Abstract
Background:
A phase III trial was conducted to compare the safety and efficacy of erlotinib with that of gefitinib in advanced non-small cell lung cancer harbouring epidermal growth factor receptor mutations in exon 19 or 21.
Methods:
Eligible patients were randomised to receive erlotinib (150 mg per day) or gefitinib (250 mg per day) orally until disease progression or unacceptable toxicity. We aimed to determine whether erlotinib is superior to gefitinib in efficacy. The primary end point was progression-free survival.
Results:
A total of 256 patients were randomised to receive erlotinib (N=128) or gefitinib (N=128). Median progression-free survival was not better with erlotinib than with gefitinib (13.0 vs 10.4 months, 95% confidence interval (CI) 0.62–1.05, P=0.108). The corresponding response rates and median overall survival were 56.3% vs 52.3% (P=0.530) and 22.9 vs 20.1 months (95% CI 0.63–1.13, P=0.250), respectively. There were no significant differences in grade 3/4 toxicities between the two arms (P=0.172).
Conclusions:
The primary end point was not met. Erlotinib was not significantly superior to gefitinib in terms of efficacy in advanced non-small cell lung cancer with epidermal growth factor receptor mutations in exon 19 or 21, and the two treatments had similar toxicities.
Similar content being viewed by others
Main
Both gefitinib and erlotinib are first-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) for advanced non-small cell lung cancer (NSCLC) patients. Two phase III trials (the Iressa Survival Evaluation in Lung Cancer study and the BR.21 trial) comparing gefitinib or erlotinib to placebo in previously treated advanced NSCLC showed that erlotinib significantly prolonged median overall survival (OS; 6.7 vs 4.7 months, hazard ratio (HR) 0.70, P<0.001; Shepherd et al, 2005), but gefitinib did not (Thatcher et al, 2005). In 2004, two milestone studies identified somatic mutations in EGFR that predicted sensitivity and response to EGFR TKIs (Lynch et al, 2004; Paez et al, 2004). The frequency of these activating mutations, including EGFR exon 19 deletions and exon 21 (L858R) point mutations, was reported to be increased in specific NSCLC populations such as women, patients of Asian origin, and patients without a history of smoking (Fukuoka et al, 2003; Giaccone et al, 2004; Shepherd et al, 2005; Thatcher et al, 2005).
Until 2009, gefitinib and erlotinib had been considered valid treatment options for patients with advanced NSCLC who had received prior treatment (on the basis of several phase III trials (Shepherd et al, 2005; Thatcher et al, 2005; Kim et al, 2008) and were registered in many countries for this indication, particularly in Asian countries (Guan et al, 2005; Maruyama et al, 2008; Uhm et al, 2009). However, it was unclear how to choose between these two EGFR TKIs in the clinic. Although some differences in the trial results for erlotinib and gefitinib led to differences in regulatory policy, no head-to-head randomised controlled trials were published to provide a final treatment strategy. In addition, there were no significant differences in progression-free survival (PFS) or OS between first-line and second-line EGFR TKI treatment in EGFR-mutant NSCLC (Massuti et al, 2009). We were faced with the challenging problem of how to customise EGFR TKI treatment for advanced NSCLC patients with EGFR activating mutations.
Robust data were lacking, so it was difficult to make an informed choice, even though these two EGFR TKIs were available in the clinic. Therefore, in July 2009, we initiated a randomised controlled trial of erlotinib vs gefitinib in advanced NSCLC harbouring EGFR exon 19 or 21 mutations and enroled patients regardless of the line of treatment (Chinese Thoracic Oncology Group (CTONG) 0901) to determine whether erlotinib is superior to gefitinib in terms of response and survival.
Patients and methods
Eligibility criteria
Eligible patients were adults aged ⩾18 years with histologically or cytologically confirmed and locally advanced or metastatic (stage IIIB without any indications for curative chemoradiation or other local treatments to stage IV) NSCLC (AJCC/UICC version 6) harbouring EGFR exon 19 or 21 mutations detected by direct DNA sequencing as previously described (Jiang et al, 2008); measurable disease according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (Eisenhauer et al, 2009); Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2; and adequate bone marrow, liver, and kidney function. Patients without exposure to any EGFR inhibitors were eligible for recruitment. Those with clinically unstable brain metastases, a history of cardiac disease, uncontrolled hypertension, other active malignancies, or any active infectious diseases were excluded.
