Summary:
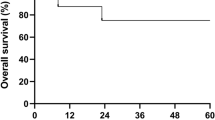
We have developed a reduced-intensity conditioning regimen for patients with severe aplastic anemia (SAA) undergoing alternative donor transplants, which includes fludarabine (120 mg/m2), cyclophosphamide (1200 mg/m2) and antithymocyte globulin (7.5 mg/kg). Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporine and methotrexate. We have enrolled 38 SAA patients in this trial: median age of 14 (3–37) years, transplanted from unrelated (n=33) or family mismatched (n=5) donors, with unmanipulated marrow (n=36) or peripheral blood (n=2). Seven patients (18%) had evidence of graft failure, 11% developed grade II–III acute GvHD and 27% developed chronic GvHD. The actuarial 2-year survival is 73%, with a median follow-up of 621 days. Younger patients (⩽14 years) had a lower risk of rejection (5%) and improved actuarial survival (84%). Causes of death were infections (n=3), graft failure (n=2), Epstein–Barr virus lymphoma (n=2) and hemorrhage (n=2). In conclusion, the actuarial 2-year survival is encouraging in young SAA patients receiving a radiation-free conditioning regimen. The significant risk of graft failure in patients 15 years or older may require modification of the conditioning regimen in adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bacigalupo A, Hows J, Gordon Smith EC et al. Bone marrow transplantation for severe aplastic anemia from donors other than HLA identical siblings: a report of the BMT working party. Bone Marrow Transplant 1988; 3: 531.
Kodera Y, Morishima Y, Kato S et al. Analysis of 500 bone marrow transplants from unrelated donors (UR-BMT) facilitated by the Japan Marrow Donor Program: confirmation of UR-BMT as a standard therapy for patients with leukemia and aplastic anemia. Bone Marrow Transplant 1999; 24: 995–1003.
Gratwohl A, Hermans J, Baldomero H . Blood and marrow transplantation activity in Europe 1995. European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 1997; 19: 407–419.
Tzeng CH, Chen PM, Fan S et al. CY/TBI-800 as a pretransplant regimen for allogeneic bone marrow transplantation for severe aplastic anemia using HLA-haploidentical family donors. Bone Marrow Transplant 1996; 18: 273–277.
Davies SM, Shu XO, Blazar BR et al. Unrelated donor bone marrow transplantation: influence of HLA A and B incompatibility on outcome. Blood 1995; 86: 1636–1642.
Sanders JE, Storb R, Anasetti C et al. Marrow transplant experience for children with severe aplastic anemia. Am J Pediatr Hematol Oncol 1994; 16: 43–49.
Hows J, Szydlo R, Anasetti C et al. Unrelated donor marrow transplants for severe acquired aplastic anemia. Bone Marrow Transplant 1992; 10 (Suppl. 1): 102–106.
Gajewski JL, Ho WG, Feig SA et al. Bone marrow transplantation using unrelated donors for patients with advanced leukemia or bone marrow failure. Transplantation 1990; 50: 244–249.
Casper JT, Truitt RR, Baxter-Lowe LA, Ash RC . Bone marrow transplantation for severe aplastic anemia in children. Am J Pediatr Hematol Oncol 1990; 12: 434–448.
Camitta B, Ash R, Menitove J et al. Bone marrow transplantation for children with severe aplastic anemia: use of donors other than HLA-identical siblings. Blood 1989; 74: 1852–1857.
Bacigalupo A, Brand R, Oneto R et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy – The European Group for Blood and Marrow Transplantation Experience. Semin Hematol 2000; 37: 69–80.
Deeg HJ, Amylon ID, Harris RE et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant 2001; 7: 208–215.
Kojima S, Matsuyama T, Kato S et al. Outcome of 154 patients with severe aplastic anemia who received transplants from unrelated donors: the Japan Marrow Donor Program. Blood 2002; 100: 799–803.
Pierga JY, Socie G, Gluckman E et al. Secondary solid malignant tumors occurring after bone marrow transplantation for severe aplastic anemia given thoraco-abdominal irradiation. Radiother Oncol 1994; 30: 55–58.
Khouri IF, Keating M, Korbling M et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 1998; 16: 2817–2824.
Dulley FL, Vigorito AC, Aranha FJ et al. Addition of low-dose busulfan to cyclophosphamide in aplastic anemia patients prior to allogeneic bone marrow transplantation to reduce rejection. Bone Marrow Transplant 2004; 33: 9–13.
Storb R, Erzioni R, Anasetti C et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood 1994; 84: 941–949.
Bacigalupo A . Antilymphocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant 2004; 35: 225–231.
Bacigalupo A, Lamparelli T, Bruzzi P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Van Esser JW, van der Holt B, Meijer E et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and qualitatively predicts EBV-lymphoproliferative disease following T cell depleted SCT. Blood 2001; 98: 972–978.
Dominietto A, Tedone E, Soracco M et al. Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplant based on molecular monitoring is predictive of lymphoproliferative disease. Bone Marrow Transplant 2004; 33: S192.
Marsh JC, Ball SE, Darbyshire P et al. British Committee for Standards in Haematology. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol 2003; 123: 782–801.
Acknowledgements
This work was supported by Associazione Italiana Ricerca contro il Cancro (AIRC) Milano grant to AB and Associazione Ricerca Trapianto Midollo Osseo (ARITMO), Fondazione Cassa di Risparmio di Genova (CARIGE), Genova.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Bacigalupo, A., Locatelli, F., Lanino, E. et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant 36, 947–950 (2005). https://doi.org/10.1038/sj.bmt.1705165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705165
Keywords
This article is cited by
-
Conditioning regimen for allogeneic bone marrow transplantation in children with acquired bone marrow failure: fludarabine/melphalan vs. fludarabine/cyclophosphamide
Bone Marrow Transplantation (2020)
-
Outcome of allogeneic hematopoietic stem cell transplantation in adult patients with hepatitis-associated aplastic anemia
International Journal of Hematology (2019)
-
Outcomes of a novel rituximab-based non-myeloablative conditioning regimen for hematopoietic cell transplantation in severe aplastic anemia
Bone Marrow Transplantation (2018)
-
Immunosuppressive therapy versus alternative donor hematopoietic stem cell transplantation for children with severe aplastic anemia who lack an HLA-matched familial donor
Bone Marrow Transplantation (2017)
-
Pharmacokinetic comparison of cyclosporin A and tacrolimus in graft-versus-host disease prophylaxis
Annals of Hematology (2017)