Abstract
Tiller angle of rice (Oryza sativa L.) is an important agronomic trait that contributes to grain production, and has long attracted attentions of breeders for achieving ideal plant architecture to improve grain yield. Although enormous efforts have been made over the past decades to study mutants with extremely spreading or compact tillers, the molecular mechanism underlying the control of tiller angle of cereal crops remains unknown. Here we report the cloning of the LAZY1 (LA1) gene that regulates shoot gravitropism by which the rice tiller angle is controlled. We show that LA1, a novel grass-specific gene, is temporally and spatially expressed, and plays a negative role in polar auxin transport (PAT). Loss-of-function of LA1 enhances PAT greatly and thus alters the endogenous IAA distribution in shoots, leading to the reduced gravitropism, and therefore the tiller-spreading phenotype of rice plants.
Similar content being viewed by others
Introduction
Gravity is one of the fundamental factors that affect a number of biological processes. Plants, to compensate their sessile nature, adapt to gravity by directing shoots upwards and roots downwards termed negative and positive gravitropisms, respectively. Significant progresses on the molecular mechanisms of plant gravitropism have been achieved in the past years using Arabidopsis as a model 1. Although genetic and phenotypic studies have indicated that the molecular mechanisms underlying the gravitropic response in roots, hypocotyles and inflorescence stems are not uniform 2, the gravitropic response is suggested to proceed through three major conserved steps: gravity perception, signal transduction and transmission, and growth response 3, 4.
In Arabidopsis, root cap and shoot endodermis are responsible for sensing gravity in plant roots and shoots, respectively 5, 6. The displacement of amyloplasts is thought to be the main event that triggers further signal transduction. Phytohormones 7, 8, 9, 10, cytosolic ions such as Ca2+ 11, 12, pH 13, 14, InsP3 15, 16, and several proteins such as DnaJ-like proteins 17, 18 were suggested to play important roles in the gravity signal transduction and transmission.
Since Cholodny and Went proposed that asymmetrical auxin distribution within plant organs might explain the mechanism of tropic growth 19, 20, auxin transport has been thought to be a central component of the gravitropic signaling. Auxin is transported along the shoot-root axis from cell to cell in a polar manner, which requires both influx and efflux carriers 21. So far, auxin carriers have been identified and well characterized in Arabidopsis, especially for the efflux carriers. Eight members of PIN proteins have been demonstrated to facilitate auxin efflux 22. Among them, PIN1 and PIN2/AGR1/EIR1/WAV6 were first identified to participate in auxin transport because the phenotypes of pin1 and pin2 were similar to those of wild-type plants grown in the presence of auxin transport inhibitors 23, 24, and these two proteins are localized on plasma membranes in an asymmetric manner 25, 26. PIN3, another regulator of auxin efflux, was subsequently reported to localize with the plasma membrane and vesicles that cycle in an actin-dependent manner and could relocalize laterally upon gravity stimulation 27. Recent studies in Arabidopsis provided strong evidence to support the view that PIN polar localization within the auxin transport-competent cells determines the intercellular auxin flow 28, 29, 30. In addition, AUX1 and AtPGP4 have been identified as auxin influx carriers in Arabidopsis 31, 32, 33.
More importantly, gravitropism is also a key factor to determine plant architecture, one of the most crucial agronomic traits that contributes to crop grain production and thus has attracted much attention of breeders for centuries. Several agravitropic mutants in crops have been reported such as lazy in maize 34, lazy (la) 35 and spk(t) (spreading type of kasalath) 36 in rice, and serpentine in barley 37. Among them, the rice la mutant has been intensively studied for decades 35, 38, 39, 40, 41, 42. Although enormous efforts have been made in the elucidation of the mechanisms underlying the la phenotype, the studies were mainly concentrated on its phenotypic description and preliminary physiological experiments, and the corresponding gene has not been identified, leading to the poor understanding on molecular mechanisms of shoot gravitropism in crops.
We report here the in-depth characterization of the rice mutant la1-ZF802 (previously termed la-2) 43. LA1, cloned through a map-based approach, is a novel grass-specific gene that functions as a negative regulator of PAT, the dysregulation of which likely leads to the reduced gravitropism and tiller-spreading phenotype of the mutant plants.
