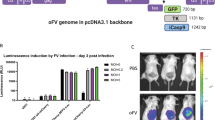
Abstract
Semliki Forest virus (SFV) vectors are promising tools for cancer gene therapy because they ensure a high level of transgene expression and a rapid and strong cytopathic effect. However, broad tissue tropism and transient expression make it more difficult to develop an optimal cancer treatment strategy. In this study, we have compared the distribution of recombinant SFV particles (recSFV) and naked viral RNA replicon (recRNA) in tumor-free and 4T1 mammary tumor-bearing mice as a consequence of different vector administration strategies. The high potential of SFV recRNA as a biosafe approach for the development of therapeutic treatment was demonstrated. Intravenous (i.v.) inoculation of recRNA provided primary brain targeting in both tumor-free and 4T1 tumor mouse models, but local intratumoral inoculation revealed a high expression level in tumors. Moreover, we observed the predominant tumor targeting of recSFV at a reduced viral dose on i.v. and intraperitoneal (i.p.) virus inoculation, whereas the dose increase led to a broad virus distribution in mice. To prolong transgene expression, we have tested several i.v. and i.p. reinoculation strategies. A detailed evaluation of vector distribution and readministration properties could have an impact on cancer gene therapy clinical trial safety and efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tani J, Faustine, Sufian JT . Updates on current advances in gene therapy. West Indian Med J 2011; 60: 188–194.
Larocca C, Schlom J . Viral vector-based therapeutic cancer vaccines. Cancer J 2011; 17: 359–371.
Lundstrom K . Semliki Forest virus vectors for large-scale production of recombinant proteins. Methods Mol Med 2003; 76: 525–543.
Lundstrom K . Alphaviruses in gene therapy. Viruses 2009; 1: 13–25.
Venticinque L, Meruelo D . Sindbis viral vector induced apoptosis requires translational inhibition and signaling through Mcl-1 and Bak. Mol Cancer 2010; 9: 37.
Glasgow GM, McGee MM, Tarbatt CJ, Mooney DA, Sheahan BJ, Atkins GJ . The Semliki Forest virus vector induces p53-independent apoptosis. J Gen Virol 1998; 79 (Part 10): 2405–2410.
Avogadri F, Merghoub T, Maughan MF, Hirschhorn-Cymerman D, Morris J, Ritter E et al. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS One 2010; 5: e12670.
Leitner WW, Hwang LN, deVeer MJ, Zhou A, Silverman RH, Williams BR et al. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat Med 2003; 9: 33–39.
Riezebos-Brilman A, Walczak M, Regts J, Rots MG, Kamps G, Dontje B et al. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther 2007; 14: 1695–1704.
Morse MA, Hobeika AC, Osada T, Berglund P, Hubby B, Negri S et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J Clin Invest 2010; 120: 3234–3241.
Johanning FW, Conry RM, LoBuglio AF, Wright M, Sumerel LA, Pike MJ et al. A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res 1995; 23: 1495–1501.
Quetglas JI, Ruiz-Guillen M, Aranda A, Casales E, Bezunartea J, Smerdou C . Alphavirus vectors for cancer therapy. Virus Res 2010; 153: 179–196.
Leung JY, Ng MM, Chu JJ . Replication of alphaviruses: a review on the entry process of alphaviruses into cells. Adv Virol 2011; 2011: 249640.
Liljestrom P, Garoff H . A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (NY) 1991; 9: 1356–1361.
Lundstrom K . Semliki Forest virus-based expression for versatile use in receptor research. J Recept Signal Transduct Res 2002; 22: 229–240.
Wahlfors JJ, Zullo SA, Loimas S, Nelson DM, Morgan RA . Evaluation of recombinant alphaviruses as vectors in gene therapy. Gene Ther 2000; 7: 472–480.
Rodriguez-Madoz JR, Prieto J, Smerdou C . Biodistribution and tumor infectivity of Semliki Forest virus vectors in mice: effects of re-administration. Mol Ther 2007; 15: 2164–2171.
Chikkanna-Gowda CP, Sheahan BJ, Fleeton MN, Atkins GJ . Regression of mouse tumours and inhibition of metastases following administration of a Semliki Forest virus vector with enhanced expression of IL-12. Gene Ther 2005; 12: 1253–1263.
Lyons JA, Sheahan BJ, Galbraith SE, Mehra R, Atkins GJ, Fleeton MN . Inhibition of angiogenesis by a Semliki Forest virus vector expressing VEGFR-2 reduces tumour growth and metastasis in mice. Gene Ther 2007; 14: 503–513.
Rodriguez-Madoz JR, Prieto J, Smerdou C . Semliki Forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol Ther 2005; 12: 153–163.
Rodriguez-Madoz JR, Liu KH, Quetglas JI, Ruiz-Guillen M, Otano I, Crettaz J et al. Semliki Forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma. J Virol 2009; 83: 12266–12278.
Ketola A, Hinkkanen A, Yongabi F, Furu P, Maatta AM, Liimatainen T et al. Oncolytic Semliki Forest virus vector as a novel candidate against unresectable osteosarcoma. Cancer Res 2008; 68: 8342–8350.
