Key Points
-
Laser use in periodontology includes the removal of intra-pocket diseased epithelium, bacterial and calculus accumulation and in the surgical correction of infra- and intra-bony pocketing.
-
Laser use should be adjunctive to good periodontal therapy and not a replacement.
-
Research into laser use in periodontology has been mixed. This may be due to the number of associated clinical parameters involved in in vivo investigations.
-
All currently available laser wavelengths have been claimed to be effective in some or all aspects of the treatment of periodontal conditions. In addition, newer or experimental wavelengths may expand therapeutic use.
-
Laser power levels must be kept to a minimum to avoid unwanted damage.
Key Points
Lasers in dentistry
-
1
Introduction, history of lasers and laser light production
-
2
Laser-tissue interaction
-
3
Low-level laser use in dentistry
-
4
Lasers and soft tissue: 'loose' soft tissue surgery
-
5
Lasers and soft tissue: 'fixed' soft tissue surgery
-
6
Lasers and soft tissue: periodontal therapy
-
7
Surgical laser use in implantology and endodontics
-
8
Surgical lasers and hard dental tissue
-
9
Laser regulation and safety in general dental practice
Abstract
Periodontology exists as a major specialty within clinical dentistry that has developed through the extensive research carried out into all parameters pertaining to a 'best practice' approach. With the advent of surgical lasers into clinical dentistry, considerable interest has been shown in the possible benefits that might be derived from the adjunctive effects of bacterial control and haemostasis that are associated with laser use. Despite the number of publications on the subject, there is still controversy over the use of lasers in periodontology. The following paper will outline the procedures that have been advocated for laser use and provide a review of the literature.
Similar content being viewed by others
Surgical lasers and periodontology
The use of surgical lasers in periodontology is explored in three areas of treatment (Fig. 1):
-
Removal of diseased pocket lining epithelium
-
Bactericidal effect of lasers on pocket organisms
-
Removal of calculus deposits and root surface detoxification.
Whatever benefits that may exist through the use of lasers, the prime responsibility of the clinician is to diagnose the existence of periodontal disease, establish and modify aggravating factors, treat the condition and seek to maintain health. As such, the use of lasers should be seen as adjunctive and supplemental to established protocols.
When integrated into a sound approach to pocket reduction, all current dental wavelengths have been advocated for the removal of diseased epithelium (Table 1). Added to the current wavelengths is the recent development of a frequency-doubled (wavelength-halved) Nd:YAG laser at 532 nm, termed the KTP laser, which has a range of action similar to that of the 810 nm diode. 'KTP' denotes potassium titanyl phosphate – the crystal used to effect the frequency doubling of the 1,064 nm wavelength.
The haemostatic advantage of using laser energy confers a controlling factor that is beneficial to both clinician and patient. Conceptually, in a periodontal pocket that is essentially supra-bony, the removal of hyperplastic soft tissue, together with a reduction in bacterial strains, renders the post-laser surgical site amenable to healing within normal limits. Where the pocket is infra-bony, a number of procedures have been advocated, including laser-ENAP1 (excisional new attachment procedure), where the Nd:YAG (1,064 nm) laser is used in a non-flap procedure to reduce pocket depths of several millimetres, through a succession of treatment appointments.
A number of studies have been carried out to support the action of laser energy on various bacterial strains implicated in chronic periodontal disease. Short wavelength lasers interact with pigmented strains, whereas longer wavelength laser energy is absorbed by cellular water, leading to fragmentation of cellular structure.
Calculus, being a non-uniform mixture of inorganic salts, organic material, bacterial strains and water, can be viewed as a ready absorber of all wavelengths. However, the close association of calculus deposits with tooth and periodontal structures does pose a potential risk of collateral damage. Of the wavelengths investigated, erbium YAG (2,940 nm), erbium YSGG (2,780 nm) and frequency-doubled alexandrite (FDA, 377 nm) have been shown to interact and remove calculus selectively, with unwanted effects of a magnitude comparable with conventional techniques involving hand-instruments.