Treatment schedule
After screening, patients were randomly assigned at a 1 : 1 ratio to receive oral erlotinib 150 mg or gefitinib 250 mg once daily. Second- and further-line treatments were defined as second line in the present study. Treatment continued until unacceptable toxicity, disease progression, or another discontinuation criterion was met. Erlotinib or gefitinib dose delays of ⩽14 days were permitted for grade ⩾3 nonhaematological toxicities until resolution to grade 1 or baseline, and treatment was reintroduced at a reduced dosage depending on the toxicity.
Tumour response was assessed by investigators according to RECIST version 1.1 (Eisenhauer et al, 2009). The initial response was assessed after 5 weeks of treatment, and the baseline assessments were repeated every 2 months. Toxicities were assessed by the investigators based on the incidence and severity of adverse events (AEs), according to the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 3.0.
Study design and objectives
In June 2009, this study was a head-to-head phase II randomised controlled trial (CTONG 0901; clinicaltrials.gov No. NCT01024413) comparing erlotinib with gefitinib for patients with exon 21 mutations. The primary end point was response rate (RR), and the secondary endpoints included PFS, OS, and safety. The sample size was 70 (35 in each arm).
However, the protocol was amended in January 2010, and the study was redesigned and approved as a phase III randomised controlled trial by the appropriate independent ethics committees at the Guangdong Lung Cancer Institute, Guangdong General Hospital; this study was conducted according to the Declaration of Helsinki. EGFR exon 19 or 21 mutation-positive patients with advanced NSCLC were allowed to be recruited into this phase III trial. All patients provided written informed consent before study participation.
In this phase III trial, the primary end point was PFS, and the secondary end points included OS, RR, and safety. An exploratory end point was efficacy between the exon 19 and 21 mutation groups.
Statistical considerations
The study hypothesis was that erlotinib would improve PFS relative to gefitinib in advanced NSCLC harbouring EGFR exon 19 or 21 mutations. Based on the median PFS of 9.5 months with gefitinib and 14.0 months with erlotinib (Mok et al, 2008; Massuti et al, 2009), 80% power to detect a HR of 0.65 at a two-sided significance level of 0.05, 12 months of enrolment, 48 months of study duration, and a 5% rate of loss to follow-up, the appropriate sample size was calculated to be 254 patients with 127 in each arm, with statistical analysis of median survival time by log-rank test.
Chi-square or Fisher’s exact tests were used to compare qualitative data. PFS was defined as the time of randomisation to the first documentation of progressive disease (PD) or death from any cause. OS was calculated from randomisation to the last visit or death from any cause. Efficacy analyses were completed for the intent-to-treat population.
The Kaplan-Meier method was used to generate survival curves. The log-rank test was used to compare survival curves among patient groups. All statistical tests were two-sided, and 0.05 was deemed to indicate statistical significance. PASS version 11.0 (NCSS, Inc., Kaysville, Utah, USA) was used for the analyses.
Results
Patient population and characteristics
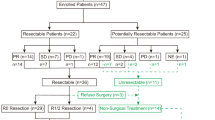
Between July 2009 and 2014, 256 patients at the Guangdong Lung Cancer Institute satisfied the inclusion or exclusion criteria and were randomly assigned (128 in each arm; Figure 1). The first and last patients with exon 21 mutations were recruited on 13 July 2009, and 11 July 2014, respectively. The first and last patients with exon 19 mutations were recruited on 26 February 2010 and 29 January 2014, respectively. In the erlotinib arm, 10 patients did not have an assessment of tumour response (three did not return to the hospital for evaluation; two discontinued treatment by themselves within one month; and two died within 1 month of treatment) or a confirmed response (three had an initial response of SD but did not undergo further imaging). In the gefitinib arm, eight patients did not undergo an assessment of tumour response (four died within 1 month of treatment, and four discontinued treatment by themselves within 1 month due to AEs or financial problems and refused to undergo imaging investigation). The compliance of the enroled patients was 95.3% (244/256).
Patient characteristics
The 256 randomized patients formed well-balanced treatment arms in terms of baseline demographics and clinicopathological characteristics (Table 1). The median age was 58.5 years (range, 30–85 years); 57.8% of the patients had EGFR exon 19 deletions, and 66.0% were in the first-line setting.
