Abstract
Background:
Obtaining tissue for pancreatic carcinoma diagnosis and biomarker assessment to aid drug development is challenging. Circulating tumour cells (CTCs) may represent a potential biomarker to address these unmet needs. We compared prospectively the utility of two platforms for CTC enumeration and characterisation in pancreatic cancer patients in a pilot exploratory study.
Patients and methods:
Blood samples were obtained prospectively from 54 consenting patients and analysed by CellSearch and isolation by size of epithelial tumour cells (ISET). CellSearch exploits immunomagnetic capture of CTCs-expressing epithelial markers, whereas ISET is a marker independent, blood filtration device. Circulating tumour cell expression of epithelial and mesenchymal markers was assessed to explore any discrepancy in CTC number between the two platforms.
Results:
ISET detected CTCs in more patients than CellSearch (93% vs 40%) and in higher numbers (median CTCs/7.5 ml, 9 (range 0–240) vs 0 (range 0–144)). Heterogeneity observed for epithelial cell adhesion molecule, pan-cytokeratin (CK), E-Cadherin, Vimentin and CK 7 expression in CTCs may account for discrepancy in CTC number between platforms.
Conclusion:
ISET detects more CTCs than CellSearch and offers flexible CTC characterisation with potential to investigate CTC biology and develop biomarkers for pancreatic cancer patient management.
Similar content being viewed by others
Main
Pancreatic adenocarcinoma is the eleventh most common cancer in the United Kingdom (Cancer Research UK, 2011, http://info.cancerresearchuk.org/cancerstats/types/pancreas/incidence/) with a 5-year survival across all disease stages of 2–3%. Medical treatment with single-agent gemcitabine or more recently FOLFIRINOX combination chemotherapy confers a survival benefit with improvement in quality of life measures (Burris et al, 1997; Conroy et al, 2011). Molecularly targeted therapies however have failed to show an additional advantage to chemotherapy. The addition of erlotinib to gemcitabine resulted in a marginal improvement in overall survival (OS), although no predictive biomarker has been identified to determine which patients benefit most from therapy (Moore et al, 2007; da Cunha Santos et al, 2010). Moreover, both cetuximab and bevacizumab have failed to demonstrate an improvement over gemcitabine monotherapy (Kindler et al, 2010; Philip et al, 2010).
The lack of stratified therapies and limitations to new drug target identification in pancreatic cancer are mirrored and underpinned by a paucity of informative biomarkers. Currently, Ca19-9 is the only clinically implemented biomarker used as a diagnostic aid, and as a surrogate marker of response and patient outcome. However, CA19-9 is not applicable to all patients (10% are not within the Lewis blood group) and can be falsely elevated (Ko et al, 2005; Ferrone et al, 2006; Goonetilleke and Siriwardena, 2007).
Substantial challenges exist in obtaining tissue from pancreatic cancer patients for histological diagnosis, and acquiring pre- and post-treatment tumour biopsies to monitor pharmacodynamic responses during clinical trials of novel treatments are even more difficult. Circulating tumour cell (CTC) analysis may yield prognostic, predictive and pharmacodynamic biomarker information. Moreover, the molecular characterisation of CTCs may provide insight on the process of metastasis per se and could facilitate discovery of new drug targets. If CTCs are demonstrated to be useful as a biomarker(s), then ultimately they could represent a less invasive alternative to tissue for diagnosis and treatment management, particularly where biopsy is considered unsafe or unfeasible.
Recent advances in technology have enabled protocol standardisation to enumerate CTCs and allow comparisons between clinical studies (Krebs et al, 2010). Circulating tumour cell number, as determined by the CellSearch technology platform (Veridex, LLC, Raritan, NJ, USA), is of prognostic significance in metastatic breast, colorectal and prostate carcinomas, and is FDA approved for prognostication in these clinical contexts (Cristofanilli et al, 2004; Cohen et al, 2008; de Bono et al, 2008). Our laboratory has demonstrated recently that CTC detection by CellSearch also has prognostic significance in lung carcinomas (Hou et al, 2009; Krebs et al, 2011b).
Detection of a CTC using the CellSearch platform is dependent on CTC expression of epithelial markers, namely epithelial cell adhesion molecule (EpCAM) for tumour cell capture and cytokeratin (CK) for tumour cell confirmation.