Risk analysis of laser use
Notwithstanding non-structural factors such as local and systemic host susceptibility and genetic and lifestyle influences, the diseased periodontal pocket remains a complicated and potentially delicate structure to treat. Added to this, most laser delivery systems depend on an axial, end-on emission of light energy, which renders the target tissue liable to a potential build-up of direct and conductive heat effects. Consequently, there exists a profound need to limit laser power values to the minimum required to establish a desired effect and to avoid unwanted interaction, both with the tooth and periodontal attachment apparatus.
The lack of tactile feedback, together with the 'blind' treatment of non-reflected periodontal flaps, renders the need for caution as paramount. A detailed and thorough record of the diseased periodontium must be obtained prior to laser use as well as a respect for the need for conventional debridement to co-exist. In this way, only the proven benefits of laser use can be employed as an adjunctive, to maximise the outcome of the treatment in general. The temptation merely to expose a periodontal pocket to any laser energy, in the expectation that a magical resolution of the condition would ensue, undermines the professional approach to the patient and is to be deprecated.
De-epithelialisation of the periodontal pocket
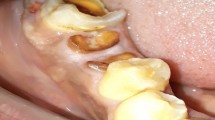
The development of the quartz optic fibre delivery system associated with the diode and Nd:YAG group of lasers, with diameters of 200-320 μm, makes access into the periodontal pocket extremely easy. Longer wavelengths, where non-quartz deliveries are required, rely on fine bore waveguide probes and sapphire hand-piece tips, which are slightly wider, but which have been designed for the purpose (Figs 2 3 4).
Following the removal of all hard and soft deposits through scaling and/or root-planing, the pocket architecture is re-assessed, especially the depth. The laser probe or fibre is measured to a distance of one to two millimetres short of the pocket depth and is inserted at an angle to maintain contact with the soft tissue wall at all times. Using laser power values sufficient to ablate the epithelial lining (approximately 0.8 W CW diode, 100 mJ/20 pps, 2.0 W Nd:YAG and Er:YAG/YSGG, 1.0 W CW CO2), the laser probe is used in a light contact, sweeping mode to cover the entire soft tissue lining. Ablation should commence near the base of the pocket and proceed upwards, by slowly removing the probe (Fig. 5).
It is often seen that some bleeding of the pocket site will occur. This may be due to disruption of the fragile inflamed pocket epithelium, but in terms of laser haemostasis, the power levels employed are low and designed to remove the epithelial surface and decontaminate. Regular inspection should be carried out to prevent the build-up of ablation debris on the fibre or probe end, which should be cleaned with damp sterile gauze. Each pocket site should be treated for 20-30 seconds, amounting possibly to two minutes per tooth site, with re-treatment at approximate weekly intervals during any maximum four-week period. Gentle pocket probing and measurement to establish benefits of treatment should be resisted during this period.
Several laser-related studies have appeared in the periodontal literature to date.2,3,4,5,6,7,8,9,10,11,12,13,14 Case reports have recommended the diode laser (810 nm), along with the Nd:YAG (1,064 nm), for treatment of periodontal pockets by laser sub-gingival curettage. However, these reports offer no evidence that these procedures are superior to conventional scaling and root planing alone. The American Academy of Periodontology, in its position statement on lasers in ENAP,15 states 'The Academy is not aware of any published data that indicates that the ENAP laser procedure is any more effective for these purposes than traditional scaling and planing'.12,16,17,18,19,20,21,22 This is sharply contrasted by reports by Gregg and McCarthy, reported in later journals.23,24,25 In 2004 in a study presented by Evans26 to review the new attachment procedure on a sample of six cases, evidence was given to show new cementum and bone growth, including periodontal ligament. What must be considered is the extent to which such treatment can be empirically assessed, when many deep periodontal lesions often merit tooth stabilisation and occlusal guarding. Furthermore, there are limited evidence-based clinical trials to substantiate the clinical benefits of laser-assisted sub-gingival curettage and the presence of root surface damage following this procedure has been reported.27 The carbon dioxide laser has been shown to enhance periodontal therapy through a de-epithelial technique in conjunction with traditional flap surgery procedures. It has been demonstrated that the CO2 laser can be used to de-epithelialise the flap during surgery and it has enhanced reduction in periodontal probing depths.4,13,14 Several controlled studies have assessed the use of laser therapy combined with conventional scaling and root planing, although these investigations demonstrated no benefit or only slightly improved treatment outcomes.28,29,30,31
Conversely, a study in 2003 by Schwarz et al.32 using an erbium laser indicated that non-surgical periodontal therapy with both an Er:YAG laser plus scaling/root planing (SRP) and an Er:YAG laser alone, led to significant improvements in all clinical parameters investigated; also, the combined treatment Er:YAG laser plus SRP did not seem to additionally improve the outcome of the therapy compared to Er:YAG laser alone.
