Abstract
We evaluated whether vitamin D insufficiency (VDI; 25(OH)D <20 ng/ml) was associated with adverse outcomes among follicular lymphoma (FL) patients using an observational prospective cohort study of 642 FL patients enrolled from 2002–2012. The median age at diagnosis was 60 years. At a median follow-up of 59 months, 297 patients (46%) had an event (progression, treatment failure), 78 had died and 42 (6.5%) had a lymphoma-related death. VDI was associated with inferior event-free survival (EFS) at 12 months (EFS12, odds ratio (OR)=2.05; 95% confidence interval (CI) 1.18–3.54), overall survival (OS, hazards ratio (HR)=2.35; 95%CI 1.37–4.02), and lymphoma-specific survival (LSS, HR=2.97; 95% CI 1.52–5.80) for the full cohort. Among patients treated with immunochemotherapy (IC), VDI was associated with inferior EFS12 (OR=3.00; 95% CI 1.26–7.13), OS (HR=2.86; 95% CI 1.39–5.85), and LSS (HR=2.96; 95% CI 1.29–6.79). For observed patients, VDI was associated with inferior OS (HR=2.85; 95% CI 1.20–6.76). For other therapies, VDI was associated with inferior OS (HR=3.06; 95% CI 1.01–9.24). Our work is the first to reveal an association of VDI with early clinical failure, and to demonstrate an association of VDI with adverse outcomes among patients who are observed or treated with therapies other than IC. Our findings suggest a potentially modifiable prognostic factor to address in patients with FL.
Similar content being viewed by others
Introduction
The survival of patients with follicular lymphoma (FL) has substantially improved over the past 20 years, and these gains are partially attributable to the use of anti-CD20 monoclonal antibody therapy, exemplified by rituximab, as a single agent or added to traditional chemotherapy regimens.1 While FL is considered an indolent lymphoma, the clinical course is highly variable, with an overall survival (OS) at 5 years in the rituximab era ranging from 90% for low-risk patients by the FL International Prognostic Index (FLIPI) to 65% for high-risk patients,2 and there is a need to identify better prognostic markers and treatments in this heterogeneous disease.1 Casulo et al.3 reported that disease progression within 24 months of diagnosis predicts shorter OS in FL patients initially treated with immunochemotherapy. For all FL patients, we recently reported that event-free survival (EFS) at 12 months (EFS12), defined as any progression, retreatment or death due to any cause within 12 months of diagnosis, is a marker of poor outcome.4 Specifically, we demonstrated that the 83% of patients in our FL cohort who achieved EFS12 had no excess mortality above that of an age- and sex-matched general population with a median follow-up of 71 months. In contrast, the remaining group of patients experienced a more aggressive course, incurring overall mortality rates 3–8-fold higher than their matched counterparts. Prognostic markers associated with failure to achieve EFS12 would therefore allow for early identification of high-risk patients at a timepoint when alternative treatment strategies may be beneficial.
In an earlier publication, we evaluated the association of vitamin D insufficiency (VDI), defined as <25 ng/ml, with prognosis across a variety of lymphoma subtypes, and found a strong prognostic association with diffuse large B-cell lymphoma and peripheral T-cell lymphoma but not with either EFS or OS in FL, although our power for OS was low.5 More recently, Kelly et al.6 demonstrated that VDI, defined as <20 ng/ml, was associated with inferior progression-free survival (PFS) and OS among patients with FL treated with IC. Further, Bittenbring et al. demonstrated that diffuse large B-cell lymphoma patients with VDI who were treated with R-CHOP experienced inferior EFS and OS as compared to patients with optimal vitamin D levels, but there was no difference in outcomes in a historical cohort of patients treated with CHOP,7 raising the question of whether VDI is an adverse prognostic factor for patients who do not receive rituximab-containing regimens. Directly extrapolating these findings to FL would imply that VDI is of little prognostic benefit for the large number of patients who are initially approached with a watchful waiting strategy or with non-IC regimens.8 However VDI has also been shown to be broadly predictive of inferior outcomes in T-cell lymphoma,5 CLL,9 multiple myeloma,10 several solid malignancies11, 12, 13 and even all-cause mortality, suggesting that VDI may instead be a more global marker of poor prognosis, regardless of initial treatment.