As EpCAM-negative CTCs are not detected using the CellSearch platform, and homogeneity of EpCAM expression on pancreatic CTCs was not established, we reasoned that an alternative approach for CTC isolation and enumeration should be investigated. Isolation by size of epithelial tumour cells (ISET) is a filtration-based, marker-independent method of CTC detection based on cell size and morphology. ISET has been used successfully to study CTCs in patients with melanoma, breast, lung and hepatocellular carcinomas (Vona et al, 2004; Pinzani et al, 2006; De Giorgi et al, 2010; Hofman et al, 2011; Hou et al, 2011).
The primary objective of this prospective, proof-of-principle exploratory study in patients with metastatic and inoperable pancreatic cancer was to examine the feasibility of CTC enumeration across this heterogeneous patient population, comparing the CellSearch and ISET platforms, in order to determine the utility of CTCs as a source of biomarkers in this disease setting. Exploratory end points were to explore whether CTC number holds potential as a prognostic biomarker relating to the clinical end points of progression-free and OS, and to inform the statistical design of future studies. We previously demonstrated substantial heterogeneity in epithelial and mesenchymal marker expression in lung cancer CTCs using ISET (Hou et al, 2011). We also sought to explore the hypothesis that pancreatic CTCs also exhibit nonuniform epithelial marker expression, altered during the process of epithelial to mesenchymal transition (EMT) that is associated with loss of cell–cell contacts, tumour cell invasion and metastasis (Polyak and Weinberg, 2009). To extend our assessment of EMT during metastasis, we compared the expression of EMT markers (EpCAM, CKs, E-Cadherin and Vimentin) in pancreatic tumours and in pancreatic CTCs.
Materials and methods
Patients
This single-centre prospective study was undertaken at the Christie Hospital, Manchester, UK. Patients with newly diagnosed or progressive metastatic or inoperable adenocarcinoma of the pancreas were eligible, requiring at least a minimum of 6-week treatment-free period. The population chosen was heterogeneous as this study was exploratory as to whether CTCs were detectable in pancreatic cancer per se. Histological diagnosis was not mandatory provided a clinico-radiological diagnosis was agreed by a specialist pancreato-biliary multidisciplinary team. Patients with a previous history of carcinomas within 5 years were excluded.
Clinical data were collected for age, gender, site of primary tumour, site and number of metastases, pathological diagnosis, prior treatment received (including surgical intervention and stent insertion), number of lines of treatment, Ca19-9 levels (at baseline and 8–12 weeks after treatment) and clinical outcomes. Overall survival and progression-free survival (PFS) were calculated from time of consent onto the study. The study was approved by the Local Research Ethics Committee. Peripheral venous blood was taken at study enrolment (before the first planned treatment) for the analysis of CTCs using both CellSearch and ISET platforms. The CellSearch platform was acquired before the ISET device and blood samples obtained from the first 19 patients enrolled were analysed using CellSearch only.
CTC enumeration by CellSearch
Blood samples were collected in CellSave tubes (Veridex), stored at room temperature and processed within 96 h according to manufacturer's instructions. Procedures relating to the use of the CellSearch platform have been previously described in detail (Allard et al, 2004). All cell images collected and displayed on the CellSearch gallery were independently reviewed by two analysts (blinded) with any discordant results rereviewed by both analysts.
CTC enrichment by ISET
The ISET process has been described previously in detail (Vona et al, 2000). Briefly, blood (10 ml) was collected into an EDTA tube (Beckton Dickinson, Franklin Lakes, NJ, USA) and within 4 h divided into 1 ml aliquots. Each aliquot was diluted 1 : 10 with red cell lysis buffer (RareCell Diagnostics, Paris, France) and was loaded into an individual well of the ISET filter module (RareCell Diagnostics) consisting of a 10-well plastic reservoir above a polycarbonate membrane perforated with 8 μm cylindrical pores. Blood samples were filtered by attaching the module to the ISET device and applying gentle regulated suction. This process produced 10 discrete ‘6 mm2 membrane ‘spots’, on which cells contained within the 1 ml aliquot of whole blood were deposited. Membranes were stored at −20°C.
