Key Points
-
Osteomyelitis should be considered as a differential diagnosis in patients complaining of chronic pain post-dental extraction or injury.
-
A good clinical and patient history is required as clinical and radiographic signs may present late.
-
Oral antibiotics appear to have minimal impact as initial treatment.
-
Cone beam CT may help conclude a diagnosis earlier.
Abstract
Chronic osteomyelitis of the jaw is a rare entity in the healthy population of the developed world. It is normally associated with radiation and bisphosphonates ingestion and occurs in immunosuppressed individuals such as alcoholics or diabetics. Two cases are reported of chronic osteomyelitis in healthy individuals with no adverse medical conditions. The management of these cases are described.
Similar content being viewed by others
Introduction
Osteomyelitis can be defined as an inflammatory condition of the bone, which begins as an infection of the medullary cavity, rapidly involves the haversian systems, and extends to involve the periosteum of the affected area.1 It is a well known entity in the historical literature where in the absence of antibiotics, compound fractures of long bones frequently failed to heal. Such cases are no longer part of modern medical experiences. In the twenty-first century osteomyelitis presents as a sub-chronic condition and is more commonly associated with debilitated, immunosuppressed or medically compromised2,3 patients and the pattern of events does not pose a diagnostic dilemma.
Classification
Acute osteomyelitis (AO) compared to chronic osteomyelitis is differentiated arbitrarily based on time: an acute process occurs up to one month after the onset of symptoms and the chronic process occurs for longer than one month.4,5 Using the nomenclature discussed by Eyrich et al.,6 primary chronic osteomyelitis (PCO) is defined as chronic non-suppurative osteomyelitis; when PCO occurs in children and adolescents it is termed 'Garré's osteomyelitis'. This is in contrast to secondary chronic osteomyelitis (SCO), which is chronic osteomyelitis with suppuration, abscess/fistula formation, and sequestration at some stage of the disease due to a defined, infectious aetiology.7
Presentation
Acute osteomyelitis is characterised by a virulent infection with intense pain, inflammation, redness and can be life threatening due to its toxic effects. If however, the bacteria are less virulent, the symptoms can differ and mimic an acute and prolonged alveolar osteitis making it difficult to diagnose and treat.
This paper outlines two examples of this condition arising from routine dental procedures, detailing their mode of presentation and the distinguishing features indicative of the condition.
Case report
Case 1
In June 2008 a 47-year-old female was referred to the Oral and Maxillofacial Department with pain and swelling following the extraction of a lower right second molar (LR7) by her general dental practitioner (GDP) a month earlier. The extraction proved difficult and required repeat injections of local anaesthesia. Her medical history was non-contributory and she had smoked approximately 20 cigarettes a day for the past five years and did not drink alcohol.
On examination the extraction socket was red and inflamed indicative of local osteitis (dry socket). A four week course of clindamycin was prescribed which delayed the symptoms initially but recurred on cessation of the medication in September 2008. The complaint was of intense uncontrollable pain and a sensation of 'loose teeth'. On examination the patient was apyrexial and intra-orally there were no signs of infection at the extraction site. A full blood profile including ESR and CRP were reported as normal. A MRI scan demonstrated a blush within the bone marrow cavity indicative of oedema but lacked evidence of extensive bone involvement. A bone scan report suggested the possibility of osteomyelitis but should be considered in conjunction with the MRI. A second more intense course of antimicrobial therapy was commenced with a mixture of IV and oral antibiotics (azithromycin, teicoplamin, co-amoxiclav, clindamycin and metronidazole) continued over four weeks. The patient responded to the treatment and became symptom free for six months.
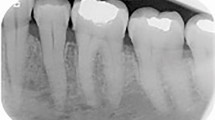
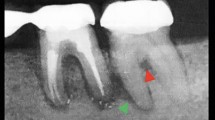
At this point she again complained of intense pain and general malaise. A Cone Beam CT (CBCT) demonstrated bony defects in the LR7/8 area compatible with chronic/recurrent osteomyelitis (Fig. 1). Further imaging was available from the CBCT showing the extent of the bone involvement in Figures 2 and 3. A biopsy of the bone was uninformative as was microbiology which reported 'there is a presence of growth of mixed anaerobes, some Viridans streptococci and Actinomyces naeslandii. This growth could be compatible with normal oral flora though Actinomyces can cause chronic osteomyelitis.' The patient was recommenced on Ceftriaxone IV and Metronidazole PO for a further four weeks. Currently this patient is symptom free and under long term review.
