Abstract
Using a mailed questionnaire, we investigated the risk of renal cell cancer in relation to different types of alcoholic beverages, and to total ethanol in a large population-based case–control study among Swedish adults, including 855 cases and 1204 controls. Compared to non-drinkers, a total ethanol intake of >620 g month−1 was significantly related to a decreased risk of renal cell cancer (odds ratio (OR) 0.6, 95% confidence interval (CI) 0.4–0.9; P-value for trend=0.03). The risk decreased 30–40% with drinking more than two glasses per week of red wine (OR 0.6, 95% CI 0.4–0.9), white wine (OR 0.7, 95% CI 0.4–1.0), or strong beer (OR 0.6, 95% CI 0.4–1.0); there was a clear linear trend of decreasing risk with increasing consumption of these beverages (P-values for trends <0.05).
Similar content being viewed by others
Main
Case–control and prospective studies have shown an inverse association between alcohol intake and risk of renal cell cancer (Goodman et al, 1986; Asal et al, 1988; Wolk et al, 1996; Parker et al, 2002; Hu et al, 2003; Nicodemus et al, 2004; Mahabir et al, 2005; Rashidkhani et al, 2005; Lee et al, 2006, 2007), but no association (Yu et al, 1986; Brownson, 1988; Maclure and Willett, 1990; Talamini et al, 1990; Benhamou et al, 1993; Hiatt et al, 1994; Muscat et al, 1995; Boeing et al, 1997; Lindblad et al, 1997; Yuan et al, 1998; Pelucchi et al, 2002) or a slightly elevated risk among the highest category of beer drinkers (McLaughlin et al, 1984) was found in others. In studies that showed a reduced risk (Goodman et al, 1986; Asal et al, 1988; Wolk et al, 1996; Parker et al, 2002; Hu et al, 2003; Nicodemus et al, 2004; Mahabir et al, 2005; Rashidkhani et al, 2005; Lee et al, 2006, 2007), the association was more pronounced for wine (Asal et al, 1988; Rashidkhani et al, 2005), wine and liquor (Goodman et al, 1986; Wolk et al, 1996), beer (Parker et al, 2002; Lee et al, 2006), or beer and liquor (Mahabir et al, 2005). These differences may be due to small sample sizes in many studies and they require further investigation.
We investigated the association of different types of alcoholic beverages and of total alcohol (ethanol) consumption with the risk of renal cell cancer in a large population-based case–control study in Sweden.
Materials and methods
We carried out a population-based case–control study of men and women aged 20–79 years without previously diagnosed renal cell cancer (ICD-9 diagnosis code 189.0), born in Sweden or any other Nordic country and resident in Sweden between 1 January 1996 and 30 June 1998 (Bergstrom et al, 2001). Through regional cancer registers, we identified all incident cases of renal cell cancer (n=1275) in five of Sweden's six hospital regions. Patients were asked to participate through their physicians. A total of 877 cases (69%) participated in the study. Non-participation was because of death (12% of identified cases), the patient being too ill or disabled (6%), or patient refusal (13%). The cancer patients were contacted at least 1 month after diagnosis and on average after 3 months. Control subjects were randomly selected from the Swedish population registry and were frequency-matched to cases by sex, age in 10-year strata, and place of residence. Of the 2046 selected control subjects, 1508 (74%) agreed to participate, with non-participation mainly because of refusal (24% of subjects). All regional ethics committees approved the study protocol.
All case and control subjects received a self-administered questionnaire on personal and medical history, as well as dietary habits and alcohol consumption 5 years before study, disregarding recent changes. We asked about the usual frequency of consumption of medium-strong beer (2.8 g ethanol per 100 g), strong beer (4.5 g ethanol per 100 g), white wine (8.9 g ethanol per 100 g), red wine (9.9 g ethanol per 100 g), strong wine (16 g ethanol per 100 g), and hard liquor (32 g ethanol per 100 g). The respondents answered in terms of number of drinks per week, month, or year, given standard portion sizes (a glass of beer=200 ml, a glass of wine=100 ml, and a glass of strong wine or hard liquor=40 ml). Light beer (1.8 g ethanol per 100 g) consumption was given in a range of nine predetermined response categories ranging from ‘never’ to ‘three times a day or more’. We converted frequency and amount of alcohol into total grams of ethanol. The questionnaire also covered education, smoking, usual adulthood weight, height, hypertension, and diabetes. If needed, we contacted subjects by telephone for missing details. Nine cases and 284 controls failed to return the mailed questionnaire and were instead interviewed only by telephone. This short telephone interview did not include the questions on alcohol consumption. Furthermore, 13 cases and 20 controls did not answer the question on alcohol consumption in the questionnaire.