Materials and Methods
Plant materials
The original tiller-spreading mutant used in this work was acquired from Dr Khush in IRRI. We introduced its spreading character into an indica cultivar ZF802 with 10 generations of backcrosses and named it as la1-ZF802. An allelic mutant la1-Shiokari was derived from Shiokari variety and used for gene transformations owing to its high transformation efficiency.
Analysis of plant gravity response
Rice gravity response under light and in the dark was measured using 5-day-old seedlings planted in plates containing 1/2 MS medium (pH 5.8). Rice seeds were dehusked and surface sterilized with 70% ethanol for 2 min and 30% bleach for 2 h, and then washed five times with autoclaved distilled water. The seedlings were then grown at 28 °C. Gravity response was determined by measuring the shoot curvature after seedlings were reoriented 90° at every 4 h interval with 20 seedlings for each time point.
DNA extraction and gel blotting
The rice genomic DNA and DNA gel blotting were performed as described 44. In brief, the isolated genomic DNA was digested with proper restriction enzymes, separated in a 0.8% agrose gel, transferred onto a Hybond N+ membrane (Amersham), and hybridized with 32P-labeled probes.
RT- or RACE-PCR analysis
Total RNA was prepared using TRIzol® reagent according to the user manual (Cat. No. 15596-026, Invitrogen). One microgram of total RNA was treated with DNase I and used for complementary DNA synthesis with RT kit (Cat. No. A3500, Promega). 5′- or 3′-RACE of LA1 was carried out by using a SMART™ RACE cDNA Amplification Kit according to the manufacturer's instruction (Cat. No. K1181-1, Clontech). The primer sequences used for the above studies were listed in Supplementary information, Table S1.
Subcellular localization of LA1
To determine the subcellular localization of LA1, CaMV35S::LA1-GFP, CaMV35S::LA1ΔN100-GFP, CaMV35S::LA1ΔNLS-GFP and CaMV35S::GFP were constructed. LA1ΔN100 is the truncated LA1 with a deletion of amino-acid residues1-100 that contain a predicted transmembrane domain. LA1ΔNLS refers to the LA1 truncated from amino-acid residues 286 to 312, a segment containing a putative nuclear localization signal (NLS) domain. The vectors were introduced into onion epidermal cells by using a bombardment-mediated gene transformation system (PDS-1000/He, BIORAD). After overnight incubation in the dark, GFP was examined under a confocal microscope at an excitation wavelength of 488 nm (FluoView 500, Olympus).
Histological analysis and mRNA in situ hybridization
The assay of β-glucuronidase (GUS) activity was performed as described 45. For detecting the GUS-staining pattern in the rice vegetative shoot, the stained samples were trimmed, fixed, sectioned into 7 to 10 mm, observed under bright field through a microscope (Leica DMR), and photographed using a Micro Color charge-coupled device (CCD) camera (Apogee Instruments).
Shoot apexes of rice seedlings at the four-leaf stage were fixed with 4% (w/v) paraformaldehyde at 4 °C overnight, followed by a series of dehydration and infiltration, and embedded in paraffin (Paraplast Plus, Sigma). RNA in situ hybridization was performed as described previously 46. The 996-1571 bp region of the LA1 gene was subcloned into the T-easy vector and used as templates to generate sense and antisense RNA probes. Digoxigenin-labeled RNA probes were prepared using a DIG Northern Starter Kit (Cat. No. 2039672, Roche), according to the manufacturer's instruction. Slides were observed under bright field through a microscope (Leica DMR), and photographed with a Micro Color charge-coupled device (CCD) camera (Apogee Instruments).
Polar auxin transport assay
The polar auxin transport assays were performed according to the method described previously with some modifications 23. Five groups of five 5-day-old dark-grown coleoptile segments (2 cm) were used for the assay. The segments were deprived of endogenous IAA by pre-incubation in 1/2 MS (pH 5.8) liquid medium for 2 h. N-1-naphthylphtalamic acid (NPA) was added to the medium as indicated. To avoid gravistimulation, the pre-incubation took place on a shaker. The apical or basal ends of the segments (for basipetal or acropetal transport assays, respectively) were then submerged in 10 μl of 1/2 MS liquid medium containing 0.35% phytogel, 500 nM 3H-IAA and 500 nM free IAA in a 1.5-ml Eppendorf tube in the dark at room temperature, and 14C-benzoic acid was applied as a control of IAA. The non-submerged ends were laid with a layer of lanolin. After 3 h, 5 mm sections from the non-submerged ends of segments were excised and washed two times with 1/2 MS liquid medium. After 18 h incubation in 2 ml scintillation liquid, the radioactivity of each section was counted by a liquid scintillation counter (1450 MicroBeta TriLux, Perkin-Elmer).