Case 2
In April 2008 a 67-year-old female was referred complaining of an intense pain in her lower jaw. The condition had been ongoing for almost four months. Medically she was fit and well.
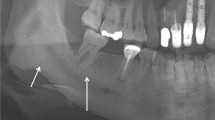
The history revealed that in January 2008 she attended her GDP in Norway for root canal treatment of a lower right first molar (LR6). Treatment was preceded by a lingually applied intra-osseous injection of local anaesthesia. The following day she developed pain and lingual swelling which was treated with antibiotics and analgesics but without resolution. She consulted a second endodontist who thought the pain was pulpitis in the adjacent tooth (LR5) and proceeded to a second root canal treatment. The chronic pain persisted and a month after presentation she developed swelling on the lingual aspect of the mandible, in the LR5 and LR6 region which was subsequently drained. The pain remained poorly controlled despite liberal quantities of Oramorph and MST. As time progressed the infection began to tract further forward and pus was evident in the gingivae of the anterior teeth. The patient sought a second surgical opinion and at this time a sinus was present on the lingual aspect of the right mandible. There was no paraesthesia but the patient complained of her 'teeth becoming loose' although this could not be demonstrated clinically. She was commenced on intravenous clindamycin for two weeks followed by co-amoxiclav for a further four weeks to which she responded well. Subsequently the infection recurred, but now the pain was in the left mandible, for the infection had run through the marrow spaces to the contralateral side of the jaw. A CBCT reported 'widespread perforation of the lingual plate consistent with sub-periosteal spread of infection from the original intra-osseous injection site across the midline to affect the left premolar region' (Fig. 4). Bone biopsies were compatible with sclerosing low-grade chronic osteomyelitis. A repeat CBCT one year later showed regeneration of the mandible and improvement in comparison to the previous CBCT (Fig. 5).
Discussion
Osteomyelitis of the jaw is a relatively uncommon inflammatory disease in developed countries.8 The aetiology is unknown and theories include bacterial infection (dental or bacteraemia from distant foci), vascular deficiency (localised endarteritis), autoimmune disease7 or trauma.9 Conditions altering the vascularity of the bone such as radiation, malignancy, osteoporosis, osteopetrosis, and Paget's disease predispose to osteomyelitis. Systemic diseases like diabetes, anaemia and malnutrition that cause concomitant alteration in host defences profoundly influence the course of osteomyelitis.10 The incidence of the disease has decreased dramatically with the introduction of antibiotics and improvement in the general health to the population together with access to medical and dental care.11,12,13
The jaws are unique from other bones of the body in that the presence of teeth creates a direct pathway for infectious and inflammatory agents to invade bone by means of caries and periodontal disease.14 Oral bone appears to be particularly resistant to infection despite exposure to oral flora.15 This further reiterates the rarity of the mandible experiencing osteomyelitis.
Microbiology
Osteomyelitis of long bones is normally attributed to Staphylococcus aureus whereas in mandibular osteomyelitis it is usually considered a polymicrobial disease.8 The search for an infectious aetiological agent of PCO has led some researchers to investigate the microbiologic samples taken from surgical specimens. Bacteriologic and serologic studies have shown Propionibacterium acnes,16 Actinomyces species, or Eikenella corrodens17 as causative agents, but cultures from the bone lesions often show negative results18,19 and no specific microorganism has been identified as a dominant aetiological agent.11,12,13 This therefore shows the differential between osteomyelitis in long bones and the mandible. Where in long bones infection is via Staphylococcus aureus which is usually transferred via the bloodstream, this has proven not to be the case when the mandible is affected.
Imaging
There remains much choice when considering imaging for osteomyelitis. A simple dental panoramic radiograph may be enough to diagnose this condition. However, the disease process may only become evident on the radiograph in the latter stages. MRI T1 weighted images are usually better as inflamed tissue creates low signal intensity in the normally bright signal of fat contained in the marrow.2 MRI does not show specific features capable of making a diagnosis, but does show the extent of the lesions and may be helpful in disease monitoring.20,21 The use of cone beam CT enables an image of high quality of a selected area. This imaging was used for the cases described above and proved to give accurate and detailed information.
Differential diagnosis
The differential diagnoses of yet to diagnose PCO includes malignant and benign entities discussed by Eyrich et al.,6 Baltensperger et al.22 and Soubrier et al.23 The benign include ossifying and non-ossifying fibroma, infection of the salivary glands (juvenile recurrent parotitis or chronic recurrent sialadenitis) and non-specific chronic lymphadenitis. The malignant entities that should be considered because of the insidious nature of PCO are Ewing's sarcoma, osteosarcoma, chondrosarcoma, non-Hodgkin's lymphoma and metastatic disease.
