Abstract
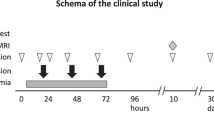
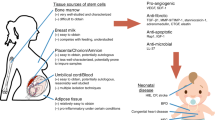
Brain injury resulting from perinatal hypoxic-ischemic encephalopathy (HIE) is a major cause of acute mortality in infants and chronic neurologic disability in surviving children. Recent multicenter clinical trials demonstrated the effectiveness of hypothermia initiated within the first 6 postnatal hours to reduce the risk of death or major neurological disabilities among neonates with HIE. However, in these trials, approximately 40% of cooled infants died or survived with significant impairments. Therefore, adjunct therapies are required to improve the outcome in neonates with HIE. Cord blood (CB) is a rich source of stem cells. Administration of human CB cells in animal models of HIE has generally resulted in improved outcomes and multiple mechanisms have been suggested including anti-inflammation, release of neurotrophic factors and stimulation of endogenous neurogenesis. Investigators at Duke are conducting studies of autologous CB infusion in neonates with HIE and in children with cerebral palsy. These pilot studies indicate no added risk from the regimens used, but results of ongoing placebo-controlled trials are needed to assess efficacy. Meanwhile, further investigations are warranted to determine the best strategies, that is, timing, dosing, route of delivery, choice of stem cells and ex vivo modulations, to attain long-term benefits of CB stem cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE . A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol 2008; 199: 587–595.
American Congress of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy. Defining the Pathogenesis 2003. American College of Obstetricians and Gynecologists, Washington, DC.
Sarnat HB, Sarnat MS . Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976; 33: 696–705.
Robertson CM, Finer NN, Grace MG . School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr 1989; 114: 753–760.
Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R . Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev 1991; 25: 135–148.
Badawi N, Felix JF, Kurinczuk JJ, Dixon G, Watson L, Keogh JM et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol 2005; 47: 293–298.
Azzopardi D, Strohm B, Edwards AD, Halliday H, Juszczak E, Levene M et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Disease Child Fetal Neonatal Ed 2009; 94: F260–F264.
National Institute for Health and Clinical Excellence Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injuries: interventional procedure guidance 347.
Cotten CM, Shankaran S . Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol 2010; 5: 227–239.
Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells 2007; 25: 918–928.
Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA . Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 2006; 12: 1259–1268.
Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008; 321: 1218–1221.
Bliss T, Guzman R, Daadi M, Steinberg GK . Cell transplantation therapy for stroke. Stroke 2007; 38 (Suppl): 817–826.
Schira J, Gasis M, Estrada V, Hendricks M, Schmitz C, Trapp T et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain 2012; 135 (Pt 2): 431–446.
Prasad VK, Kurtzberg J . Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant 2008; 41: 99–108.
Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood 2008; 112: 2979–2989.
Liao Y, Geyer MB, Yang AJ, Cairo MS . Cord blood transplantation and stem cell regenerative potential. Exp Hematol 39: 393–412.
Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion 2005; 45: 842–855.
McLean C, Ferriero D . Mechanisms of hypoxic-ischemic injury in the term infant. Seminars Perinatol 2004; 28: 425–432.
Johnston MV, Fatemi A, Wilson MA, Northington F . Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol 2011; 10: 372–382.
Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, Yager J . Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediat Neurol 2009; 40: 215–226.
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD et al. Delayed ("secondary") cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48 h studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 1994; 36: 699–706.
Freeman JP . Brain injury in the term neonate. Semin Perinatol 2004; 28: 415–424.
Mehmet H, Yue X, Squier MV, Lorek A, Cady E, Penrice J et al. Increased apoptosis in the cingulate sulcus of newborn piglets following transient hypoxia-ischaemia is related to the degree of high energy phosphate depletion during the insult. Neurosci Lett 1994; 181: 121–125.
Fellman V, Raivio KO . Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 1997; 41: 599–606.
Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JD . Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab 1999; 19: 1099–1108.
Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ et al. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res 1996; 39: 791–797.
Gluckman PD, Guan J, Williams C, Scheepens A, Zhang R, Bennet L et al. Asphyxial brain injury—the role of the IGF system. Mol Cell Endocrinol 1998; 140: 95–99.
Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D et al. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev Med Child Neurol 1992; 34: 285–295.
Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C . Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull 1998; 46: 281–309.
Ferriero DM . Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995.
Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK . Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab 2000; 20: 1294–1300.
Edwards AD, Yue X, Squier MV, Thoresen M, Cady EB, Penrice J et al. Specific inhibition of apoptosis after cerebral hypoxia-ischaemia by moderate post-insult hypothermia. Biochem Biophys Res Commun 1995; 217: 1193–1199.
Rothstein RP, Levison SW . Gray matter oligodendrocyte progenitors and neurons die caspase-3 mediated deaths subsequent to mild perinatal hypoxic/ischemic insults. Dev Neurosci 2005; 27: 149–159.
Geddes R, Vannucci RC, Vannucci SJ . Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci 2001; 23: 180–185.
Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ . Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol 2008; 64: 535–546.
Miller SP, Ferriero DM . From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 2009; 32: 496–505.
Imitola J . Prospects for neural stem cell-based therapies for neurological diseases. Neurotherapeutics 2007; 4: 701–714.
Martino G, Pluchino S . The therapeutic potential of neural stem cells. Nat Rev Neurosci 2006; 7: 395–406.
Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA . Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA 2000; 97: 13883–13888.
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A . Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999; 97: 703–716.
Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM . Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis 2004; 16: 585–595.
Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z et al. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci 2006; 26: 4359–4369.
Yang Z, You Y, Levison SW . Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol 2008; 511: 19–33.
Blaschke AJ, Staley K, Chun J . Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 1996; 122: 1165–1174.
Gunn AJ, Gunn TR . The ‘pharmacology’ of neuronal rescue with cerebral hypothermia. Early Hum Dev 1998; 53: 19–35.
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD . Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997; 99: 248–256.
Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD . Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 1998; 102: 1098–1106.
Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR . Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res 1999; 46: 274–280.
Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I . Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed 1996; 74: F3–F9.
Fukuda H, Tomimatsu T, Watanabe N, Mu JW, Kohzuki M, Endo M et al. Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res 2001; 910: 187–191.
Thoresen M, Satas S, Puka-Sundvall M, Whitelaw A, Hallstrom A, Loberg EM et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8: 3359–3362.
Edwards AD, Azzopardi DV . Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed 2006; 91: F127–F131.
Schulzke SM, Rao S, Patole SK . A systematic review of cooling for neuroprotection in neonates with hypoxic ischemic encephalopathy - are we there yet? BMC Pediatr 2007; 7: 30.
Shah PS, Ohlsson A, Perlman M . Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med 2007; 161: 951–958.
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P . Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007 (e-pub ahead of print 17 October 2007; doi:10.1002/14651858.CD003311).
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361: 1349–1358.
Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med 2005; 352: 2069–2081.
Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S et al. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 2010; 50: 1980–1987.
Cotten CM, Goldberg R, Smith PB, Grotegut C, Goldstein RF, Fisher KA et al. Autologous cord blood cells for infants with hypoxic-ischemic encephalopathy (HIE): a feasibility study. Pediatric Academic Societies.. Denver, CO, 2011 pp E-PAS20113833.327.
McGuckin C, Jurga M, Ali H, Strbad M, Forraz N . Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc 2008; 3: 1046–1055.
Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia 2007; 21: 297–303.
van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS . The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp Hematol 2007; 35: 1753–1765.
Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 2004; 200: 123–135.
Greschat S, Schira J, Kury P, Rosenbaum C, de Souza Silva MA, Kogler G et al. Unrestricted somatic stem cells from human umbilical cord blood can be differentiated into neurons with a dopaminergic phenotype. Stem Cells Dev 2008; 17: 221–232.