Lateral auxin transport assay
Five-day-old coleoptiles of seedlings grown in the dark were used for the assay of auxin lateral transport as described 47 with some modifications. Coleoptiles (1 cm) were harvested and deprived of endogenous IAA as mentioned above. The coleoptiles were laid horizontally on microscope slides with their apical ends contacting with 0.4 × 0.4 × 0.2 cm agar blocks that contain 500 nM 3H-IAA and 500 nM IAA. After transport in the dark at room temperature for 2.5 h, sections of the 5-10 mm segments from the apex were evenly split into upper and lower halves. After 18 h incubation in 2 ml scintillation liquid, the radioactivity of each half (n = 15) was counted as described above.
Results
Characterization of rice spreading-grown mutant la1-ZF802
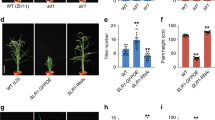
Although the phenotype of rice la mutant has been described in previous studies 35, 40, 48, the background of the materials used was not uniform and the analysis was not systematic and comprehensive. We therefore performed an in-depth analysis of the la1-ZF802 mutant. Morphological analysis showed that the la1-ZF802 mutant plant exhibited a tiller-spreading phenotype at different growth stages (Figure 1A and 1B) and the phenotype was not affected by planting densities (Figure 1C). Close examination revealed that the mutant phenotype was caused by the loss of ability to grow upright during the elongation of primary and tiller shoots, consistent with the previous report that the prostrate phenotype resulted from agravitropism of rice main shoots and tillers 41. The gravitropic response was greatly reduced in both light- and dark-grown la1-ZF802 mutant seedlings (Figure 1E and 1F), which was further confirmed by kinetic studies (Figure 1G). In addition, mutant plants also exhibited a larger leaf angle than those of wild type, especially at the reproductive stage (Figure 1D). However, mutant roots showed normal gravitropism (Figure 1H), indicating that LA1 may be involved only in shoot and tiller gravitropism of rice plants.
Phenotype of the la1-ZF802 mutant. (A, B) Comparison of the tiller angle between ZF802 (left) and la1-ZF802 (right) at the (A) tillering stage and (B) ripening stage. (C) Tiller angles at different planting densities. (D) Leaf angles of ZF802 (left) and la1-ZF802 (right). (E, F) Five-day-old light-grown (E) and dark-grown (F) ZF802 (left) and la1-ZF802 (right) seedlings on stimulation of gravity for 24 h. The arrow marked with g indicates the direction of gravity. (G, H) Kinetic analyses of the gravitropic responses of shoots (G) and roots (H). Error bars indicate ± SE (n = 18).
Map-based cloning of LA1
Our previous studies placed the LA1 gene in an interval of 0.4 cM between the AC35795 and AC6330 markers, near the centromere region of Chromosome 11 43. To fine-map the LA1 locus, we generated three large F2 mapping populations derived from crosses between la1-ZF802 and three local cultivars in China. Of the F2 plants, ∼30 000 mutant individuals were used for fine-mapping. Screening with molecular markers (see Supplementary information, Table S2) placed LA1 in a 68-kb region that was covered by two BAC clones (AC136378 and AC136787) between the pd56 and M265 markers, and no recombinants were found between the M13F marker and the LA1 locus (Figure 2A). Within this region, there are 17 predicted genes/ORFs. Sequencing these genes/ORFs of la1-ZF802 revealed an 8-bp deletion at the fourth exon of a putative gene/ORF, which leads to protein premature termination (Figure 2A and Supplementary information, Figure S1). No mutation could be found in the other predicted genes/ORFs (data not shown). Mutations were also identified in the la1-Shiokari allele with a prostrate phenotype similar to la1-ZF802 (see Supplementary information, Figure S2). The predicted ORF was entirely lost in la1-Shiokari (Figure 2A), which was confirmed by DNA gel blot analysis (see Supplementary information, Figure S3). These results indicated that the predicted ORF was very probably the LA1 gene.