Pathogenesis
The varied treatments for PCO reflect the lack of understanding of the aetiology of this disease. It is thought the relatively avascular and ischaemic nature of the infected region and sequestrum produces an area of lowered oxygen tension as well as an area that antibiotics cannot penetrate. The lowered oxygen tension effectively reduces the bacteriocidal activities of polymorpholeukocytes and also favours the conversion of a previously aerobic infection to one that is anaerobic. The diffusion rate of antibiotics into dead bone is so low that frequently it is impossible to reach the organisms regardless of the external concentration. This may lead to ineffective antibiotic concentrations at the site of infection despite serum levels indicating therapeutic concentrations.24
Treatment
Treatment varies from a range of simple non-invasive approaches to more invasive and radical treatment. The nonsurgical approach includes: antibiotics,23 NSAIDS,23 hyperbaric oxygen therapy,25 bisphosphonate treatment,15,23 and muscle relaxants.18 Following the failure of a non-surgical approach a surgical intervention to consider include decortications alone,25 decortication with bone grafting,26 partial (marginal) resection,27 and segmental resection.23,27 Unfortunately, conservative management invariably could lead to multiple recurrences of the disease, and aggressive management may lead to significant co-morbidity with subsequent need for reconstructive surgery7 therefore leaving the clinician with a dilemma.
The outstanding clinical characteristics of the two cases were the intense and uncontrollable nature of the pain with little or no accompanying physical signs. Inflammatory indicators were normal. The disparity between signs and symptoms were so great as to make the clinician doubt the veracity of the patient's history. The combination of MRI and CBCT examination were helpful in distinguishing changes in the bone. The lesson drawn from these cases is that in the early stages of chronic osteomyelitis, the identification of the disease depends largely on clinical judgement rather than haematological and radiographic tests. Another characteristic was the reluctance of the infection to respond to standard regimen of oral antibiotics possibly due to the pathogenesis theory proposed earlier. Rather long courses of IV antibiotics are required to resolve the infection. Oral antibiotics seem ineffective.
The role of an intra-osseous injection in the induction of osteomyelitis remains unclear. Published literature has stated symptoms of pain and swelling post administration of a intra-osseous injection post-operatively.28,29,30 Furthermore Replogle et al.29 reported purulence following intra-osseous injection which resolved up to 14 days post administration without any morbidity. This form of analgesia has not been associated with osteomyelitis in the medical literature. However, it was obvious as the instigating factor in the second case. It remains a mystery why a healthy adult patient should develop osteomyelitis after a simple intra-oral injection.
Conclusion
Osteomyelitis remains a rare entity in medically fit and well individuals. The clinical features in these patients are not typical of those seen in the traditional debilitated patient and can pose a diagnostic problem. Osteomyelitis should always be considered in the presence of intense and poorly controlled pain following injury to the jaw. Clinicians should remember that osteomyelitis responds poorly to antibiotics and may require long term IV and oral doses, possibly even as multiple courses. Finally, consideration of CBCT as part of radiological examination may help conclude a diagnosis earlier due to the localisation of the imaging.
References
Topazian R G . Osteomyelitis of jaws. In Topazian R G, Goldberg M H (eds). Oral and maxillofacial infections, 3rd ed. pp 251–286. Philadelphia, PA: Saunders, 1994.
Eckman M H, Greenfield S, Mackey W C, Wong J B et al. Foot infections in diabetic patients. Decision and cost-effectiveness analyses. JAMA 1995; 273: 712–720.
Grayson M L, Gibbons G W, Balogh K, Levin E, Karchmer A W . Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995; 273: 721–723.
Marx R E . Chronic osteomyelitis of the jaws. Oral Maxillofac Surg Clin North Am 1991; 3: 367.
Mercuri L G . Acute osteomyelitis of the jaws. Oral Maxillofac Surg Clin North Am 1991; 3: 355.
Eyrich G K H, Baltensperger M M, Bruder E et al. Primary chronic osteomyelitis in childhood and adolescence: a retrospective analysis of 11 cases and review of the literature. J Oral Maxillofac Surg 2003; 61: 561–573.
Bevin C R, Inwards C Y, Keller E E . Surgical management of primary chronic osteomyelitis: a long-term retrospective analysis. J Oral Maxillofac Surg 2008; 66: 2073–2085.
Scolozzi P, Lombardi T, Edney T, Jaques B . Enteric bacteria mandibular osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: E42–46.
Lucchesi L, Kwok J . Long term antibiotics and calcitonin in the treatment of chronic osteomyelitis of the mandible: case report. Br J Oral Maxillofac Surg 2008; 46: 400–402.