Enumeration of CTCs by ISET
Enumeration of CTCs was performed after ISET filtration and negative selection to exclude CD45 positively stained leukocytes by IHC alongside confirmation of characteristic tumour cell morphology. Individual membrane spots were allowed to equilibrate to room temperature before incubating in pH6 citrate antigen retrieval buffer (S1699, Dako, Glostrup, Denmark) in a 99 °C water bath for 40 min. Membranes were washed briefly in tris-buffered saline (TBS) before placing in 0.2% Triton for 10 min. Membranes were washed in TBS and incubated in a 3% solution of hydrogen peroxide in methanol for 30 min. Membranes were washed again in distilled water before the primary CD45 antibody (1 : 30 dilution clone T29/33, Dako) in S0809 antibody diluent (Dako) was added and incubated overnight at 4 °C. Envision Liquid DAB + Substrate Chromagen System (Dako) were used according to manufacturer's instructions to visualise CD45 staining. Counterstaining with 1 × Gill's haematoxylin was performed for 3 min. The membrane spots were mounted on glass slides using Fairmount aqueous mounting medium (Dako) and coverslips were applied. The Bioview duet microscope system (Olympus BX52 microscope (Olympus) and image analysis software, Bioview, Rehovot, Israel) was used to scan membranes at × 40 magnification. Manual image review and scoring of tumour cells was performed by one analyst (blinded).
CD45-negative cells with a high nuclear to cytoplasmic ratio, irregular shaped, hyperchromatic nuclei and diameter >10 μm were designated as CTCs. Four of the 10 membrane spots obtained were used for CTC enumeration. Mean CTC count was extrapolated to 7.5 ml for direct comparison with the standardised CellSearch enumeration procedure. The mean ISET CTC count from four spots was demonstrated to provide a robust CTC count (Krebs et al, 2011a; see Supplementary Figure 2).
Molecular characterisation of CTCs
Molecular characterisation of pancreatic cancer CTCs was performed for the expression of five epithelial or mesenchymal markers. The number of markers analysed was dependent upon the number of membrane spots per patient sample where CTCs were detected. The following IHC analyses were performed using the procotol described above for CD45 and the following antibodies: EpCAM (1 : 100 #MS-144, Labvision, Kalamazoo, MI, USA), anti-CK C-11 antibody to CKs 4,5,6,8,10,13,18 (1 : 100 #MS-149, Labvision), Vimentin (1 : 100, clone v9, Dako) CK 7, as a pancreatic-specific CK marker (1 : 50, clone ov-TL 12/30, Dako) and E-Cadherin (1 : 1000, clone 36/E-Cadherin, BD Biosciences, Franklin Lakes, NJ, USA). EpCAM expression was assessed in 30 patients, Pan CK (C-11) in 19 patients, CK 7 in 25 patients, E-Cadherin in 30 patients and Vimentin in 31 patients.
IHC of tumour biopsies
Paraffin-embedded tumour blocks were obtained wherever possible to allow correlation of tumour differentiation status with epithelial and mesenchymal marker expression, and to explore which platform might be more suitable for pancreatic CTC detection, that is, whether CellSearch should be able to detect a majority of CTCs that are EpCAM, CK double positive or whether the marker-independent ISET platform would be more appropriate if epithelial markers were likely to be expressed in a minority sub-population of CTCs only. Sections (4 μm) were stained with haematoxcylin and eosin, EpCAM and CK, dewaxed in xylene, and rehydrated using serial washes in 100–70% alcohol. The same IHC protocol and antibodies used for ISET CTC analysis was adopted to determine tumour EpCAM and CK expression in tumour samples. Tumour differentiation and scoring for staining intensity was assessed independently in a blinded fashion, by an experienced histopathologist (LM).
Statistical analysis
Statistical analysis was performed using SPSS (IBM, Hampshire, UK) for Windows where P-values of ⩽0.05 were considered significant. Graphpad prism was used to produce Kaplan–Meier survival curves.
Variables were positively skewed and were log transformed before analysis to stabilise the sample variance and nonparametric tests were used to satisfy the assumptions of variance between the sample groups. The association of CTCs with individual clinical characteristics, including presence of a stent, number of metastatic sites, site of primary, and degree of primary tumour differentiation and performance status were compared by Fisher's exact test or χ2 test. Univariate survival analysis was performed using the Kaplan–Meier method with data categorised into (median) middle quartiles that were compared using the log-rank (Mantel–Cox) test (serial CTC thresholds were tested as cut off levels to stratify patients), with the median CTC count chosen as the cut off level for all analysis. Survival was calculated from time of study enrolment to progression (PFS) or death (OS). The Mann–Whitney U test was used to determine whether CellSearch and ISET were significantly different, that is, they detected different numbers of CTCs.