To determine whether VDI is prognostic of inferior clinical outcomes in FL regardless of initial therapy as well as in selected treatment subgroups, we examined the association of circulating 25(OH)D levels with OS and lymphoma-specific survival (LSS) in an expanded analysis of our prior cohort (increased from 285 to 642 FL patients), and also for the first time examined whether VDI is associated with early clinical failure, defined as failure to achieve EFS12.
Materials and methods
Study population
As previously described, all patients included in this study were enrolled in the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence.5 This study was approved by the human subjects institutional review board and written informed consent was obtained from all participants. Since September 2002, we have offered enrollment to consecutive, newly diagnosed (within 9 months) patients with NHL who were evaluated at Mayo Clinic Rochester and the University of Iowa, were age 18 years or older, and were a resident of the United States. Exclusion criteria included known HIV infection, and unwillingness or inability to provide written informed consent. All pathology was reviewed by a lymphoma hematopathologist to verify the diagnosis and the classification of FL according to WHO criteria.14, 15 A blood sample was obtained at enrollment, and a standard protocol was used to abstract baseline clinical, laboratory and treatment data from medical records. Patients were contacted every 6 months for the first 3 years, and then annually thereafter, and reports of disease progression, retreatment and death were verified by medical record review.
Vitamin D measurement
Measurements of 25(OH)D were performed as described previously.5 25(OH)D was quantified by the gold-standard liquid chromatography-tandem mass spectrometry method, and represented the sum of 25(OH)D2 and 25(OH)D3 fractions. Measurements of 25(OH)D were made by deuterated stable isotope [d6-25(OH)D]-dilution liquid chromatography-tandem mass spectrometry on an API 4000 instrument (Applied Biosystems, Forest City, CA, USA), with sample introduction performed by a cohesive four-channel multiplexed system (Thermo-Fisher, Waltham, MA, USA). Calibration utilized a six-point standard curve over a concentration range of 0–200 ng/ml. Intra- and interassay coefficients of variation have been previously reported, and are less than 7%. All 642 samples were successfully assayed. We defined VDI as serum 25(OH)D level <20 ng/ml, as levels above this are considered sufficient by the US Institute of Medicine.16
Statistical analysis
Our primary endpoints were EFS12, OS, and lymphoma-specific survival (LSS). EFS was defined as time from diagnosis to the first event of progression, retreatment or death; EFS12 was defined using EFS status at 12 months from diagnosis. LSS was defined as the time from diagnosis to death due to disease, and OS was defined as the time from diagnosis to death due to any cause. Patients without an event or death were censored at time of last known follow-up. X2 and Fisher’s exact tests were used to assess the association of 25(OH)D levels with baseline clinical and demographic characteristics. Associations of 25(OH)D levels with LSS and OS were estimated using Kaplan–Meier curves, and adjusted hazard ratios (HRs) and 95% confidence intervals (95%CI) were estimated from Cox regression models. Associations with EFS12 were estimated from logistic regression models, using odds ratios (ORs) and 95%CI. These models were adjusted for FLIPI,17 body mass index and season of blood draw (by calendar quarter). Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R version 3.1.1 (http://www.r-project.org/).
Results
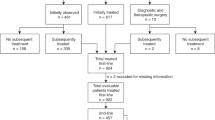
From 2002 through 2012, 920 patients with FL grade 1-3a were enrolled in the MER cohort. 278 patients were excluded for not having a serum sample available within 120 days of diagnosis, leaving 642 patients available for analysis. The median age at diagnosis of these patients was 60 years (range 23–93 years) and 52% were male. For initial treatment, 252 received combination IC (118 received rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP); 85 received rituximab, cyclophosphamide, vincristine and prednisone (R-CVP); 45 received rituximab with bendamustine (BR); and four received rituximab, cyclophosphamide and dexamethasone). Two hundred eighteen were observed, 76 received rituximab monotherapy and 96 received other therapies.