Laser bacterial reduction
Among the bacteria most implicated in periodontal disease and bone loss are Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Bacteroides forsythus. Other bacteria associated with periodontal disease are Treponema denticola, T. sokranskii and Prevotella intermedia. These latter bacteria, together with P. gingivalis, are frequently present at the same sites and are associated with deep periodontal pockets. Most studies reported in the literature focus on the in vitro action of various laser wavelengths on these selected bacterial species.
The effectiveness of any laser wavelength is dependant upon the absorption characteristics of the target bacterial structure (water, pigment) being matched by the incident beam. In addition, in vivo, the indeterminate existence of definable parameters of laser energy dosage, concentration of bacterial colonies and accuracy of exposure, may give rise to some scepticism as to the predictability of this therapy. However, the conjunctive use of lasers within conventional periodontal therapy, both in vitro and in vivo, does support the clinical picture of a beneficial role of lasers in pocket decontamination.
Many studies have been carried out to demonstrate the effectiveness of laser energy on bacterial strains found in the diseased pocket.33,34,35,36 Some studies have reported on the additional role of laser use in conjunction with scaling and root planing and locally-applied antibiotic preparations.37,38 It is evident from the numerous studies undertaken in this field that the levels of incident energy employed are essentially sufficient to ablate bacterial cellular structure; what appears to be difficult to quantify is the protocol required to render any periodontal pocket 'sterile'.
A recent study by Bornstein39 cites an innovative use of a diode (810-830 nm) laser, in conjunction with methylene blue, to address some of the difficulties of using this wavelength within the confines of the periodontal pocket. All too often, the build-up of char and de-natured protein material on the delivery fibre of the (emitting CW) diode laser, results in the development of a carbonised tip, with the temperature rising in excess of 700°C. If not removed, this leads to an attenuation of the subsequent laser beam, replaced by the secondary emission of radiant thermal energy from the carbonised deposits ('hot-tip effect'). The conductive heat effects that result lead to unwanted damage to the delicate tissue structure. The use of a chemical mediator, such as methylene blue, serves to act as a heat sink for the thermal energy and to enhance bacteriocidal action. This proposal, along with extension of the concept of photo-activated disinfection in cavity preparation, remains the subject of further investigation.
Lasers and calculus removal
The predominance of Nd:YAG and CO2 laser wavelengths in dentistry until 1994 gave good ground to the viewpoint that calculus removal using laser energy was either incomplete or fraught with damage potential to surrounding tissues.23,40,41,42 The development of Er:YAG and Er,Cr:YSGG, together with innovative near-UV wavelengths such as frequency-doubled alexandrite (FDA, 377 nm), has given encouragement to the safe use of these lasers in calculus removal.
In order to provide access to calculus deposits, specific laser hand-piece tips have been developed for use with the mid-infrared erbium wavelengths (Fig. 6).
The shorter FDA wavelength is delivered through an optic fibre (Fig. 7) and to date, remains a developmental machine. However, further investigation is anticipated into the use of diode-based lasers of wavelengths in the region of 400 nm, which would still prove interactive with calculus, but avoid some claims that the 377 nm wavelength might give rise to ionising effects in target tissue.