Results
Recruitment and clinical characteristics of patients
Fifty-four patients aged 35–85 years (29 males and 25 females) were enrolled between July 2008 and October 2009. Ten patients had relapsed or progressive disease, 4 patients relapsed following surgery (one of whom had received adjuvant chemotherapy) and 6 patients had progressive metastatic disease having had previous palliative chemotherapy. The remaining patients were newly diagnosed. Fifty-three patients were evaluable by CellSearch, and of these, 31 patients had paired blood samples for ISET assessment and EMT marker expression. Direct comparison of CTC number between the ISET and CellSearch platforms was possible for 27 patients. Patient clinical characteristics are shown in Table 1.
CTC enumeration using CellSearch and ISET
Of the 53 patient blood samples assessed using the CellSearch platform, 21 patients had detectable CTCs defined as ⩾1 CTC (Figure 1). The majority of patients had between 1 and 2 CTCs and five patients had >5 CTCs. Isolation by size of epithelial tumour cells detected higher CTC numbers compared with CellSearch and over a greater dynamic range. The direct comparison of CTC/7.5 ml detected by CellSearch vs ISET for the 27 patients with matched samples revealed that the mean CTC/7.5 ml was 2 vs 26, the median CTC/7.5 ml was 0 vs 9 and that the range was 0–15 vs 0–240, respectively. Direct comparison of CTC numbers per patient is shown in Table 2. A nonparametric Mann–Whitney U test showed a P-value 0.000002 indicating that the CTC numbers detected by CellSearch and by ISET were significantly different to each other.
CTC number and clinical outcomes
Compared with those without detectable CTCs, pancreatic cancer patients with a CTC ⩾1/7.5 ml blood, measured by CellSearch, had poorer performance status (50% were performance status 2), higher burdens of disease (54% had more than one site of disease) and had primaries situated in the head or tail of the pancreas (38% and 55%, respectively). However, perhaps due to small sample size none of these variables reached statistical significance (Table 1), and a larger powered study would be needed to demonstrate this formally. The PFS and OS for patients without vs those with CTCs was 140 vs 94 days (P=0.13 log rank, hazard ratio 0.63 (95% confidence interval: 0.34–1.14)) and 164 vs 127 days (P=0.26 log-rank, hazard ratio 0.7 (95% confidence interval 0.38–1.29)) (Figures 2A and B), respectively. These differences show a nonsignificant trend towards decreased PFS and OS for patients with detectable CTCs (irrespective of what CTC threshold was used) On the basis of these results, 199 patients would be required to determine the prognostic significance of CTC number by CellSearch analysis in a future study.
PFS and OS for patients without and with CTCs. (A) Progression-free survival of patients without CTCs vs those with CTCs using the CellSearch platform. Median survival (days) 140 vs 94 hazard ratio 0.63. P=0.13 (log rank). (B) Overall survival of patients without CTCs vs those with CTCs using the CellSearch platform. Median survival (days) 164 vs 127 hazard ratio 0.7. P=0.26 (log rank).
Circulating tumour cell/7.5 ml measured by ISET (using serial CTC thresholds) did not correlate with either OS or PFS in this small sample set (data not shown). Only nine patients were recruited onto the study with locally advanced disease, but even when these patients were excluded clinical end points of PFS and OS remained non-statistically significant with regard to CTC/7.5 ml.
Univariate Cox regression results for PFS and OS were obtained for CellSearch CTC/7.5 ml, ISET CTC/7.5 ml, baseline and mid-treatment CA19-9 levels, and percentage change in CA19-9 (between baseline and after 8–12 weeks of treatment). Circulating tumour cell/7.5 ml determined using either CellSearch or ISET platforms was not significant for PFS or OS: CellSearch PFS P=0.2, OS P=0.19; ISET PFS P=0.85, OS P=0.36. The percentage change in CA19-9 levels was statistically significant for both PFS and OS (P=0.0003 and P=0.01, respectively) confirming previous studies (Ko et al, 2005).
Expression of epithelial markers EpCAM and CKs in pancreatic tumours
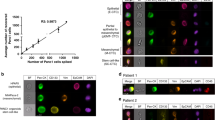
We hypothesised that patients whose tumours expresed EpCAM and CK would be more likely to have CellSearch detectable CTCs. Epithelial cell adhesion molecule expression was assessed by IHC in the tumour specimens available from 21 patients within the cohort. The expression of Pan CK (C-11) was also evaluated where sufficient biospy allowed in 13/21 cases. Tumours, reported by a certified pathologist (LM), were graded as either well, moderately or poorly differentiated. All tumours expressed EpCAM; EpCAM expression was graded as 1+ (n=6), 2+ (n=7) or 3+ (n=8) (Figure 3, panels A (3+), B (2+) and C (1+). Cytokeratin expression was graded as positive, weakly positive or negative (Figures 3D–F). There was intratumour heterogeneity in CK expression but only 4/13 tumours were CK positive. There was no correlation between the degree of tumour differentiation, level of expression of EpCAM or CK in tumour and CTC number/7.5 ml by CellSearch. When both the CellSearch capture antigen (EpCAM) and the detection antigen (CK) are expressed in tumour, the CellSearch detected CTC number can be zero. A summary of marker expression is given per patient in Table 3. Positive and negative controls for IHC markers are shown in Supplementary Figure 1.