Colmenero P, Berglund P, Kambayashi T, Biberfeld P, Liljestrom P, Jondal M . Recombinant Semliki Forest virus vaccine vectors: the route of injection determines the localization of vector RNA and subsequent T cell response. Gene Ther 2001; 8: 1307–1314.
Vignuzzi M, Gerbaud S, van der WS, Escriou N . Naked RNA immunization with replicons derived from poliovirus and Semliki Forest virus genomes for the generation of a cytotoxic T cell response against the influenza A virus nucleoprotein. J Gen Virol 2001; 82: 1737–1747.
Saxena S, Sonwane AA, Dahiya SS, Patel CL, Saini M, Rai A et al. Induction of immune responses and protection in mice against rabies using a self-replicating RNA vaccine encoding rabies virus glycoprotein. Vet Microbiol 2009; 136: 36–44.
Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum Gene Ther 2001; 12: 235–252.
Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM et al. Cancer therapy using a self-replicating RNA vaccine. Nat Med 1999; 5: 823–827.
Wong CW, Song C, Grimes MM, Fu W, Dewhirst MW, Muschel RJ et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol 2002; 161: 749–753.
Ryan MD, Drew J . Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J 1994; 13: 928–933.
Zajakina A, Kozlovska T, Bruvere R, Aleksejeva J, Pumpens P, Garoff H . Translation of hepatitis B virus (HBV) surface proteins from the HBV pregenome and precore RNAs in Semliki Forest virus-driven expression. J Gen Virol 2004; 85: 3343–3351.
Tomayko MM, Reynolds CP . Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 1989; 24: 148–154.
Quetglas JI, Fioravanti J, Ardaiz N, Medina-Echeverz J, Baraibar I, Prieto J et al. A Semliki Forest virus vector engineered to express IFNalpha induces efficient elimination of established tumors. Gene Ther 2012; 19: 271–278.
Zhang YQ, Tsai YC, Monie A, Wu TC, Hung CF . Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol Ther 2010; 18: 692–699.
Chen B, Timiryasova TM, Haghighat P, Andres ML, Kajioka EH, Dutta-Roy R et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother 2001; 24: 46–57.
Murphy AM, Morris-Downes MM, Sheahan BJ, Atkins GJ . Inhibition of human lung carcinoma cell growth by apoptosis induction using Semliki Forest virus recombinant particles. Gene Ther 2000; 7: 1477–1482.
Ren H, Boulikas T, Lundstrom K, Soling A, Warnke PC, Rainov NG . Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki Forest virus vector carrying the human interleukin-12 gene--a phase I/II clinical protocol. J Neurooncol 2003; 64: 147–154.
Ulmer JB, Mason PW, Geall A, Mandl CW . RNA-based vaccines. Vaccine 2012; in: press.
Yu Q, Pecchia DB, Kingsley SL, Heckman JE, Burke JM . Cleavage of highly structured viral RNA molecules by combinatorial libraries of hairpin ribozymes. The most effective ribozymes are not predicted by substrate selection rules. J Biol Chem 1998; 273: 23524–23533.
Naslund TI, Kostic L, Nordstrom EK, Chen M, Liljestrom P . Role of innate signalling pathways in the immunogenicity of alphaviral replicon-based vaccines. Virol J 2011; 8: 36.
Johansson DX, Ljungberg K, Kakoulidou M, Liljestrom P . Intradermal electroporation of naked replicon RNA elicits strong immune responses. PLoS One 2012; 7: e29732.
Meruelo D . Systemic gene therapy by Sindbis vectors: a potentially safe and effective targeted therapy for identifying and killing tumor cells in vivo. Discov Med 2004; 4: 54–57.
Tseng JC, Levin B, Hurtado A, Yee H, Perez de Castro I, Jimenez M et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotechnol 2004; 22: 70–77.
Tseng JC, Granot T, DiGiacomo V, Levin B, Meruelo D . Enhanced specific delivery and targeting of oncolytic Sindbis viral vectors by modulating vascular leakiness in tumor. Cancer Gene Ther 2010; 17: 244–255.
Unno Y, Shino Y, Kondo F, Igarashi N, Wang G, Shimura R et al. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin Cancer Res 2005; 11: 4553–4560.
Acknowledgements
We thank Professor A Merits and his group for sharing the pSFV/Enh.Luc DNA plasmid, and for critically reading the manuscript and useful suggestions. We also acknowledge Professor P Pumpens and his lab members for helpful discussions and excellent technical assistance. We are grateful to Dr I Shestakova and her group for useful advice in 4T1 model development. The publishing expenses were covered by ERDF project Nr. 2010/0196/2DP/2.1.1.2.0/10/APIA/VIAA/004. This study was supported by The Latvian National Research Programme 2010–2013, “BIOMEDICINE”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vasilevska, J., Skrastina, D., Spunde, K. et al. Semliki Forest virus biodistribution in tumor-free and 4T1 mammary tumor-bearing mice: a comparison of transgene delivery by recombinant virus particles and naked RNA replicon. Cancer Gene Ther 19, 579–587 (2012). https://doi.org/10.1038/cgt.2012.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.37