Cloning and functional confirmation of LA1. (A) Fine mapping and a schematic representation of LA1. Δ indicates the 8-bp deletion in la1-ZF802 and the deletion of the entire LA1 gene in la1-Shiokari is also shown. The start codon (ATG) and stop codon (TGA) are indicated. Boxes indicate the coding sequences and lines between boxes indicate introns. (B) Complementation plasmids containing the entire (pLA) or truncated (pLAt) LA1. (C) Phenotypes of transgenic lines of pLAt (left) and pLA (right).
The identity of LA1 was further confirmed by genetic complementation. The plasmid pLA, containing the entire ORF, and pLAt, containing the partial coding region of the ORF (Figure 2B), were introduced into the la1-Shiokari mutant, and 8 and 11 independent lines were generated from these two constructs, respectively. All the eight lines containing the LA1 transgene showed a complementation of the la1-Shiokari phenotype, whereas all the 11 lines of pLAt failed to rescue the la1-Shiokari mutant (Figure 2C). We therefore conclude that we have cloned the rice LA1 gene, which is responsible for the tiller-spreading phenotype of mutant plants.
LA1 knockout/knockdown transgenic lines were generated by introducing the specific RNA interference (RNAi) construct into wild-type plants. The LA1 RNAi transgenic plants displayed larger tiller angles compared to the wild plants (Figure 3A), and the phenotype was correlated with endogenous LA1 expression levels (Figure 3B), suggesting that LA1 is a potential useful target for rice tiller angle modification in molecular breeding.
LA1 encodes a novel grass-specific protein
Sequence analysis of 5′- and 3′- RACE cDNA products indicated that the LA1 cDNA is 1 606 bp long, with an ORF of 1 251-bp, a 35-bp 5′-untranslated region (UTR), and a 320-bp 3′-UTR (see Supplementary information, Figure S1). Sequence comparison between genomic and complementary DNAs revealed that LA1 is composed of five exons that encode a 416 amino-acid protein (Figures 2B and 4A). BlastP analysis revealed that LA1 is a novel protein, sharing no homology with any functionally known protein. However, homology analysis against TIGR plant transcript assemblies showed that LA1 shares high identities with deduced proteins only in cereal plants, including Sorghum bicolor, Zea mays and Triticum aestivum (Figure 4A), suggesting that LA1 might be a grass-specific protein.
Sequence comparison and subcellular localization of LA1. (A) Alignment of deduced amino-acid sequence of LA1 with its homologs in Sorghum bicolor, Zea mays and Triticum aestivum. Shaded letters indicate the identical amino-acid residues. Asterisks indicate the predicted transmembrane domain and the squared letters indicate the putative NLS domain. (B-F) Subcellular localization of (B) CaMV35S:GFP, CaMV35S:LA1-GFP (C) before and (D) after plasmolysis, (E) CaMV35S:LA1ΔN100-GFP, and (F) CaMV35S:LA1ΔNLS-GFP in onion epidermic cells.
Using the TMpred 49 and PredictNLS 50 programs, we found that the LA1 protein contains a transmembrane domain (amino-acid residues 62-83) and an NLS domain (amino-acid residues 286-312; Figure 4A), which were confirmed by transient expression analysis in onion epidermal cells (Figure 4B-F). In contrast to the GFP control that was distributed everywhere in onion epidermal cells (Figure 4B), the LA1-GFP fusion protein was mainly localized to the plasma membrane and nucleus (Figure 4C and 4D). However, the truncated LA1 without the predicted transmembrane domain (deletion of the amino-acid residues 1-100) was delocalized from the plasma membrane (Figure 4E), and that without the NLS-containing region was unable to direct GFP to the nucleus (Figure 4F). These results indicate that both predicted domains are functional.