The poorly-calcified deposits, together with higher water content, has rendered supra- and sub-gingival calculus susceptible to de-fragmentation through photo-mechanical ablation with the erbium group.43,44,45 Potentially, this enables deposits to be removed using laser energy levels less than those required for ablation of dental hard tissue (Figs 8 9 10 11 12 13). This is borne out in a study by Aoki et al.,46 where laser power levels as low as 0.3 Watts have been shown to be sufficient to ablate calculus. Intriguingly, the same centre reported that the efficiency of Er:YAG in calculus removal was less than that of ultrasonic instrumentation.47
The advantage of using the 377 nm laser is based on studies that have shown the differential increased absorption of this laser by calculus, as opposed to cementum and dentine.48,49,50
In addition to the treatment of periodontal disease, erbium YAG and erbium YSGG lasers can be used to carry out bone remodelling. Whilst the effectiveness of these wavelengths on bone is discussed in greater detail in the later article on lasers and hard tissue, the clinical results obtained within the management of the alveolus/periodontium complex are most promising (Figs 14 15 16 17 18).
Conclusion
Considerable debate continues as to the effectiveness and/or efficiency of lasers in the field of periodontology. In those geographical areas of the world where hygienists and other auxiliaries are able to carry out surgical pocket debridement, there is considerable enthusiasm for use. Generally, conventional opinion remains unequivocal as to laser usage, despite the number of studies carried out. The many anecdotal reports as to beneficial use of lasers serve only to establish an opinion as to laser effectiveness and certainly there is agreement amongst protagonists as to the improvement in tissue health following laser treatment. The difficulties in establishing a series of protocols, addressing differences in periodontal pocket architecture, presence and extent of disease and deposits, laser power parameters and which laser wavelength is at all superior, will only serve to allow the debate to continue. What is quite evident is that, whilst any 'closed' procedure within the pocket demands skill and consideration, the use of any laser should be adjunctive and thorough knowledge of potential damaging factors appreciated. Perhaps nowhere else is the maxim 'minimal power to achieve the desired effect' more appropriate that in this field of dentistry. From the review of the literature, it is personally felt that there is a need for greater control of studies that reflect objectivity and reduce subjectivity, in order to provide confidence for practitioners in maximising the benefits of lasers. Through this approach, laser use can be anticipated to gain greater acceptance in the field of periodontology.
References
Millennium Dental Technologies, Inc. Dent Prod Rep 1999; 33: 40.
Spencer P, Cobb C M, Wieliczka D M, Glaros A G, Morris P J . Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontol 1998; 69: 1278–1282.
Fujii T, Baehni P C, Kawai O, Kwawkami T, Matsuda K, Kowashi Y . Scanning electron microscopic study of the effects of Er:YAG laser on root cementum. J Periodontol 1998; 69: 1283–1290.
Rossmann J A, Cobb C M . Lasers in periodontal therapy. Periodontol 2000 1995; 9: 150–164.
Israel M, Rossmann J A, Froum S J . Use of the carbon dioxide laser in retarding epithelial migration: a pilot histological human study utilizing case reports. J Periodontol 1995; 66: 197–204.
Williams T M, Cobb C M, Rapley J W, Killoy W J . Histologic evaluation of alveolar bone following CO2 laser removal of connective tissue from periodontal defects. Int J Periodontics Restorative Dent 1995; 15: 497–506.
Wilder-Smith P, Arrastia A A, Schell M J, Liaw L H, Grill G, Berns M W . Effect of Nd:YAG laser irradiation and root planing on the root surface: structural and thermal effects. J Periodontol 1995; 66: 1032–1039.
Rizoiu I M, Eversole L R, Kimmel A I . Effects of an erbium, chromium:yttrium, scandium, gallium garnet laser on mucocutaneous soft tissues. Oral Surg Oral Med Oral Pathol 1996; 82: 386–395.
Yamaguchi H, Kobayashi K, Reiko O et al. Effects of irradiation of an erbium:YAG laser on root surfaces. J Periodontol 1997; 68: 1151–1155.
Israel M, Cobb C M, Rossmann J A, Spencer P . The effects of the CO2, Nd:YAG and Er:YAG lasers with and without surface coolant on the tooth root surfaces: an in vitro study. J Clin Periodontol 1997; 24: 595–602.
Krause L S, Cobb C M, Rapley J W, Killoy W J, Spencer P . Laser irradiation of bone: I. An in vitro study concerning the effects of the CO2 laser on oral mucosa and subjacent bone. J Periodontol 1997; 68: 872–880.
Gopin B W, Cobb C M, Rapley J W, Killoy W J . Histologic evaluation of soft tissue attachment to CO2 laser treated root surfaces: an in vivo study. Int J Periodontics Restorative Dent 1997; 17: 317–325.