The distribution of vitamin D levels (Supplementary Figure S1) was similar to that seen previously in North American cohorts,18 with an overall median serum 25(OH)D level of 29 ng/ml (s.d. 11.9 ng/ml). Median vitamin D levels were similar across treatment groups (Table 1). Using a threshold of <20 ng/ml, the overall prevalence of VDI was 19% (120/642). Within specific treatment groups, the prevalence of VDI was 23% for patients treated with IC (58/252), 17% for observed patients (36/218), 14% for patients treated with rituximab monotherapy (11/76) and 16% for patients treated with other therapies (15/96). Prevalence of VDI was not associated with sex, age, geographic residence at time of diagnosis, season of blood draw, Ann Arbor Stage, bone marrow involvement or FLIPI. VDI was associated with non-white race (P=0.03), obesity (P=0.001) and lower performance status (P=0.001); these associations also persisted within some specific treatment groups (Table 1).
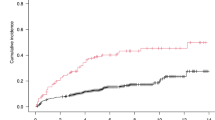
At a median follow-up of 59 months (range 1–144), 297 patients (46%) had an event, 78 had died (12%) and 42 (6.5%) had a lymphoma-related death. The EFS12 rate per Kaplan–Meier was 84% (95% CI: 82–87%). In all patients, VDI was associated with inferior EFS12 (OR=2.05; 95% CI 1.18–3.54), OS (HR=2.35; 95% CI 1.37–4.02), and LSS (HR=2.97; 95% CI 1.52–5.80) after adjustment for treatment, FLIPI, BMI and season of blood draw (Table 2 and Figures 1 and 2). Univariate results are shown in Supplementary Table SI.
For patients treated with IC, VDI was associated with inferior EFS12 (OR=3.00; 95% CI 1.26–7.13), OS (HR=2.86; 95% CI 1.39–5.85) and LSS (HR=2.96; 95%CI 1.29–6.79) after adjusting for FLIPI, body mass index and season of blood draw. For observed patients, VDI was associated with inferior OS (HR=2.85; 95% CI 1.20–6.76) but was not significantly associated with inferior EFS12 (OR=1.71; 95% CI 0.70–4.17); LSS could not be calculated due to insufficient deaths from lymphoma (N=7).
For patients who were initially treated with rituximab monotherapy, VDI was not significantly associated with EFS12 (OR=0.63; 95% CI 0.06–6.49). OS and LSS could not be calculated due to insufficient deaths (N=5), or deaths due to lymphoma (N=2).
Finally, for patients who had received other therapies, VDI was also associated with inferior OS both before (HR=3.29; 95% CI 1.12–9.62) and after adjustment for FLIPI, body mass index and season of blood draw (HR=3.06; 95% CI 1.01–9.24). There was also a trend towards a significant association of VDI with EFS12 (OR=3.18; 95% CI 0.91–11.08); LSS could not be calculated due to insufficient deaths from lymphoma (N=7).
When we combined all patients treated with any rituximab-containing therapy, VDI was associated with inferior EFS12 (OR=2.40; 95% CI 1.12–5.14), OS (HR=2.99; 95% CI 1.50–5.97) and LSS (HR=3.48; 95% CI 1.55–7.83). For comparison to our prior publication,5 we repeated our analyses using the threshold of 25 ng/ml; this did not materially affect our conclusions (Supplementary Table SII). Associations were also modeled using continuously distributed 25(OH)D concentrations, which revealed associations of inferior outcomes with 25(OH)D levels <20 ng/ml, with some suggestion of inferior outcome associations for levels as high as 25 ng/ml (Supplementary Figure S2). Finally, associations between VDI, defined as 20 ng/ml, and inferior outcomes were similar to the above findings when using EFS24 (approximately equivalent to POD24(ref. 3) as the end point (Supplementary Table SIII).