EpCAM and CK expressions in CTCs detected by ISET
The low number of CTCs detected by CellSearch could arise due to low or no CTC expression of EpCAM and/or CK. Of the 30 patients’ CTCs analysed for EpCAM expression, 9 had EpCAM-negative CTCs, 3 had CTCs that were all weakly positive for EpCAM and in the remaining 18 patients EpCAM expression was heterogenous, that is, only a proportion of CTCs were EpCAM postive (Figures 4A–C). Of the 19 patients whose CTCs were analysed for CK, 1 patient had strong expression of CKs in their CTCs, 3 patients had homogeneously CK-negative CTCs, 2 patients had CTCs all of which had weak expression of CKs; (Figures 4D–F) and for the remaining 13 patients their CTCs showed intra-patient heterogeneity in CK expression. However, there was no obvious correlation between the observed CellSearch CTC/7.5 ml and the ISET-detected CTC expression profiles of EpCAM and CKs, which was perhaps not surprising considering that intra-patient heterogeneity in these markers was the predominant finding.
Examples of differential expression of EpCAM and Pan CK C-11 staining of CTCs. (A) Epithelial cell adhesion molecule-negative staining in patient 60, (B) EpCAM weakly positive staining in patient 54, (C) EpCAM-positive staining in patient 51. (D) C-11-negative staining in patient 51, (E) C-11 weakly positive staining in patient 42, (F) C-11-positive staining in patient 50.
Circulating tumour microemboli in pancreatic cancer patients
Tumour cells can circulate in the bloodstream as single cells or in groups termed microemboli. Our previous studies of patients with SCLC showed that circulating tumour microemboli (CTM) were detectable using the ISET but not the CellSearch platform (Hou et al, 2009). Similarly, we found that no CTM were observed in pancreatic cancer patients using the CellSearch platform The ISET device detected CTM in three patients, patients 39, 50 and 58, (Figures 5 and 6) where single CTCs were also detected. Pancreatic tumour specimens were available for 2 of 3 patients presenting with CTM: patient 50 had a poorly differentiated EpCAM (3+)-positive carcinoma, with CK-positive cells within CTM by ISET (Figure 6A). Patient 58 had no EpCAM-positive cells within CTM, some CK-positive CTCs by ISET and three CTCs measured by CellSearch. Similar cell morphology was observed when comparing these CTCs and CTM with matched tumour samples (Figures 5D and E). Epithelial to mesenchymal transition is thought to be a dynamic process relevant to CTC extravasation and metastases formation. Cells within CTM were heterogeneous in EMT marker expression (Figures 6A–D). Both CK-positive and -negative tumour cells coexisted within a CTM (Figure 6A), and this within CTM heterogeneity was also observed for both E-Cadherin and Vimentin (Figures 6B–D). Cytokeratin 7 was not expressed in any of the CTCs examined from 10 pancreatic cancer patients (Figure 6E).
CTCs and CTMs captured on ISET filters. (A–C) Three different patient samples, filtered through ISET membranes (pores are dark circles) and stained with CD45 antibody so that leukocytes can be excluded; these are shown in brown. Circulating tumour cells were counterstained with haematoxylin. (C) A large CTM. The membrane pores are all 8 μm in diameter, which gives scale to these images. (D) A CTM from patient 39 (pancreatic head primary and liver, lung and abodminal wall metastases) demonstrates similar morphology to (E). (E) A haematoxylin and eosin (H&E)-stained tumour block from the same patient photographed at the same magnification.
Discussion
Biomarkers are required to develop better therapies for pancreatic cancer patients where access to tumour biopsies is challenging. We undertook a pilot exploratory study comparing the enumeration of CTCs using two technology platforms (CellSearch and ISET) to assess the feasibility of CTCs as a useful source for biomarker research in this disease.