Centty I G, Blank L W, Levy B A et al. Carbon dioxide laser for de-epithelialization of periodontal flaps. J Periodontol 1997; 68: 763–769.
Israel M, Rossmann J A . An epithelial exclusion technique using the CO2 laser for the treatment of periodontal defects. Compend Contin Educ Dent 1998; 19: 1238–1245.
American Academy of Periodontology. Statement regarding use of dental lasers for excisional new attachment procedure (ENAP). Chicago: AAP, 1999.
Trylovich D J, Cobb C M, Pippin D J, Spencer P, Killoy W J . The effects of the Nd:YAG laser on in vitro fibroblast attachment to endotoxin-treated root surfaces. J Periodontol 1992; 63: 626–632.
Morlock B J, Pippin D J, Cobb C M, Killoy W J, Rapley J W . The effect of Nd:YAG laser exposure on root surfaces when used as an adjunct to root planing. J Periodontol 1992; 63: 637–641.
Spencer P, Trylovich D J, Cobb C M . Photoacoustic FTIR spectroscopy of lased cementum surfaces. J Periodontol 1992; 63: 633–636.
Spencer P, Cobb C M, McCollum M H, Wieliczka D M . The effects of CO2 laser and Nd:YAG with and without water/air surface cooling on tooth root structure: correlation between FTIR spectroscopy and histology. J Periodont Res 1996; 31: 453–462.
Thomas D, Rapley J W, Cobb C M, Spencer P, Killoy W J . Effects of the Nd:YAG laser and combined treatments on in vitro fibroblast attachment to root surfaces. J Clin Periodontol 1994; 21: 38–44.
Cobb C M, Spencer P, McCollum M H . Histologic comparison of the CO2 and Nd:YAG lasers with and without water/air surface cooling on tooth root structure. Proc SPIE 1995; 2394: 20–31.
Radvar M, Creanor S L, Gilmour W H et al. An evaluation of the effects of an Nd:YAG laser on subgingival calculus, dentine and cementum. An in vitro study. J Clin Periodontol 1995; 22: 71–77.
Gregg R H, McCarthy D K . Laser ENAP for periodontal bone regeneration. Dent Today 1998; 17(5): 88–91.
Gregg R H, McCarthy D K . Laser ENAP for periodontal ligament regeneration. Dent Today 1998; 17(11): 86–89.
Gregg R H, McCarthy D K . Laser economics: periodontal therapy. Dent Econ 1998; 88: 42–44.
Yukna R A, Evans G, Vastardis S, Carr R L . Human periodontal regeneration following laser assisted new attachment procedure. Proc IADR/AADR/CADR 82nd General Session. Hawaii, 2004.
Cobb C M, McCawley T K, Killoy W J . A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol 1992; 63: 701–707.
Yilmaz S, Kuru L, Noyan U, Argun D, Kadir T . Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 2002; 30: 60–66.
Liu C M, Hou L T, Wong M Y, Lan W H . Comparison of Nd:YAG laser versus scaling and root planing in periodontal therapy. J Periodontol 1999; 70: 1276–1282.
Schwarz F, Sculean A, Georg T, Reich E . Periodontal treatment with an Er:YAG laser compared to scaling and root planning. A controlled clinical study. J Periodontol 2001; 72: 361–367.
Neill M E, Mellonig J T . Clinical efficacy of the Nd:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent 1997; 9: 1–5.
Schwarz F, Sculean A, Berakdar M, Georg T, Reich E, Becker J . Clinical evaluation of an Er:YAG laser combined with scaling and root planing for non-surgical periodontal treatment. A controlled, prospective clinical study. J Clin Periodontol 2003; 30: 26–34.
Harris D M, Yessik M . NdYAG better than diode. Lasers Surg Med 2004; 35: 206–213.
Grassi R F, Pappalardo S, Frateiacci A et al. Antibacterial effect of Nd:YAG laser in periodontal pockets decontamination: an in vivo study. Minerva Stomatol 2004; 53: 355–359 [article in Italian].
Moritz A, Schoop U, Goharkhay K et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med 1998; 22: 302–311.