Unconditional logistic regression models were used to calculate odds ratios (OR) as estimates of relative risk and 95% confidence intervals (95% CI). Data were explored in models including only sex and age (categorised as <40, 40–49, 50–59, 60–69, 70–79 years) in addition to models with sex, age and the following covariates: cigarette smoking (never smoked, smokers stratified as low (⩽16.7 pack-years) or high (>16.7 pack-years)); usual adulthood body mass index (BMI, weight height−2 (kg m−2), stratified into quartiles); years of education (<10, 10–12, >12); hypertension (yes/no); diabetes (yes/no). Subjects who had quit smoking were classified as low (67%) or high (33%), according to their pack-years consumption. The effect of different types of alcoholic beverage was examined in separate logistic regression models in which non-drinkers of the alcoholic beverage in question served as the reference category. Median values of exposure categories were entered as continuous variables into multivariate regression models to assess the significance of linear trend with increasing exposure.
Results
A total of 855 cases and 1204 controls reported their alcohol consumption and were included in the analyses. The distributions according to age, cigarette smoking, BMI, education, hypertension, and diabetes among case and control subjects are shown in Table 1. Cases and controls were broadly similar in distribution by sex (59% male cases and 61% male controls), and age (64.3 years among cases and 64.4 among controls). The prevalence of hypertension and diabetes was higher among case subjects. Control subjects had a somewhat lower BMI, but there was no major difference in the prevalence of smoking or education. Control subjects with missing alcohol consumption had a higher BMI than control subjects who were included in the analyses. No differences in characteristics were observed between cases with missing information on alcohol consumption and those included in the analyses.
Fifteen per cent of the study population (136 cases and 179 control subjects) reported not drinking alcohol (including light beer) while two-thirds drank different types of alcoholic beverage, 11% drank only beer, 3% only wine, and 4% only strong wine or hard liquor. Consumption of white wine and red wine was correlated (Spearman correlation coefficient=0.58; 95% CI 0.55–0.60). Correlation coefficient between white wine and strong beer was 0.29 (95% CI 0.25–0.33) and between red wine and strong beer was 0.27 (95% CI 0.24–0.32). Alcohol intake was not related to BMI (r=−0.01; 95% CI −0.05 to 0.03). Control subjects drank more alcohol than cases, especially more wine. Men consumed beer and hard liquor more often and in greater quantities than women. Smokers drank more alcohol than non-smokers among both cases and controls.
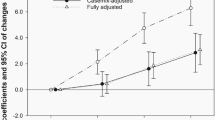
Total ethanol intake was statistically significantly associated with a decreased risk of renal cell cancer (Table 2). The multivariate OR for more than 650 g month−1 (approximately 21 g day−1) of ethanol compared to non-users of alcohol was 0.6 (95% CI 0.4–0.9). Although a statistically significant association was observed only for the >620 g month−1 category, there was a significant inverse trend (P for trend=0.03).
Odds ratios for different types of alcoholic beverage and total ethanol intake, and risk of renal cell cancer are presented in Table 3. In multivariate models including age, sex, BMI, and cigarette smoking (Model 1), consumption of more than two glasses of red wine per week was associated with a 40% (OR 0.6, 95% CI 0.4–0.9) reduction in risk compared to non-drinkers of red wine. An inverse association was also observed, albeit of borderline statistical significance, among those drinking more than two glasses per week of white wine (OR 0.7, 95% CI 0.4–1.0) or strong beer (OR 0.6, 95% CI 0.4–1.0). The risk of renal cell cancer decreased with increasing intake frequencies of white wine (P for trend=0.02), red wine (P for trend=0.01), and strong beer (P for trend=0.04). We found no relation between renal cell cancer risk and drinking of either light beer, medium-strong beer, strong wine, or hard liquor. Further adjustment of the multivariate models (Model 1) for education, hypertension, and diabetes did not affect the risk estimates (data not shown).