CellSearch detected low CTC numbers in pancreatic cancer patients in comparison with other epithelial carcinomas such as breast, prostate, colorectal and small cell lung cancer (Cristofanilli et al, 2004; Cohen et al, 2008; de Bono et al, 2008; Hou et al, 2009) Two previous studies of pancreatic cancer CTCs using CellSearch reported low CTC numbers in agreement with our data. Kurihara et al (2008) reported on 26 patients, 11 of whom had CTCs (range 1–105, mean CTC/7.5 ml 17, median, 5) with a mean and median CTC number for all 26 patients of 7 and 0, respectively. Negin et al (2010) reported on 48 patients with median CTC/7.5 ml <1, range 0–20. In the Kurihara study, CTC number was significant regarding OS (with vs without CTCs 110.5 vs 375.8 days, respectively, P<0.001) (Kurihara et al, 2008); but in the Negin study CTC number did not correlate significantly with OS (with vs without CTCs 191 vs 269 days, respectively, P=0.5) (Negin et al, 2010). In our study, ISET detected CTCs in more patients than CellSearch (93% vs 40%) and in higher numbers (median CTCs/7.5 ml, 9 (range 0–240) vs 0 (range 0–144)), respectively.
The relatively low CTC number reported for gastrointestinal cancers may result from CTC sequestration by the liver as blood passes through the portal circulation into the systemic circulation (Jiao et al, 2009; Wind et al, 2009). Decreased vascularity is associated with tumour aggressiveness in pancreatic cancer and this may reflect the low CTC number reported (Komar et al, 2009; Neesse et al, 2011). Primaries in the body and tail of the pancreas are more prone to haematogenous spread than primaries of the pancreatic head (Mao et al, 1995), and this is consistent with the data presented here, though further study is warranted to be definitive.
There was no cross-platform correlation in CTC number consistent with their detection of different subsets of CTCs suggested by the heterogeneity of EpCAM and CK expression in both pancreatic cancer patients’ CTCs and tumours and consistent with EMT. This is consistent with findings from two previous studies directly comparing the ISET and CellSearch platforms for CTC detection in resectable non-small cell lung cancer (Hofman et al, 2010) and metastatic carcinomas (breast, prostate and lung cancer) (Farace et al, 2011). Isolation by size of epithelial tumour cells detected more CTCs and identified Vimentin-positive CTCs in patients who did not have detectable CTCs by CellSearch (Hofman et al, 2010). Discrepancies between the platforms appeared to differ between different carcinomas (Farace et al, 2011). Epithelial cell adhesion molecule is expressed on normal epithelial cells and overexpressed in a variety of epithelial carcinomas, including pancreatic cancers where heterogeneous EpCAM expression is observed (Baeuerle and Gires, 2007; Trzpis et al, 2007; Fong et al, 2008; Munz et al, 2009). Epithelial cell adhesion molecule expression differs between carcinomas with either a good or poor prognostic indication depending on the carcinoma (Baeuerle and Gires, 2007). The relevance of EpCAM expression to CTC behaviour and metastatic potential is unknown. Epithelial cell adhesion molecule is also cleaved upon activation (Maetzel et al, 2009) and this may have implications for CTC capture by the CellSearch kit EpCAM antibody.
Speculative interpretation of the trend seen for CellSearch, but not for ISET regarding clinical outcome, is that the EpCAM/CK-positive CTC sub-population detected by CellSearch is necessary for successful metastases formation as shown in a mouse model (Tsuji et al, 2008). Larger studies with balanced patient numbers for each platform will now have to be conducted to assess the prognostic significance of CTC number in pancreatic cancer, and this pilot established that 199 patients will be required to power these future studies.