Temporal and spatial expression of LA1
Expression analysis with RT-PCR showed that LA1 transcripts were abundant in stems and etiolated coleoptiles, less in pulvini, and undetectable in panicles, mature leaves, sheaths and roots (Figure 5A). LA1 mRNA in situ hybridization in longitudinal sections through the seedling apexes demonstrated that LA1 was mainly expressed in vascular cells at the adaxial parts of the junctions of young rice leaves and stems (Figure 5B), consistent with the expectation for the LA1 action sites in regulating the angles of tillers. Cross-sections further showed that LA1 was expressed specifically in the cells at the inner side of the vascular bundles of young leaf sheaths (Figure 5C and 5D) and peripheral cylinders of vascular bundles in the unelongated stems (Figure 5C and 5E). Taken together, we propose that LA1 is a finely regulated temporally and spatially expressed gene, and that the region of its specific expression may play an important role in controlling the rice tiller angle.
Expression pattern of LA1. (A) LA1 expression pattern in various organs, including roots (R), stems (S), leaves (L), sheaths (Sh), pulvini (P), panicles (Pa), and etiolated coleoptiles (C). (B-E) LA1 expression patterns revealed by mRNA in situ hybridization. (D, E) Magnification images of the squared and circled regions in (C), respectively. L, leaf; T, tiller; S, stem; V, vascular bundle; CB, peripheral cylinder of vascular bundles. Arrows show the LA1 expression sites. (C) Bars = 200 μm and (B, D, E) 100 μm.
Involvement of LA1 in auxin transport
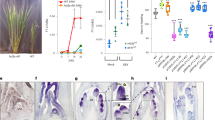
Aberrations associated with gravitropism in la1-ZF802 suggested that auxin homeostasis may be somehow perturbed in the mutant. Considering that the cereal coleoptile represents a model system for studying the regulation of cell growth and tropisms 51, 52, we measured the basipetal and acropetal transports of IAA in etiolated coleoptiles of wild type and mutant plants. We found that the basipetal PAT in la1-ZF802 was highly elevated up to ∼230% compared with that in the wild type, whereas the acropetal PAT of 3H-IAA, basipetal transport of 3H-IAA treated with a PAT inhibitor (30 μM NPA), or basipetal transport of a control compound 14C-benzoic acid displayed no differences between wild type and mutant plants (Figure 6A). To find out whether the enhanced PAT caused by the deficiency in LA1 affects the distribution of endogenous IAA, we visualized the difference in the endogenous IAA distribution by comparing GUS expression levels in the transgenic plants that carry the auxin reporter DR5::GUS in the background of la1-Shiokari and wild-type Shiokari, respectively. In Shiokari coleoptiles, a higher GUS expression level was detected in the apical than that in the middle or basal part (Figure 6B), whereas in la1-Shiokari coleoptiles the GUS expression level in the apical part turned out to be lower than that in the middle or basal part (Figure 6C). Moreover, in longitudinal sections through apexes of DR5::GUS transgenic seedlings, the altered GUS expression profile could also be observed in la1-Shiokari (Figure 6D and 6E). In wild-type plants, GUS expression was mainly accumulated at the peripheral area of shoots (Figure 6D); in la1-Shiokari, however, the GUS expression was more intense and covered a broader area in the unelongated stem (Figure 6E), indicating that the enhanced basipetal PAT resulted in altered endogenous IAA distribution in the la1-Shiokari mutant plant. Furthermore, we measured the lateral IAA transport in rice coleoptiles upon gravity stimulation. After 2.5-h gravistimulation, the radioactivity ratio between the lower and upper halves of the coleoptiles in la1-ZF802 was significantly lower than that in ZF802 (Figure 6F), suggesting that the asymmetric IAA distribution is impaired by the LA1 mutation. The structures of wild type and la1-ZF802 coleoptiles are very similar (Figure 6G and 6H), which rules out the possibility that the difference in IAA transport arises from their structural difference.
Comparison of auxin transport between wild-type and la1-ZF802 plants. (A) Comparison of PAT between ZF802 and la1-ZF802 in dark-grown coleoptiles. Error bars indicate ± SE (n = 5). (B, C) DR5::GUS expression patterns in dark-grown coleoptiles of (B) wild type and (C) la1-Shiokari. (D, E) DR5::GUS expression patterns in wild type (D) and la1-Shiokari (E) unelongated stems. (F) Comparison of lateral IAA transport in coleoptiles between ZF802 and la1-ZF802. Error bars indicate ± SE (n = 15). The cpm ratio represents the radioactivity ratio between the lower half and the upper half of coleoptiles upon gravity stimulation. (G, H) Cross-sections of (G) ZF802 and (H) la1-ZF802 coleoptiles. (B, C) Bars = 1 mm and (D, E, G, H) 200 μm.