When we additionally adjusted for the other six beverages in the multivariate regression model, this mutual adjustment did not change risk estimates for each specific alcoholic beverage (Model 2), suggesting that strong beer, white wine, and red wine were each responsible for the reduction in risk of renal cell cancer.
To investigate if there is an effect modification by sex, we performed analyses of men and women separately. In the multivariate model, significant and nonsignificant inverse associations for total ethanol, strong beer, white wine, and red wine were observed in both men and women, but were less apparent in women (data not shown). Similarly, in analyses stratified by BMI (<25.0 vs ⩾25.0 kg m−2), smoking (ever/never), and hypertension (yes/no), there were no differences between the subgroups (data not shown).
Discussion
In this population-based case–control study, we observed an inverse association between moderate alcohol intake and risk of renal cell cancer. Consumption of red wine, white wine, and strong beer was associated with a lower risk. However, there were no clear associations with light and medium beer, strong wine, or hard liquor, perhaps due to chance or differences in other risk factors related to specific types of alcoholic drink. For example, the large variation in other risk factors such as smoking and occupation could explain why hard liquor was not associated with renal cell cancer risk although we controlled for known risk factors.
The major strengths of our study are its population-based design and the large number of cases. The Swedish regional cancer registers made it possible to ascertain virtually all incident cases of renal cell cancer and the National Population Registry enabled random selection of frequency-matched population controls. In this case–control study, both cases and controls were selected from 19 counties in Sweden (covering 79% of the population) and the participation rate was relatively high.
Nevertheless, a possible limitation might be selection bias due to non-participation or non-response. Although a substantial number of cancer patients (12%) died before they could be included or were too ill to participate (6%), this would influence the results only if alcohol consumption is associated with short-term prognosis of renal cell cancer. Refusing to participate could influence the results if this was associated with alcohol consumption. Another concern is that 97% (855) of the cases but only 80% (1204) of the control subjects in the study population answered the question on alcohol consumption. This difference is mainly due to the fact that alcohol consumption was not included in the short telephone interview with the 284 control subjects who failed to answer the mailed full questionnaire. Control subjects with missing alcohol consumption had a higher BMI than those who were included in the analyses, but alcohol intake was not related to BMI in our data. Any selection bias, therefore, has probably limited influence on our findings.
We cannot rule out the possibility that misclassification of alcohol intake affected our results given that under-reporting of alcohol consumption has often been reported. However, our observed associations are not likely to be fully explained by misclassification of alcohol intake because validation studies demonstrate that self-reported alcohol assessment methods yield most realistic levels of intake if both the frequency and amount of consumption are asked for different types of alcoholic beverages separately (Feunekes et al, 1999). Any non-differential misclassification between cases and controls, or incorrect recalling of consumption, may lead to underestimation of the true association (Rothman and Greenland, 1998). If cases tended to under-report their intake more than controls, it would distort the observed OR towards a seemingly protective effect. However, inverse associations between alcohol intake and risk of renal cell cancer observed in prospective studies (Nicodemus et al, 2004; Mahabir et al, 2005; Rashidkhani et al, 2005; Lee et al, 2006, 2007) suggest that our results are not fully explained by such bias. Even though cigarette smoking is a risk factor for renal cell cancer (Hunt et al, 2005), it did not confound the associations with alcoholic beverages in our study.
The inverse association with alcohol mentioned in the above prospective studies, and in some case–control studies (Goodman et al, 1986; Asal et al, 1988; Wolk et al, 1996; Parker et al, 2002; Hu et al, 2003), corresponds with our results, although the findings for each specific beverage varied across studies.
A shortcoming in other studies is the inability to clearly disentangle the effect of different types of alcoholic beverage owing to limited number of only/mainly drinkers of wine, beer, or hard liquor. However, in our multivariate model with mutual adjustment for individual beverages, risk estimates did not markedly change. Because we asked for alcohol consumption 5 years before the study, we cannot account for variation in consumption over time or identify ex-drinkers in our analyses. Also, we did not ask for alcohol drinking patterns. We had insufficient information to examine associations separately by histological type of renal cell cancer.