Coffelt D W, Cobb C M, MacNeill S, Rapley J W, Killoy W J . Determination of energy density threshold for laser ablation of bacteria. An in vitro study. J Clin Periodontol 1997; 24: 1–7.
Miyazaki A, Yamaguchi T, Nishikata J et al. Effects of Nd:YAG and CO2 laser treatment and ultrasonic scaling on periodontal pockets of chronic periodontitis patients. J Periodontol 2003; 74: 175–180.
Noguchi T, Sanaoka A, Fukuda M, Suzuki S, Aoki T . Combined effects of Nd:YAG laser irradiation with local antibiotic application into periodontal pockets. J Int Acad Periodontol 2005; 7: 8–15.
Bornstein E . Method and dosimetry for thermolysis and removal of biofilm in the periodontal pocket with near-infrared diode lasers: a case report. Dent Today 2005; 24(4): 60, 62, 64–70.
Arcoria C J, Vitasek-Arcoria B A. The effects of low-level energy density Nd:YAG irradiation on calculus removal. J Clin Laser Med Surg 1992; 10: 343–347.
Cobb C M . Lasers in periodontics: use and abuse. Compend Contin Educ Dent 1997; 18: 847–852, 854–855, 858–859.
Tucker D, Cobb C M, Rapley J W, Killoy W J . Morphologic changes following in vitro CO2 laser treatment of calculus-ladened root surfaces. Lasers Surg Med 1996; 18: 150–156.
Folwaczny M, Mehl A, Haffner C, Benz C, Hickel R . Root substance removal with Er:YAG laser radiation at different parameters using a new delivery system. J Periodontol 2000; 71: 147–155.
Frentzen M, Braun A, Aniol D . Er:YAG laser scaling of diseased root surfaces. J Periodontol 2002; 73: 524–530.
Eberhard J, Ehlers H, Falk W, Acil Y, Albers H K, Jepsen S . Efficacy of subgingival calculus removal with Er:YAG laser compared to mechanical debridement: an in situ study. J Clin Periodontol 2003; 30: 511–518.
Aoki A, Ando Y, Watanabe H, Ishikawa I . In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser. J Periodontol 1994; 65: 1097–1106.
Aoki A, Miura M, Akiyama F et al. In vitro evaluation of Er:YAG laser scaling of subgingival calculus in comparison with ultrasonic scaling. J Periodont Res 2000; 35: 266–277.
Pilgrim C, Rechmann P, Goldin D, Hennig T . Measurement of efficiency in calculus removal with a frequency-doubled alexandrite laser on pigs' jaws. Proc SPIE 2000; 3910: 50–58.
Rechmann P, Hennig T, Reichart P . Periodontal treatment with the frequency-doubled alexandrite laser in dogs. Proc SPIE 2000; 3910: 35–41.
Rechmann P, Hennig T, Hamid M, Sadegh M, Goldin D . Light and scanning electron microscope investigations comparing calculus removal using an Er:YAG laser and a frequency-doubled alexandrite laser. Proc SPIE 1997; 2973: 53–59.
Acknowledgements
Permission granted by Dr Donald Coluzzi, Redwood City, California, USA to reproduce his clinical photographs of crown lengthening treatment is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Parker, S. Lasers and soft tissue: periodontal therapy. Br Dent J 202, 309–315 (2007). https://doi.org/10.1038/bdj.2007.224
Published:
Issue Date:
DOI: https://doi.org/10.1038/bdj.2007.224
This article is cited by
-
Different application procedures of Nd:YAG laser as an adjunct to scaling and root planning in smokers with stage III grade C periodontitis: a single-blind, randomized controlled trial
Irish Journal of Medical Science (1971 -) (2023)
-
Efficacy of antimicrobial photodynamic therapy as an adjuvant in periodontal treatment in Down syndrome patients
Lasers in Medical Science (2016)
-
Cytomorphometric and clinical investigation of the gingiva before and after low-level laser therapy of gingivitis in children
Lasers in Medical Science (2012)
-
Chronic gingivitis: the prevalence of periodontopathogens and therapy efficiency
European Journal of Clinical Microbiology & Infectious Diseases (2012)