Discussion
Gravitropism, a complicated multi-step process that directs plants to adapt to the fundamental environment factor gravity, has fascinated plant biologists for more than a century. Although significant progress in elucidating mechanisms involved in gravitropism has been made recently in Arabidopsis, the mechanisms in monocot remain largely unknown.
Plant shoots typically grow upwards and lateral organs are generated at a defined angle termed gravitropic set-point angle, which determines plant architecture at large extent and is mainly maintained by gravitropism 53. Tiller angle, the angle between the main culm and its primary tillers, is an important agronomic trait that contributes to the rice plant architecture 54. Neither the extremely spreading nor the compact plant type is beneficial to rice grain production 54, 55. Two single recessive mutations, lazy (la) and erecta (er), were reported to confer spreading and compact phenotypes, respectively 35, 56. However, no corresponding genes have been cloned and the molecular mechanism controlling rice tiller angle remains unclear. In this article, we described molecular genetic characterization of the tiller-spreading mutant la1-ZF802 and the isolation and functional analyses of the LA1 gene. By in-depth analysis of the rice classical mutant la1 and map-based cloning of LA1, we have shown that LA1 plays an essential role in regulating rice shoot gravitropism, which in turn controls the tiller angle, an important agronomic trait determining rice grain production.
The expression pattern of LA1 is consistent with the morphological phenotype of la1 mutant. LA1 is specifically expressed at the adaxial parts of the junctions of young rice leaves and stems (Figure 5B), where the shoot bending happens, and in the cells at the inner side of the vascular bundles of young leaf sheaths (Figure 5C and 5D) and peripheral cylinders of vascular bundles in the unelongated stems (Figure 5C and 5E), where it correlates with PAT. Additionally, LA1 expression strictly depends on the developmental process and its expression level is correlated with the degree of tiller angles (Figure 3).
Although LA1 is a novel protein that shows no homology to any functionally known proteins, proteins sharing high homology with LA1 are encoded by corresponding cDNAs in grass species (Figure 4A), implicating that the grass species may respond to gravity and transduce the signal through a distinct pathway from that in dicots. The transient expression in onion epidermal cells demonstrates that LA1 contains both the transmembrane and NLS domains (Figure 4A-F). The LA1-GFP fusion protein was mainly found in the plasma membrane and nucleus, whereas the truncated LA1 lacking the transmembrane domain or without the NLS-containing region was unable to direct the GFP signal to the membrane or nucleus. Therefore, LA1 may represent a new type of regulating proteins that shuttle between the plasma membrane and the nucleus. Further investigations of LA1 functions will allow for a better understanding of the mechanism underlying the monocotyledonous shoot gravitropism.
The Cholodny-Went hypothesis suggested that auxin might be the main phytohormone involved in gravitropism 19, 20. So far, ample evidences that have been gathered with the asymmetrical distribution and polar or lateral transport of auxin support its major role in gravitropism, especially in Arabidopsis roots. Consistently, our results showed that the reduced gravitropism in la1-ZF802 shoots results from the defective PAT. As shown in Figure 6A, the capacity of basipetal auxin transport in la1-ZF802 is about two-fold higher than that in wild type, leading to an alteration in IAA distribution in the mutant as visualized by the DR5::GUS analysis (Figure 6B-E). Furthermore, IAA lateral transport upon gravity stimulation is impaired in la1-ZF802 (Figure 6F). These results are consistent with the previous finding that the unequal distribution of auxin on either side of the organ is the premise of its bending upon gravistimulation 47, 52, 57. Therefore, we conclude that the mutation in LA1 results in a significant increase in PAT and thus impairs the IAA differential distribution in la1-ZF802, which ultimately leads to a reduced gravitropic response in the mutant shoots.
Our findings demonstrate that LA1 is an essential regulator of tiller angle of rice, opening a promising way for breeders to develop elite rice cultivars and other cereal crops with optimal plant architecture.
Accession number
LA1 sequence data from this article can be found in the GenBank data libraries under accession number DQ855268.