Efficacy analysis
The last follow-up was on 30 June 2015, and the median follow-up time was 22.1 months. When 218 progression events (85.2%) and 184 survival events (71.9%) had occurred, both PFS and OS were mature. PFS did not differ significantly between the treatment arms in the intent-to-treat population (HR 0.81, 95% CI 0.62–1.05, P=0.108; Figure 2A). Median PFS was 13.0 (95% CI 11.1–14.9) vs 10.4 (95% CI 8.8–11.9) months for the erlotinib and gefitinib arms, respectively (Figure 2A). Similarly, OS was not significantly different (median OS: erlotinib, 22.9 months; gefitinib, 20.1 months; HR 0.84, 95% CI 0.63–1.13, P=0.250; Figure 2B). Post-discontinuation therapies are listed in Supplementary Table S1. There was no significant difference in RR between the two arms in the intent-to-treat population (56.3% (76/128) vs 52.3% (67/128), P=0.530). The waterfall plots for the best percentage change in target lesion size are shown for the two arms (Figure 3).
Kaplan-Meier curves of PFS and OS in 256 patients.(A) Median PFS in the erlotinib and gefitinib arms. (B) Median OS in the erlotinib and gefitinib arms. (C) Median PFS in the EGFR exon 19 and 21 mutation arms. (D) Median OS in the EGFR exon 19 and 21 mutation arms. EGFR, epidermal growth factor receptor.
Baseline demographic characteristics for the EGFR exon 19 and 21 mutation arms are shown in Table 2. Except for age and line of EGFR TKI treatment, the other baseline demographics were well-balanced between the two arms. Upon receiving erlotinib or gefitinib treatment, patients with EGFR exon 19 mutations were superior to those with exon 21 mutations in terms of median OS (22.9 vs 17.8 months, HR 0.71, 95% CI 0.53–0.95, P=0.022; Figure 2D) and RR (62.2% vs 43.5%, P=0.003; Table 3), even though there was no significant difference in median PFS (11.4 vs 11.2 months, HR 0.82, 95% CI 0.63–1.08, P=0.160; Figure 2C).
However, in the first-line setting, the erlotinib and gefitinib arms had an RR of 58.0% (47/81) vs 52.4% (44/84) (P=0.466), a median PFS of 13.2 vs 11.1 months (HR 0.96, 95% CI 0.69–1.35, P=0.827), and a median OS of 22.4 vs 20.7 months (HR 0.98, 95% CI 0.67–1.42, P=0.902).
Safety
In the safety population of 256 patients who received any dose of study drug, no significant difference was observed in the frequency of Grade ⩾3 AEs in the erlotinib and gefitinib arms (5.4% vs 1.6%, P=0.172). No cases of interstitial lung disease were recorded. The treatment-emergent AEs that were observed in ⩾10% of the patients in each arm are shown in Table 4.
Discussion
To the best of our knowledge, the present study was the first head-to-head phase III randomised controlled trial comparing erlotinib with gefitinib in EGFR activating mutation-positive NSCLC. The results did not support the hypothesis described in the study design, namely, that the primary end point, PFS, would be significantly prolonged with erlotinib compared with gefitinib. These two EGFR TKIs produced similar results for RR, OS, and toxicity. To some extent, the conclusion of the present study was almost the same as that of the earlier randomised phase II study, in which gefitinib and erlotinib showed similar efficacy and tolerable toxicity profiles as second-line treatments for molecularly selected (EGFR activating mutations accounted for 17.7% (17/96) of all enroled patients) or clinically selected populations of patients with NSCLC (Kim et al, 2012). However, patients with EGFR exon 19 or 21 mutations in any line setting were enroled in the present study, leading to precision medicine in advanced NSCLC. In addition, the present study had a larger sample size (N=256; 128 in each arm) than the previous phase II study (N=96; 48 in each arm; Kim et al, 2012).
A prospective study showed no significant difference in PFS (14.0 vs 13.0 months, P=0.62) or OS (28.0 vs 27.0 months, P=0.67) between first- and second-line erlotinib treatment in EGFR-mutant NSCLC (Massuti et al, 2009; Rosell et al, 2009). Furthermore, in recent years, several phase III randomised controlled trials have demonstrated no significant difference in OS between first-line EGFR TKIs and chemotherapy for patients with EGFR-mutant advanced NSCLC, probably owing to subsequent EGFR TKI treatment for those receiving first-line chemotherapy (Mok et al, 2009; Maemondo et al, 2010; Mitsudomi et al, 2010; Zhou et al, 2011; Rosell et al, 2012; Sequist et al, 2013; Wu et al, 2014). Taken together, the design of the present study, comparing erlotinib with gefitinib in both first- and second-line settings, was evidence-based and could be rationalised in 2009.