Topazian R G . Osteomyelitis of jaws. In Topazian R G, Goldberg M H (eds). Oral and maxillofacial infections, 3rd ed. pp 251–286. Philadelphia, PA: Saunders, 1994.
Koorbusch G F, Fotos P, Goll K T . Retrospective assessment of osteomyelitis. Etiology, demographics, risk factors, and management in 35 cases. Oral Surg Oral Med Oral Pathol 1992; 74: 149–154.
Hudson J W . Osteomyelitis of the jaws: a 50 year perspective. J Oral Maxillofac Surg 1993; 51: 1294–1301.
Storoe W, Haug R H, Lillich T T . The changing face of odontogenic infections. J Oral Maxillofac Surg 2001; 59: 739–748.
Lee L . Inflammatory lesions of the jaws. In White S C, Pharoah M J (eds). Oral radiology: principles and interpretation, volume 3, 4th ed. pp 338–354. Missouri: Mosby, 2000.
Montonen M, Kalso E, Pylkkaren L et al. Disodium clodronate in the treatment of diffuse sclerosing osteomyelitis (DSO) of the mandible. Int J Oral Maxillofac Surg 2001; 30: 313–317.
Jacobsson S . Diffuse sclerosing osteomyelitis of the mandible. Int J Oral Surg 1984; 13: 363–385.
Pell G J, Shafer W G, Gregory T, Ping R S, Spear L B . Garré's osteomyelitis of the mandible; report of case. J Oral Surg (Chic) 1955; 13: 248–252.
Van Merkesteyn J P, Groot R H, Bras J et al. Diffuse sclerosing osteomyelitis of the mandible: clinical radiographic and histologic findings in twenty-seven patients. J Oral Maxillofac Surg 1988; 46: 825–829.
Malmstrom M, Fyhrquist F, Kosunen T U et al. Immunological features of patients with chronic sclerosing osteomyelitis of the mandible. Int J Oral Surg 1983; 12: 6–13.
Beretta-Piccoli B C, Sauvain M J, Gal I, Schibler A et al. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: a report of ten cases and review of the literature. Eur J Pediatr 2000; 159: 594–601.
Job-Deslandre C, Krebs S, Kahan A . Chronic recurrent multifocal osteomyelitis: five-year outcomes in 14 pediatric cases. Joint Bone Spine 2001; 68: 245–251.
Baltensperger M, Gratz K, Bruder E et al. Is primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J Craniomaxillofac Surg 2004; 32: 43–50.
Soubrier M, Dubost J J, Ristori J M et al. Pamidronate in the treatment of diffuse sclerosing osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92: 637–640.
Eckardt J J, Wirganowicz P Z, Mar T . An aggressive surgical approach to the management of chronic osteomyelitis. Clin Orthop Relat Res 1994; /298: 229–239.
Garcia-Marin F, Iriarte-Ortabe J I, Reychler H . Chronic diffuse sclerosing osteomyelitis of the mandible or mandibular location of SAPHO syndrome. Acta Stomatol Belg 1996; 93: 65–71.
Ogawa A, Miyate H, Nakamura Y et al. Treating chronic diffuse sclerosing osteomyelitis of the mandible with saucerization and autogenous bone grafting. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91: 390–394.
Suei Y, Tanimoto K, Miyauchi M, Ishikawa T . Partial resection of the mandible for the treatment of diffuse sclerosing osteomyelitis: report of four cases. J Oral Maxillofac Surg 1997; 55: 414–415.
Coggins R, Reader A, Nist R, Beck M, Meyers W J . Anesthetic efficacy of the intraosseous injection in maxillary and mandibular teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81: 634–641.
Replogle K, Reader A, Nist R, Beck M et al. Anesthetic efficacy of the intraosseous injection of 2% lidocaine (1: 100,000 adrenaline) and 3% mepivacaine in mandibular first molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 83: 30–37.
Dunbar D, Reader A, Nist R, Beck M, Meyers W J . Anesthetic efficacy of the intraosseous injection after an inferior alveolar nerve block. J Endod 1996; 22: 481–486.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Patel, V., Harwood, A. & McGurk, M. Osteomyelitis presenting in two patients: a challenging disease to manage. Br Dent J 209, 393–396 (2010). https://doi.org/10.1038/sj.bdj.2010.927
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2010.927
This article is cited by
-
Diabetic Maxillary Osteomyelitis: A Worrisome Vulnerability—Our Experience
Journal of Maxillofacial and Oral Surgery (2022)