Xia G, Hong X, Chen X, Lan F, Zhang G, Liao L . Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med 38: 215–221.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ . Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 24: 387–393.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ . Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci 30: 9603–9611.
Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J et al. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant 2006; 15: 231–238.
Yasuhara T, Hara K, Maki M, Mays RW, Deans RJ, Hess DC et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J Cereb Blood Flow Metab 2008; 28: 1804–1810.
Buzanska L, Jurga M, Stachowiak EK, Stachowiak MK, Domanska-Janik K . Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev 2006; 15: 391–406.
Jurga M, Lipkowski AW, Lukomska B, Buzanska L, Kurzepa K, Sobanski T et al. Generation of functional neural artificial tissue from human umbilical cord blood stem cells. Tissue Eng Part C Methods 2009; 15: 365–372.
Tracy ET, Zhang CY, Gentry T, Shoulars KW, Kurtzberg J . Isolation and expansion of oligodendrocyte progenitor cells from cryopreserved human umbilical cord blood. Cytotherapy 2011; 13: 722–729.
Sanberg PR, Park DH, Kuzmin-Nichols N, Cruz E, Hossne NA, Buffolo E et al. Monocyte transplantation for neural and cardiovascular ischemia repair. J Cell Mol Med 2010; 14: 553–563.
Gornicka-Pawlak el B, Janowski M, Habich A, Jablonska A, Drela K, Kozlowska H et al. Systemic treatment of focal brain injury in the rat by human umbilical cord blood cells being at different level of neural commitment. Acta Neurobiol Exp 2011; 71: 46–64.
Tan S, Drobyshevsky A, Jilling T, Ji X, Ullman LM, Englof I et al. Model of cerebral palsy in the perinatal rabbit. J Child Neurol 2005; 20: 972–979.
Derrick M, Drobyshevsky A, Ji X, Tan S . A model of cerebral palsy from fetal hypoxia-ischemia. Stroke 2007; 38 (Suppl): 731–735.
Jacobson Misbe EN, Richards TL, McPherson RJ, Burbacher TM, Juul SE . Perinatal asphyxia in a nonhuman primate model. Dev Neurosci 2011; 33: 210–221.
Juul SE, Aylward E, Richards T, McPherson RJ, Kuratani J, Burbacher TM . Prenatal cord clamping in newborn Macaca nemestrina: a model of perinatal asphyxia. Dev Neurosci 2007; 29: 311–320.
Rice JE, Vannucci RC, Brierley JB . The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131–141.
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res 1999; 55: 158–163.
Dobbing J, Sands J . Comparative aspects of the brain growth spurt. Early Hum Dev 1979; 3: 79–83.
Romijn HJ, Hofman MA, Gramsbergen A . At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev 1991; 26: 61–67.
Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res 2006; 59: 244–249.
Geissler M, Dinse HR, Neuhoff S, Kreikemeier K, Meier C . Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS One 2011; 6: e20194.
Pimentel-Coelho PM, Magalhaes ES, Lopes LM, deAzevedo LC, Santiago MF, Mendez-Otero R . Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev 19: 351–358.
Yasuhara T, Hara K, Maki M, Xu L, Yu G, Ali MM et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med 14: 914–921.
de Paula S, Vitola AS, Greggio S, de Paula D, Mello PB, Lubianca JM et al. Hemispheric brain injury and behavioral deficits induced by severe neonatal hypoxia-ischemia in rats are not attenuated by intravenous administration of human umbilical cord blood cells. Pediatr Res 2009; 65: 631–635.
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001; 32: 2682–2688.
Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res 2003; 73: 296–307.
Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T et al. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke 2004; 35: 2390–2395.
Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR . Stroke-induced migration of human umbilical cord blood cells: time course and cytokines. Stem Cells Dev 2005; 14: 576–586.
Borlongan CV, Hadman M, Sanberg CD, Sanberg PR . Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004; 35: 2385–2389.