Discussion
The current report of 642 patients with FL consecutively enrolled in a systematic prospective cohort study is the first to demonstrate an association of VDI with inferior LSS and OS regardless of initial treatment and after adjustment for FLIPI and other clinical factors, as well as the first to demonstrate that VDI is associated with early clinical failure, as defined by EFS12. Our results are congruent with those of Kelly et al.6 who demonstrated an association of VDI with inferior OS and PFS using two clinical trial cohorts from the US and Europe. While all of the patients included in that study had been treated with CHOP-based IC, our findings extend these observations to other FL patients initially observed or who received other therapies. Although no clear associations in our study were identified for patients treated with rituximab monotherapy, we had low power in this treatment group. We chose a threshold of 20 ng/ml to define VDI, which is the level at which the Institute of Medicine has defined vitamin D insufficiency for the general US population. Importantly, vitamin D distributions vary with latitude of residence, time spent in the midday sun, race, body mass index, diet and other factors, and it is not clear if this cutpoint would be optimal in other populations.6, 7
Previous studies of patients with diffuse large B-cell lymphoma revealed that VDI led to inferior outcomes only among those patients treated with rituximab-including regimens, possibly due to vitamin D-mediated enhancement of antibody-dependent cellular cytotoxicity, a crucial mediator of rituximab efficacy.19 Our most robust findings were in IC treated patients, and were slightly stronger for any rituximab-containing regimens; however, elevated risk of a similar magnitude was also observed among observed patients and patients treated with other (non-IC) therapies for both EFS12 and OS, although estimates were imprecise and not statistically significant. This suggests that in the setting of FL, vitamin D-mediated enhancement of rituximab efficacy may not impart any additional clinical benefit, but further data are needed to fully address this question.
The discovery of factors identifying FL patients at high risk for early clinical failure has been cited as a significant unmet clinical need.20 By demonstrating an association of VDI with EFS12, we provide valuable new prognostic information that can better inform clinicians counseling newly diagnosed patients, as well as aid in shaping future clinical trial design. Importantly, VDI represents the first modifiable adverse prognostic factor in FL, supporting the need to test the impact of Vitamin D repletion in insufficient patients. Future research should also assess whether certain subgroups of FL patients have greater benefit from repletion.
One ongoing clinical trial (clinicaltrials.gov #NCT01787409) is examining whether vitamin D replacement can improve EFS12 among patients newly diagnosed with diffuse large B-cell lymphoma, peripheral T-cell lymphoma or early stage CLL. Initial results demonstrated that an enteral replacement strategy can achieve vitamin D sufficiency in 97% of such patients within 12 weeks.21 These findings suggest rapid vitamin D repletion may also be achievable in the context of FL. Randomized controlled trials should be performed examining the impact of vitamin D repletion on prognosis in FL, and should consider using EFS12 and OS as primary outcomes.
Strengths of this study include the prospective cohort design, consecutive enrollment of newly diagnosed patients with FL, large sample size, assessment of early events, central pathology review, medical record validation and our virtually complete follow-up of events. Additionally, we used liquid chromatography-tandem mass spectrometry for 25(OH)D quantification, which is considered the gold standard. Our cohort includes all patients with FL and available serum enrolled in the parent MER cohort, reducing selection bias. Limitations include the study’s observational design, as well as the median follow-up of 59 months, which is relatively modest given the slowly progressive nature of FL.
In conclusion, VDI in FL is predictive of early clinical failure among all patients and a subset of patients treated with IC, and is also predictive of inferior longer-term prognosis among all patients, as well as in subsets who are observed, treated with IC, or non-rituximab-containing regimens. The results of this study support the incorporation of serum 25(OH)D measurement into routine studies performed at the time of diagnosis to help identify patients at risk of early clinical failure, as well as to inform longer-term prognosis. Further investigations are needed to determine whether outcomes could be improved in FL by supplementation with this readily available vitamin.