In the present study, subgroup analyses showed that patients with EGFR exon 19 mutations had a significantly higher RR (62.2% vs 43.5%, P=0.003) and longer median OS (22.9 vs 17.8 months, P=0.022) than those with exon 21 mutations treated with erlotinib or gefitinib, similar to the results of several retrospective studies in which better efficacy was observed in patients with EGFR exon 19 deletions than in those with exon 21 L858R mutations (Jackman et al, 2006; Riely et al, 2006; Rosell et al, 2012); however, the present study had a relatively large sample size (N=148 vs N=108) and a prospective design based on our translational data (Zhu et al, 2008). Recently, an analysis of OS data from two randomised phase III trials suggested that EGFR del19-positive disease might be distinct from L858R-positive disease and that these subgroups should be analysed separately in future trials (Sequist et al, 2013; Wu et al, 2014; Yang et al, 2015). However, the above differences were found in subgroup analyses. Therefore, it could be very challenging to draw a definitive conclusion.
Recently, first-line gefitinib was approved by the FDA for patients with EGFR-mutant advanced NSCLC (FDA approves targeted therapy for first-line treatment of patients with a type of metastatic lung cancer, 2015). The present study identified no significant differences in efficacy or toxicity profile between first-line erlotinib and gefitinib for patients with EGFR-mutant disease, and these results could be considered globally. Recently, in the LUX LUNG 7 trial, first-line afatinib (an irreversible ErbB family blocker) significantly improved PFS vs gefitinib in EGFR-mutant patients (HR 0.73, 95% CI 0.57–0.95, P=0.0165; Park et al, 2016). However, the LUX LUNG 7 trial was a global randomised phase IIb study, and the results will be validated by future phase III trials. The question remains as to the acceptable length of the survival benefit in the clinic. More investigations are warranted to determine which generation EGFR TKIs will be the best choice for the treatment of EGFR-mutant patients.
There are a few limitations to the present study. First, it took 5 years to complete recruitment at a single centre, and several competitive trials might have affected enrolment during this long period, possibly leading to an enrolment bias. Second, the present study was not sponsored by any pharmaceutical companies, and patients self-paid for study drugs and imaging investigations, so a few were not fully compliant. Finally, the efficacy data for erlotinib and gefitinib indicated that a much larger sample size was necessary in the present study.
In conclusion, the primary end point was not met in the present study, and erlotinib was not significantly superior to gefitinib in advanced NSCLC with EGFR exon 19 or 21 mutations in terms of response or survival, and it had similar toxicity. Meanwhile, upon treatment with erlotinib or gefitinib, patients with exon 19 mutations had markedly better outcomes than those with exon 21 mutations.
Change history
28 February 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45 (2): 228–247.
FDA approves targeted therapy for first-line treatment of patients with a type of metastatic lung cancer (2015) Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm454678.htm (accessed 13 July).
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J (2003) Multiinstitutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial). J Clin Oncol 21 (12): 2237–2246.
Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, Gatzemeier U, Grous J, Ochs JS, Averbuch SD, Wolf MK, Rennie P, Fandi A, Johnson DH (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 22 (5): 777–784.
Guan ZZ, Zhang L, Li LY, Jiang GL, Liu XY, Chu DT, Zhao HY, Li W (2005) Efficacy of gefitinib on Chinese patients with locally advanced or metastatic non-small cell lung cancer: a clinical trial. Ai Zheng 24 (8): 980–984.
Jackman DM, Yeap BY, Sequist LV, Lindeman N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS, Haber DA, Lynch TJ, Meyerson M, Johnson BE, Jänne PA (2006) Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res 12 (13): 3908–3914.
Jiang SX, Yamashita K, Yamamoto M, Piao CJ, Umezawa A, Saegusa M, Yoshida T, Katagiri M, Masuda N, Hayakawa K, Okayasu I (2008) EGFR genetic heterogeneity of non-small cell lung cancers contributing to acquired gefitinib resistance. Int J Cancer 123 (11): 2480–2486.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY (2008) Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet 372 (9652): 1809–1818.
Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim SW, Jung SH, Park YH, Ahn JS, Park K, Ahn MJ (2012) Randonmized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer 75 (1): 82–88.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350 (21): 2129–2139.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362 (25): 2380–2388.