The identification of viable CTCs by CellSearch is defined as a (minimum) 4 × 4-μm nucleated cell staining positively for EpCAM, CK, and DAPI positivity, and negative for CD45 expression. There are events that do not fit these criteria yet cannot be identified as CTCs despite being CD45 negative. Such events (presumed to be nonviable CTCs or CTC fragments) have been identified in prostate cancer and found to have prognostic significance (Coumans et al, 2010). We did not identify any such fragments in this cohort (data not shown). The finding of events classified as CTCs by CellSearch in individuals without cancer is rare. Only 1 out of 344 healthy volunteers and patients with non-malignant diseases had ⩾2 CTCs (Allard et al, 2004), and subsequent studies confirmed that ⩾1 CTC in (a presumed) non-cancer population was extremely rare (Cristofanilli et al, 2004). Previous ISET studies on healthy populations have been negative for CTCs (Vona et al, 2000; De Giorgi et al, 2010; Hofman et al, 2010, 2011). The identification of CTCs on ISET membranes was based on cell morphology (and compared with primary tumour morphology), CD45-negative selection (for enumeration as leukocytes are the main contaminant, see Supplementary Figure 3) and tumour-associated marker IHC expression, which is consistent with previous ISET studies. It may be that some of these cells are contaminating endothelial or other non-malignant cells. Real-time PCR analysis of nucleic acid extracted from ISET membranes for KRAS for example may support their identification as CTCs, but establishing true tumourigenicity of isolated CTCs would require a mouse model. Simultaneous multimarker assessment of presumed CTCs would also be more discriminatory but is technically challenging on ISET membranes. We have recently developed a four-colour immunofluorescence assay for ISET-filtered CTCs that allows simultaneous analysis of CK and Vimentin, with DAPI-stained nuclei and negative selection for CD45 (see Supplementary Figure 4). In future, this assay will be deployed in a larger study of pancreas cancer patients to explore further the heterogeneity of their CTCs.
To our knowledge, this is the first study to begin to evaluate the ISET platform for pancreatic CTC detection, enumeration and characterisation. Epithelial to mesenchymal transition is thought to occur as cells extravasate into the circulation with the reverse process; mesenchymal to epithelial transition (MET) necessary for cells to intravasate and subsequently form secondary tumours These are most likely to be dynamic events and not necessarily ‘all or nothing’ binary processes. Here, we have begun to reveal EMT marker heterogeneity in CTCs from the pancreatic cancer patients, and a more comprehensive study of their biology with simultaneous assessment of multiple markers is now underway. Epithelial to mesenchymal transition may explain the discrepancies in CTC number between CellSearch and ISET. The finding of both individual CTCs and, more rarely, CTM in pancreatic cancer patients raises questions regarding the mechanisms of cell migration and extravasation, evoking the possibility of collective migration where cells maintain their contacts to invade (Friedl and Wolf, 2003). It remains unclear, however, as to whether tumour cells extravasate as CTM or whether CTM form intravascularly, a phenomenon that has been reported in a preclinical model (Al-Mehdi et al, 2000). If CTM form intravascularly this might explain how CTM (containing as many as 50 cells) are found in a peripheral blood sample given that they would otherwise be expected to be trapped within pulmonary capillary beds. Cellular phenotype within CTMs may differ and the overall picture gained from this exploratory study was one of considerable heterogeneity in EMT markers in CTCs and within CTM.
The detection of pancreatic CTCs/CTM on ISET membranes and their downstream characterisation highlight potential for exploiting CTCs/CTM as a surrogate marker of the primary tumour, in a disease where it is notoriously difficult to biopsy tumour diagnostically or to assess tumour evolution or early indication of drug responses. The wider dynamic range of CTC number detected by ISET may be appropriate for pharmacodynamic monitoring to aid dose selection in early clinical trials. The ability to characterise CTCs downstream of ISET filtration in a flexible manner may facilitate measurement of predictive biomarkers allowing stratification of pancreatic cancer patients most likely to benefit from novel targeted therapies, for example, KRAS and EGFR mutation analysis.