References
Kahl BS, Yang DT . Follicular lymphoma: evolving therapeutic strategies. Blood 2016; 127: 2055–2063.
Nooka AK, Nabhan C, Zhou X, Taylor MD, Byrtek M, Miller TP et al. Examination of the follicular lymphoma international prognostic index (FLIPI) in the National LymphoCare study (NLCS): a prospective US patient cohort treated predominantly in community practices. Ann Oncol 2013; 24: 441–448.
Casulo C, Byrtek M, Dawson KL, Zhou X, Farber CM, Flowers CR et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol 2015; 33: 2516–2522.
Maurer MJ, Bachy E, Ghesquieres H, Ansell SM, Nowakowski GS, Thompson CA et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol 2016; 91: 1096–1101.
Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN et al. Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. J Clin Oncol 2010; 28: 4191–4198.
Kelly JL, Salles G, Goldman B, Fisher RI, Brice P, Press O et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J Clin Oncol 2015; 33: 1482–1490.
Bittenbring JT, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M et al. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol 2014; 32: 3242–3248.
Nabhan C, Zhou X, Day BM, Dawson K, Zelenetz AD, Friedberg JW et al. Disease, treatment, and outcome differences between men and women with follicular lymphoma in the United States. Am J Hematol 2016; 91: 770–775.
Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood 2011; 117: 1492–1498.
Ng AC, Kumar SK, Rajkumar SV, Drake MT . Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol 2009; 84: 397–400.
Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol 2008; 26: 2984–2991.
Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N . Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol 2009; 27: 3757–3763.
Robsahm TE, Schwartz GG, Tretli S . The Inverse Relationship between 25-Hydroxyvitamin D and Cancer Survival: Discussion of Causation. Cancers 2013; 5: 1439–1455.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H eds. International Agency for Research on Cancer: Lyon, 2008.
Jaffe ES, Harris NL, Stein H, Vardiman JW eds. World Health Organization of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2001.
Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US): Washington (DC), 2011.
Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R et al. Follicular lymphoma international prognostic index. Blood 2004; 104: 1258–1265.
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013; 369: 1991–2000.
Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004; 199: 1659–1669.
Friedberg JW, Kahl BS, Leonard JP . The roadmap forward in follicular lymphoma: time for a precision approach. ASH Clin News 2015; 1: 29–30.
Sfeir JG, Drake MT, LaPlant BR, Maurer MJ, Link BK, Berndt TJ et al. Validation of a vitamin D replacement strategy in vitamin D-insufficient patients with lymphoma or chronic lymphocytic leukemia. Blood Cancer J 2017; 7: e526.
Acknowledgements
We thank Ms Sondra Buehler for editorial assistance. This work was supported in part by the National Institutes of Health (P50 CA97274) to the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence and the Henry J. Predolin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Tracy, S., Maurer, M., Witzig, T. et al. Vitamin D insufficiency is associated with an increased risk of early clinical failure in follicular lymphoma. Blood Cancer J. 7, e595 (2017). https://doi.org/10.1038/bcj.2017.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2017.70
This article is cited by
-
25-Hydroxy vitamin D deficiency is an inferior predictor of peripheral T-cell lymphomas
Annals of Hematology (2024)
-
Pediatric primary cutaneous anaplastic large-cell lymphoma with associated hypovitaminosis D
Archives of Dermatological Research (2023)
-
Risk factors for POD24 in patients with previously untreated follicular lymphoma: a systematic review and meta-analysis
Annals of Hematology (2022)
-
Prognostic value of 25-hydroxy vitamin D in extranodal NK/T cell lymphoma
Annals of Hematology (2021)
-
Mikronährstoffe
Der Onkologe (2021)