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26 (26): 4244–4252.
Massuti B, Morán T, Porta R, Queralt C, Cardenal F, Mayo C, Camps C, Majem M, Tarón M, Rosell R (2009) Multicenter prospective trial of customized erlotinib for advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: final results of the Spanish Lung Cancer Group (SLCG) trial. J Clin Oncol 27: 15s.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11 (2): 121–128.
Mok T, Wu Y-L, Thongprasert S, Yang C-H, Chu D, Saijo N, Jiang H, Watkins C, Armour A, Fukuoka M (2008) Phase III, randomised, open-label, first-line study of gefitinib (G) vs carboplatin/paclitaxel (C/P) in clinically selected patients (PTS) with advanced non-small-cell lung cancer (NSCLC) (IPASS). Ann Oncol 19 (S8): viii1–viii4.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361 (10): 947–957.
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304 (5676): 1497–1500.
Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, Kim DW, Arvis CD, Kölbeck K, Laurie SA, Tsai CM, Shahidi M, Kim M, Massey D, Zazulina V, Paz-Ares L (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17 (5): 577–589.
Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA (2006) Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12 (3Pt1): 839–844.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13 (3): 239–246.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M (2009) Screening for epidermal growth factor receptor mutation in lung cancer. N Engl J Med 361 (10): 958–967.
Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31 (27): 3327–3334.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353 (2): 123–132.
Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366 (9496): 1527–1537.
Uhm JE, Park BB, Ahn MJ, Lee J, Ahn JS, Kim SW, Kim HT, Lee JS, Kang JH, Cho JY, Song HS, Park SH, Sohn CH, Shin SW, Choi JH, Park K (2009) Erlotinib monotherapy for stage IIIB/IV non-small cell lung cancer: a multicenter trial by the Korean Cancer Study Group. J Thorac Oncol 4 (9): 1136–1143.
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15 (2): 213–222.
Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, Lu S, Huang Y, Geater SL, Lee KY, Tsai CM, Gorbunova V, Hirsh V, Bennouna J, Orlov S, Mok T, Boyer M, Su WC, Lee KH, Kato T, Massey D, Shahidi M, Zazulina V, Sequist LV (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16 (2): 141–151.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12 (8): 735–742.
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL, Zhang YF, An SJ, Mok TS, Wu YL (2008) Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 265 (2): 307–317.
Acknowledgements
We thank the patients, investigators, study nurses, and the leadership of the CTONG who participated in this study, as well as the laboratory that conducted the EGFR mutation testing (South China Clinical Center for Genetic Tests, Guangzhou, China). We are grateful to the families of all the recruited patients. This study was supported by the following: (1) Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (No. 2012A061400006); (2) special fund for research in the public interest from the National Health and Family Planning Commission of People’s Republic of China (No. 201402031); (3) research fund from the Guangzhou Science and Technology Bureau (Nos. 2011Y2-00014 and 2014Y2-00545) (to Yi-Long Wu); and (4) the Project of the National Natural Science Funding of China (No. 81472207).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
YLW received speaker fees from Eli Lilly, Roche, AstraZeneca, Pfizer, and Sanofi. The remaining authors declare that they have no competing interest.
Additional information
Data from this study were previously accepted for a mini-oral presentation at the 16th World Conference on Lung Cancer (WCLC), Denver, United States of America, 6–9 September 2015.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Yang, J., Zhou, Q., Yan, H. et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 116, 568–574 (2017). https://doi.org/10.1038/bjc.2016.456
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.456
Keywords
This article is cited by
-
The difference between dacomitinib and afatinib in effectiveness and safety in first-line treatment of patients with advanced EGFR-mutant non-small cell lung cancer: a real-world observational study
BMC Cancer (2024)
-
Monitoring of T790M in plasma ctDNA of advanced EGFR-mutant NSCLC patients on first- or second-generation tyrosine kinase inhibitors
BMC Cancer (2023)
-
Mutational landscape of cancer-driver genes across human cancers
Scientific Reports (2023)
-
Overall survival analysis of patients enrolled in a randomized phase III trial comparing gefitinib and erlotinib for previously treated advanced lung adenocarcinoma (WJOG5108LFS)
International Journal of Clinical Oncology (2023)
-
Molecular pathways, resistance mechanisms and targeted interventions in non-small-cell lung cancer
Molecular Biomedicine (2022)