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Accession codes
References
Perrin RM, Young LS, Murthy UMN, et al. Gravity signal transduction in primary roots. Ann Bot (Lond) 2005; 96:737–743.
Tasaka M, Kato T, Fukaki H . The endodermis and shoot gravitropism. Trends Plant Sci 1999; 4:103–107.
Morita MT, Tasaka M . Gravity sensing and signaling. Curr Opin Plant Biol 2004; 7:712–718.
Perbal G, Driss-Ecole D . Mechanotransduction in gravisensing cells. Trends Plant Sci 2003; 8:498–504.
Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M . Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 1998; 14:425–430.
Tsugeki R, Olson ML, Fedoroff NV . Transposon tagging and the study of root development in Arabidopsis. Gravit Space Biol Bull 1998; 11:79–87.
Moore I . Gravitropism: lateral thinking in auxin transport. Curr Biol 2002; 12:452–454.
Kim SK, Chang SC, Lee EJ, et al. Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol 2000; 123:997–1004.
Gutjahr C, Riemann M, Muller A, Duchting P, Weiler EW, Nick P . Cholodny-Went revisited: a role for jasmonate in gravitropism of rice coleoptiles. Planta 2005; 222:575–585.
Aloni R, Langhans M, Aloni E, Ullrich CI . Role of cytokinin in the regulation of root gravitropism. Planta 2004; 220:177–182.
Heilmann I, Shin J, Huang J, Perera IY, Davies E . Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini. Plant Physiol 2001; 127:1193–1203.
Belyavskaya NA . Changes in calcium signalling, gravitropism, and statocyte ultrastructure in pea roots induced by calcium channel blockers. J Gravit Physiol 2004; 11:P209–P210.
Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S . Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 2001; 13:907–921.
Monshausen GB, Sievers A . Basipetal propagation of gravity-induced surface pH changes along primary roots of Lepidium sativum L. Planta 2002; 215:980–988.
Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB . A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol 2001; 125:1499–1507.
Perera IY, Hung CY, Brady S, Muday GK, Boss WF . A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol 2006; 140:746–760.
Guan C, Rosen ES, Boonsirichai K, Poff KL, Masson PH . The ARG1-LIKE2 gene of Arabidopsis functions in a gravity signal transduction pathway that is genetically distinct from the PGM pathway. Plant Physiol 2003; 133:100–112.
Sedbrook JC, Chen R, Masson PH . ARG1 (Altered Response to Gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA 1999; 96:1140–1145.
Went FW, Thimann KV, eds. Phytohormones. New York: Macmillan, 1937.
Richard D, Firn, Wagstaff C, Digby J . The use of mutants to probe models of gravitropism. J Exp Bot 2000; 51:1323–1340.
Sieberer T, Leyser O . Plant science: auxin transport, but in which direction? Science 2006; 312:858–860.
Petrasek J, Mravec J, Bouchard R, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006; 312:914–918.
Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y . Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991; 3:677–684.
Luschnig C, Gaxiola RA, Grisafi P, Fink GR . EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 1998; 12:2175–2187.
Galweiler L, Guan C, Muller A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998; 282:2226–2230.
Muller A, Guan C, Galweiler L, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 1998; 17:6903–6911.
Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K . Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; 415:806–809.
Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R . The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 2001; 128:4057–4067.
Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS . Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature 2003; 423:999–1002.
Wisniewska J, Xu J, Seifertova D, et al. Polar PIN localization directs auxin flow in plants. Science 2006; 312:883.
Marchant A, Kargul J, May ST, et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 1999; 18:2066–2073.
Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E . High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 2006; 16:1123–1127.
Terasaka K, Blakeslee JJ, Titapiwatanakun B, et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 2005; 17:2922–2939.
Overbeek JV . “Lazy”, an a-geotropic form of maize. J Heredity 1936; 27:93–96.
Jones JW, Adair CR . A “lazy” mutation in rice. J Heredity 1938; 28:315–318.
Maiyata M, Komori T, Yamamoto T, Ueda T, Yano M, Nitta N . Fine scale and physical mapping of Spk(t) controlling spreading stub in rice. Breed Sci 2005; 55:237–239.