Newcomb JD, Ajmo CT, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE . Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant 2006; 15: 213–223.
Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev 2005; 14: 595–604.
Jiang L, Womble T, Saporta S, Chen N, Sanberg CD, Sanberg PR et al. Human umbilical cord blood cells decrease microglial survival in vitro. Stem Cells Dev 19: 221–228.
de Paula S, Greggio S, DaCosta JC . Use of stem cells in perinatal asphyxia: from bench to bedside. J Pediatr 86: 451–464.
Pimentel-Coelho PM, Mendez-Otero R . Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev 19: 299–310.
Risdon G, Gaddy J, Broxmeyer HE . Allogeneic responses of human umbilical cord blood Blood Cells 1994; 20: 566–570 discussion 571–572.
Rainsford E, Reen DJ . Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol 2002; 116: 702–709.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ . Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res 2010; 68: 419–422.
Lee JA, Kim BI, Jo CH, Choi CW, Kim EK, Kim HS et al. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr Res 2010; 67: 42–46.
Xia G, Hong X, Chen X, Lan F, Zhang G, Liao L . Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med 2010; 38: 215–221.
Lee IS, Jung K, Kim M, Park KI . Neural stem cells: properties and therapeutic potentials for hypoxic-ischemic brain injury in newborn infants. Pediatr Int 52: 855–865.
Nodari LR, Ferrari D, Giani F, Bossi M, Rodriguez-Menendez V, Tredici G et al. Long-term survival of human neural stem cells in the ischemic rat brain upon transient immunosuppression. PLoS One 2010; 5: e14035.
Park KI, Teng YD, Snyder EY . The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol 2002; 20: 1111–1117.
Katsuragi S, Ikeda T, Date I, Shingo T, Yasuhara T, Ikenoue T . Grafting of glial cell line-derived neurotrophic factor secreting cells for hypoxic-ischemic encephalopathy in neonatal rats. Am J Obstet Gynecol 2005; 192: 1137–1145.
Katsuragi S, Ikeda T, Date I, Shingo T, Yasuhara T, Mishima K et al. Implantation of encapsulated glial cell line-derived neurotrophic factor-secreting cells prevents long-lasting learning impairment following neonatal hypoxic-ischemic brain insult in rats. Am J Obstet Gynecol 2005; 192: 1028–1037.
Lee HJ, Lim IJ, Lee MC, Kim SU . Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res 88: 3282–3294.
Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY . Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol 2006; 199: 179–190.
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA 2004; 101: 11839–11844.
Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal "reporter" neural stem cells. Exp Neurol 2006; 199: 156–178.
Lappalainen RS, Narkilahti S, Huhtala T, Liimatainen T, Suuronen T, Narvanen A et al. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci Lett 2008; 440: 246–250.
Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q et al. Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. J Cereb Blood Flow Metab 2010; 30: 653–662.
Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008; 39: 1569–1574.
Modo M, Stroemer RP, Tang E, Patel S, Hodges H . Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke 2002; 33: 2270–2278.
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ . Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 2010; 24: 387–393.
Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke 2010; 41: 516–523.
Acknowledgements
We thank Erin Morris, RN, for her excellent assistance in the preparation of the manuscript. This work was supported in part by the Pediatric Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liao, Y., Cotten, M., Tan, S. et al. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Transplant 48, 890–900 (2013). https://doi.org/10.1038/bmt.2012.169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.169
Keywords
This article is cited by
-
Umbilical cord milking versus delayed cord clamping in term infants: a systematic review and meta-analysis
Journal of Perinatology (2021)
-
Placental transfusion: may the “force” be with the baby
Journal of Perinatology (2021)
-
Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety
Scientific Reports (2020)
-
Umbilical cord milking for neonates who are depressed at birth: a randomized trial of feasibility
Journal of Perinatology (2018)
-
Rat umbilical cord blood cells attenuate hypoxic–ischemic brain injury in neonatal rats
Scientific Reports (2017)