Increased insulin sensitivity might be a mechanism by which alcohol intake reduces renal cell cancer risk. Light to moderate intake is associated with improved insulin sensitivity (Facchini et al, 1994; Kiechl et al, 1996; Lazarus et al, 1997; Davies et al, 2002) and with a lower risk of diabetes (Howard et al, 2004). Because obesity is a risk factor for renal cell cancer (Calle and Kaaks, 2004), and diabetics are at higher risk than those without diabetes (Wideroff et al, 1997; Lindblad et al, 1999), it is possible that improved insulin sensitivity lowers renal cell cancer risk.
A reduced risk associated with consumption of wine and beer might be due to the phenolics they contain as these possess antioxidant and antimutagenic properties (Elattar and Virji, 1999; Denke, 2000) or increase plasma antioxidant capacity in human (Ghiselli et al, 2000). However, the lower risk that we observed for three different alcoholic beverages and total ethanol intake suggests that alcohol itself rather than a particular type of drink is responsible for the reduction in risk. However, it is unclear why we observed an inverse association only for strong beer and not for medium–strong, or light beer, although this might be due to the lower ethanol content of light (1.8%) and medium-strong (2.8%) beer compared to strong beer (4.5%).
In conclusion, we found that moderate alcohol intake was associated with a lower risk of renal cell cancer. In particular, intake of wine, both red and white, and strong beer was associated with a reduced risk of renal cell cancer in this Swedish population.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Asal NR, Risser DR, Kadamani S, Geyer JR, Lee ET, Cherng N (1988) Risk factors in renal cell carcinoma: I. Methodology, demographics, tobacco, beverage use, and obesity. Cancer Detect Prev 11: 359–377
Benhamou S, Lenfant MH, Ory-Paoletti C, Flamant R (1993) Risk factors for renal-cell carcinoma in a French case–control study. Int J Cancer 55: 32–36
Bergstrom A, Lindblad P, Wolk A (2001) Birth weight and risk of renal cell cancer. Kidney Int 59: 1110–1113
Boeing H, Schlehofer B, Wahrendorf J (1997) Diet, obesity and risk for renal cell carcinoma: results from a case–control study in Germany. Z Ernahrungswiss 36: 3–11
Brownson RC (1988) A case–control study of renal cell carcinoma in relation to occupation, smoking, and alcohol consumption. Arch Environ Health 43: 238–241
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4: 579–591
Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR (2002) Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 287: 2559–2562
Denke MA (2000) Nutritional and health benefits of beer. Am J Med Sci 320: 320–326
Elattar TM, Virji AS (1999) The effect of red wine and its components on growth and proliferation of human oral squamous carcinoma cells. Anticancer Res 19: 5407–5414
Facchini F, Chen YD, Reaven GM (1994) Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care 17: 115–119
Feunekes GI, van 't Veer P, van Staveren WA, Kok FJ (1999) Alcohol intake assessment: the sober facts. Am J Epidemiol 150: 105–112
Ghiselli A, Natella F, Guidi A, Montanari L, Fantozzi P, Scaccini C (2000) Beer increases plasma antioxidant capacity in humans. J Nutr Biochem 11: 76–80
Goodman MT, Morgenstern H, Wynder EL (1986) A case–control study of factors affecting the development of renal cell cancer. Am J Epidemiol 124: 926–941
Hiatt RA, Tolan K, Quesenberry Jr CP (1994) Renal cell carcinoma and thiazide use: a historical, case-control study (California, USA). Cancer Causes Control 5: 319–325
Howard AA, Arnsten JH, Gourevitch MN (2004) Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med 140: 211–219
Hu J, Mao Y, White K (2003) Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control 14: 705–714
Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P (2005) Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 114: 101–108
Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, Bonora E (1996) Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study). BMJ 313: 1040–1044
Lazarus R, Sparrow D, Weiss ST (1997) Alcohol intake and insulin levels. The normative aging study. Am J Epidemiol 145: 909–916