Although larger studies are now needed to demonstrate whether the number of CTCs is sufficient for in depth and systemic biomarker assessment, and to exploit the potential of pancreatic CTCs/CTM, this study has highlighted several promising avenues for exploration.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ (2000) Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 6: 100–102
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897–6904
Baeuerle PA, Gires O (2007) EpCAM (CD326) finding its role in cancer. Br J Cancer 96: 417–423
Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213–3221
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX vs gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825
Coumans FA, Doggen CJ, Attard G, de Bono JS, Terstappen LW (2010) All circulating EpCAM+CK+CD45− objects predict overall survival in castration-resistant prostate cancer. Ann Oncol 21: 1851–1857
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781–791
da Cunha Santos G, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, Squire J, Parulekar W, Moore MJ, Tsao MS (2010) Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer 116: 5599–5607
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14: 6302–6309
De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, Janowska A, Grazzini M, Wechsler J, Orlando C, Santucci M, Lotti T, Pazzagli M, Massi D (2010) Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol 130: 2440–2447
Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, Le Moulec S, Andre F, Fizazi K, Soria JC, Vielh P (2011) A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 105: 847–853
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL (2006) Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 24: 2897–2902
Fong D, Steurer M, Obrist P, Barbieri V, Margreiter R, Amberger A, Laimer K, Gastl G, Tzankov A, Spizzo G (2008) Ep-CAM expression in pancreatic and ampullary carcinomas: frequency and prognostic relevance. J Clin Pathol 61: 31–35
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3: 362–374
Goonetilleke KS, Siriwardena AK (2007) Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 33: 266–270
Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Flejou JF, Lantuejoul S, Piaton E, Mourad N, Butori C, Selva E, Poudenx M, Sibon S, Kelhef S, Venissac N, Jais JP, Mouroux J, Molina TJ, Hofman P (2011) Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 17: 827–835
Hofman V, Ilie MI, Long E, Selva E, Bonnetaud C, Molina T, Venissac N, Mouroux J, Vielh P, Hofman P (2010) Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small cell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer 129: 1651–1660
Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A, Ranson M, Lorigan P, Dive C, Blackhall FH (2009) Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 175: 808–816
Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C (2011) Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 178: 989–996
Jiao LR, Apostolopoulos C, Jacob J, Szydlo R, Johnson N, Tsim N, Habib NA, Coombes RC, Stebbing J (2009) Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol 27: 6160–6165
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28: 3617–3622
Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA (2005) Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 93: 195–199
Komar G, Kauhanen S, Liukko K, Seppanen M, Kajander S, Ovaska J, Nuutila P, Minn H (2009) Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res 15: 5511–5517
Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C (2010) Circulating tumour cells:their utility in cancer management and predicting outcomes. Ther Adv Med Oncol 2: 351–365
Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, Ranson M, Blackhall F, Dive C (2011a) Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol; e-pub ahead of print 14 December 2011, doi:10.1097/JTO.0b013e31823c5c16
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH (2011b) Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29: 1556–1563
Kurihara T, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, Kawai T, Sakai Y, Moriyasu F (2008) Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg 15: 189–195
Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O (2009) Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 11: 162–171
Mao C, Domenico DR, Kim K, Hanson DJ, Howard JM (1995) Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch Surg 130: 125–134
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25: 1960–1966
Munz M, Baeuerle PA, Gires O (2009) The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res 69: 5627–5629
Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA (2011) Stromal biology and therapy in pancreatic cancer. Gut 60: 861–868
Negin BP, Meropol NJ, Alpaugh RK, Ruth K, McAleer C, Halbherr T, Bingham C, Fittipaldi P, Cohen SJ (2010) Characterization and prognostic significance of circulating tumor cells in the peripheral blood of patients with metastatic pancreatic cancer. ASCO Meeting Abstr 28: 4127
Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese JL, Blanke CD (2010) Phase III study comparing gemcitabine plus cetuximab vs gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-Directed Intergroup Trial S0205. J Clin Oncol 28: 3605–3610
Pinzani P, Salvadori B, Simi L, Bianchi S, Distante V, Cataliotti L, Pazzagli M, Orlando C (2006) Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol 37: 711–718
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9: 265–273
Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC (2007) Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol 171: 386–395
Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF (2008) Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 68: 10377–10386
Vona G, Estepa L, Beroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, Brechot C, Paterlini-Brechot P (2004) Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 39: 792–797
Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, Lacour B, Brechot C, Paterlini-Brechot P (2000) Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 156: 57–63
Wind J, Tuynman JB, Tibbe AG, Swennenhuis JF, Richel DJ, van Berge Henegouwen MI, Bemelman WA (2009) Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol 35: 942–950
Acknowledgements
We thank Dr Tim Ward and Cong Zou for sharing their expertise during this study, and to all the nursing staff and patients who participated in this study. LK was supported by an educational grant for a clinical pharmacology fellowship from the Cancer Research UK and AstraZeneca Ltd (c147/A12328a).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Khoja, L., Backen, A., Sloane, R. et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 106, 508–516 (2012). https://doi.org/10.1038/bjc.2011.545
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.545
Keywords
This article is cited by
-
Cancer-related cells and oncosomes in the liquid biopsy of pancreatic cancer patients undergoing surgery
npj Precision Oncology (2024)
-
Pancreatic Cancer Biomarkers: Oncogenic Mutations, Tissue and Liquid Biopsies, and Radiomics—A Review
Digestive Diseases and Sciences (2023)
-
Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication
Molecular Cancer (2021)
-
Cell-free DNA and circulating tumor cell kinetics in a pre-clinical head and neck Cancer model undergoing radiation therapy
BMC Cancer (2021)
-
Negative enrichment of circulating tumor cells from unmanipulated whole blood with a 3D printed device
Scientific Reports (2021)