in Nagasaki atomic bomb survivors from 1945 through 1959. Bull World Health Organ 1962; 26: 619–631.
Radivoyevitch T, Kozubek S, Sachs RK . Biologically based risk estimation for radiation-induced CML. Inferences from BCR and ABL geometric distributions. Radiat Environ Biophys 2001; 40: 1–9.
Radivoyevitch T, Hlatky L, Landaw J, Sachs RK . Quantitative modeling of chronic myeloid leukemia: insights from radiobiology. Blood 2012; 119: 4363–4371.
Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR et al. Sex differences in the incidence of chronic myeloid leukemia. Radiat Environ Biophys 2014; 53: 55–63.
Gale RP, Hlatky L, Sachs RK, Radivoyevitch T . Why is there so much therapy-related AML and MDS and so little therapy-related CML? Leuk Res 2014; 38: 1162–1164.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N Engl J Med 2014; 37: 2488–2498.
Varadarajan R, Ford L, Sait SN, Block AW, Barcos M, Wallace PK et al. Metachronous and synchronous presentation of acute myeloid leukemia and lung cancer. Leuk Res 2009; 33: 1208–1211.
Pirani M, Marcheselli R, Marcheselli L, Bari A, Federico M, Sacchi S . Risk for second malignancies in non-Hodgkin's lymphoma survivors: a meta-analysis. Ann Oncol 2011; 22: 1845–1858.
Wallner LP, Wang R, Jacobsen SJ, Haque R . Androgen deprivation therapy for treatment of localized prostate cancer and risk of second primary malignancies. Cancer Epidemiol Biomarkers Prev 2013; 22: 313–316.
Carreras E, Kovats S . Girl power: estrogen promotes HSC self-renewal. Cell Stem Cell 2014; 14: 137–138.
Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 2014; 505: 555–558.
Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S et al. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat Res 1994; 137: S17–S67.
Cox DR . Regression models and life tables. J R Stat Soc B 1972; 34: 187–220.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Acknowledgements
We thank Drs Li Li and Cornelis Van Noorden for suggestions and the R community for software. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We used data obtained from the Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan. RERF is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE), the latter in part through DOE award DE-HS0000031 to the National Academy of Sciences. Conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of RERF or its funding agencies. This work was supported by: the Cleveland Clinic, the National Aeronautics and Space Administration (NNJ13ZSA001N) (TR), the National Institute for Health Research Biomedical Research Centre funding scheme (RPG), and the Academic Medical Center of Amsterdam (RJM).
Author contributions
JPM initiated and motivated the study. TR developed the software and wrote the first draft. RKS, DJB and RPG helped with the A-bomb data analyses. RJM helped with SEER*Stat comparisons. RPG, JPM, MAS, BTH, RJM, HEC and SM contributed feedback/guidance and writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Radivoyevitch, T., Sachs, R., Gale, R. et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia 30, 285–294 (2016). https://doi.org/10.1038/leu.2015.258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.258
This article is cited by
-
Therapy-related myeloid neoplasms with different latencies: a detailed clinicopathologic analysis
Modern Pathology (2022)
-
Role of Germline Predisposition to Therapy-Related Myeloid Neoplasms
Current Hematologic Malignancy Reports (2022)
-
Incidence rate and factors associated with the development of secondary cancers after radioiodine therapy in differentiated thyroid cancer: a multicenter retrospective study
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
Effects of exposure to low-dose ionizing radiation on changing platelets: a prospective cohort study
Environmental Health and Preventive Medicine (2021)
-
Risk of hematologic malignancies after breast ductal carcinoma in situ treatment with ionizing radiation
npj Breast Cancer (2021)