Lee JE, Giovannucci E, Smith-Warner SA, Spiegelman D, Willett WC, Curhan GC (2006) Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev 15: 1204–1211
Lee JE, Hunter DJ, Spiegelman D, Adami HO, Albanes D, Bernstein L, van den Brandt PA, Buring JE, Cho E, Folsom AR, Freudenheim JL, Giovannucci E, Graham S, Horn-Ross PL, Leitzmann MF, McCullough ML, Miller AB, Parker AS, Rodriguez C, Rohan TE, Schatzkin A, Schouten LJ, Virtanen M, Willett WC, Wolk A, Zhang SM, Smith-Warner SA (2007) Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst 16: 801–810
Lindblad P, Chow WH, Chan J, Bergstrom A, Wolk A, Gridley G, McLaughlin JK, Nyren O, Adami HO (1999) The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia 42: 107–112
Lindblad P, Wolk A, Bergstrom R, Adami HO (1997) Diet and risk of renal cell cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 6: 215–223
Maclure M, Willett W (1990) A case-control study of diet and risk of renal adenocarcinoma. Epidemiol 1: 430–440
Mahabir S, Leitzmann MF, Virtanen MJ, Virtamo J, Pietinen P, Albanes D, Taylor PR (2005) Prospective study of alcohol drinking and renal cell cancer risk in a cohort of finnish male smokers. Cancer Epidemiol Biomarkers Prev 14: 170–175
McLaughlin JK, Mandel JS, Blot WJ, Schuman LM, Mehl ES, Fraumeni Jr JF (1984 A population-based case–control study of renal cell carcinoma. J Natl Cancer Inst 72: 275–284
Muscat JE, Hoffmann D, Wynder EL (1995) The epidemiology of renal cell carcinoma. A second look. Cancer 75: 2552–2557
Nicodemus KK, Sweeney C, Folsom AR (2004) Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer 108: 115–121
Parker AS, Cerhan JR, Lynch CF, Ershow AG, Cantor KP (2002) Gender, alcohol consumption, and renal cell carcinoma. Am J Epidemiol 155: 455–462
Pelucchi C, La Vecchia C, Negri E, Talamini R, Franceschi S (2002) Alcohol drinking and renal cell carcinoma in women and men. Eur J Cancer Prev 11: 543–545
Rashidkhani B, Åkesson A, Lindblad P, Wolk A (2005) Alcohol consumption and risk of renal cell carcinoma - A prospective study of Swedish women. Int J Cancer 117: 848–853
Rothman KJ, Greenland S (1998) Precision and validity in epidemiologic studies. In Modern Epidemiology, Rothman KJ, Greenland S (eds) pp 115–134. Philadelphia: Lippincott-Raven
Talamini R, Baron AE, Barra S, Bidoli E, La Vecchia C, Negri E, Serraino D, Franceschi S (1990) A case–control study of risk factor for renal cell cancer in northern Italy. Cancer Causes Control 1: 125–131
Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH (1997) Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 89: 1360–1365
Wolk A, Gridley G, Niwa S, Lindblad P, McCredie M, Mellemgaard A, Mandel JS, Wahrendorf J, McLaughlin JK, Adami HO (1996) International renal cell cancer study. VII. Role of diet. Int J Cancer 65: 67–73
Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE (1986) Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst 77: 351–356
Yuan JM, Gago-Dominguez M, Castelao JE, Hankin JH, Ross RK, Yu MC (1998) Cruciferous vegetables in relation to renal cell carcinoma. Int J Cancer 77: 211–216
Acknowledgements
This study was supported by grants from the Swedish Cancer Foundation.
We thank the five regional cancer registers and the 110 participating clinics for collaboration in the study. Moreover, we are indebted to all participating women and men, whose efforts enabled this study.
Dr Bergström was supported by a grant from the Swedish Foundation for International Cooperation in Research and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Greving, J., Lee, J., Wolk, A. et al. Alcoholic beverages and risk of renal cell cancer. Br J Cancer 97, 429–433 (2007). https://doi.org/10.1038/sj.bjc.6603890
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603890
Keywords
This article is cited by
-
Alcohol intake and renal cell cancer risk: a meta-analysis
British Journal of Cancer (2012)
-
Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study
British Journal of Cancer (2011)
-
Kidney cancer mortality in Spain: geographic patterns and possible hypotheses
BMC Cancer (2008)