Suge H, Turkan I . Can plants normally produce seeds under microgravity in space? Jpn J Crop Sci 1991; 60:427–433.
Yamazaki Y . Lazy-rice gotten up with tryptophan. Bot Mag 1985; 98:193–198.
Abe K . Role of gravitropic response in the dry matter production of rice (Oryza sativa L.): an experiment with a line having lazy gene. J Plant Res 1993; 106:337–343.
Abe K, Takahashi H, Suge H . Graviresponding sites in shoots of normal and “lazy” rice seedlings. Physiol Plant 1994; 92:371–374.
Abe K, Takahashi H, Suge H . Lazy gene (la) responsible for both an agravitropism of seedlings and lazy habit of tiller growth in rice (Oryza sativa L.). J Plant Res 1996; 109:381–386.
Godbole H, Takahashi H, Hertel R . The lazy mutation in rice affects a step between statoliths and gravity-induced lateral auxin transport. Plant Biol 1999; 1:379–381.
Li P, Zeng D, Liu X, et al. Mapping and characterization of a tiller-spreading mutant lazy-2 in rice. Chin Sci Bull 2003; 48:2715–2717.
Mou Z, He Y, Dai Y, Liu X, Li J . Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 2000; 12:405–418.
Scarpella E, Rueb S, Meijer AH . The RADICLELESS1 gene is required for vascular pattern formation in rice. Development 2003; 130:645–658.
Li X, Qian Q, Fu Z, et al. Control of tillering in rice. Nature 2003; 422:618–621.
Park K, Brigger W . Transport of indole-3-acetic acid during gravitropism in intact maize coleoptiles. Plant Physiol 1990; 94:1763–1769.
Abe K, Takahashi H, Suge H . Localization of cells containing sedimented amyloplasts in the shoots of normal and lazy rice seedlings. Biol Sci Space 1994; 8:221–225.
Hofmann K, Stoffel W . TMbase – a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler 1993; 374:166.
Cokol M, Nair R, Rost B . Finding nuclear localization signals. EMBO Rep 2000; 1:411–415.
Haga K, Takano M, Neumann R, Iino M . The rice coleoptile phototropism1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell 2005; 17:103–115.
Philippar K, Fuchs I, Luthen H, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 1999; 96:12186–12191.
Digby J, Firn RD . The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ 1995; 18:1434–1440.
Xu Y, Mccouch SR, Sheng Z . Transgressive segregation of tiller angle caused by complementary action of genes in crosses between indica varieties of rice Oryza sativa L. Crop Sci 1998; 38:12–19.
Qian Q, He P, Teng S, Zeng D, Zhu L . QTLs analysis of tiller angle in rice (Oryza sativa L.). Yi Chuan Xue Bao 2001; 28:29–32.
Takahashi M, Kinoshita T, Takeda K . Genetical studies of rice plant. XXXIII. Character expression and caudal genes of some mutants in rice plant. J Fac Agr Hokkaido Univ 1968; 55:496–512.
Rashotte AM, DeLong A, Muday GK . Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 2001; 13:1683–1697.
Acknowledgements
We thank G S Khush (International Rice Research Institute, the Philippines) for providing the original la1 mutant, B Han (National Center for Gene Research, China) for the BAC clone AC136787, and B Zhang (ColdSpring Science Corporation, China) for technical assistance of microscope. This work was supported by grants from the Ministry of Science and Technology of China (2005CB1208) and the National Natural Science Foundation of China (30330040 and 30570161).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Wang, Y., Qian, Q. et al. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res 17, 402–410 (2007). https://doi.org/10.1038/cr.2007.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2007.38
Keywords
This article is cited by
-
IGT/LAZY genes are differentially influenced by light and required for light-induced change to organ angle
BMC Biology (2024)
-
OsNAC103, a NAC Transcription Factor, Positively Regulates Leaf Senescence and Plant Architecture in Rice
Rice (2024)
-
Different contributions of PROG1 and TAC1 to the angular kinematics of the main culm and tillers of wild rice (Oryza rufipogon)
Planta (2024)
-
Substitution Mapping and Allelic Variations of the Domestication Genes from O. rufipogon and O. nivara
Rice (2023)
-
Genome-wide identification of peanut IGT family genes and their potential roles in the development of plant architecture
Scientific Reports (2023)