Abstract
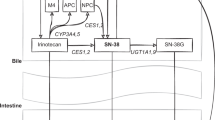
Neutropenia is a common dose-limiting toxicity associated with irinotecan treatment. Although UGT1A1 variants have been associated with neutropenia, a fraction of neutropenia risk remains unaccounted for. To identify additional genetic markers contributing to variability in irinotecan pharmacokinetics and neutropenia, a regression analysis was performed in 78 irinotecan-treated patients to analyze comprehensively three hepatic efflux transporter genes (ABCB1, ABCC1 and ABCG2). rs6498588 (ABCC1) and rs12720066 (ABCB1) were associated with increased SN-38 exposure, and rs17501331 (ABCC1) and rs12720066 were associated with lower absolute neutrophil count nadir. rs6498588 and a variant in high linkage disequilibrium are located in transcriptionally active regions or are predicted to alter transcription factor binding sites. While enhancer activity was not evident in vitro for genomic regions containing these single-nucleotide polymorphisms, rs6498588 was significantly associated with ABCC1 expression in human liver. These results suggest that genetic variation in ABCC1 and ABCB1 may contribute to irinotecan-induced neutropenia by altering expression of transporters involved in irinotecan metabolite disposition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Forni M, Bugat R, Chabot GG, Culine S, Extra JM, Gouyette A et al. Phase I and pharmacokinetic study of the camptothecin derivative irinotecan, administered on a weekly schedule in cancer patients. Cancer Res 1994; 54: 4347–4354.
Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR . Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 2003; 21: 807–814.
Slatter JG, Su P, Sams JP, Schaaf LJ, Wienkers LC . Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos 1997; 25: 1157–1164.
Ramchandani RP, Wang Y, Booth BP, Ibrahim A, Johnson JR, Rahman A et al. The role of SN-38 exposure, UGT1A1*28 polymorphism, and baseline bilirubin level in predicting severe irinotecan toxicity. J Clin Pharmacol 2007; 47: 78–86.
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–1388.
Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genom 2007; 17: 497–504.
Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE et al. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 2006; 24: 2237–2244.
Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 1998; 101: 847–854.
Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 2000; 60: 6921–6926.
Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenom J 2002; 2: 43–47.
Rouits E, Boisdron-Celle M, Dumont A, Guerin O, Morel A, Gamelin E . Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clin Cancer Res 2004; 10: 5151–5159.
McLeod HL, Parodi L, Sargent DJ, Marsh S, Green E, Abreu P et al. UGT1A1*28, toxicity and outcome in advanced colorectal cancer: results from trial N9741. J Clin Oncol 2006; 24 (Suppl 18): abstract 3520.
Côté JF, Kirzin S, Kramar A, Mosnier JF, Diebold MD, Soubeyran I et al. UGT1A1 polymorphism can predict hematologic toxicity in patients treated with irinotecan. Clin Cancer Res 2007; 13: 3269–3275.
Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 2009; 27: 2604–2614.
Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009; 27: 2530–2535.
Crona DJ, Ramirez J, Qiao W, de Graan AJ, Ratain MJ, van Schaik RH et al. Clinical validity of new genetic biomarkers of irinotecan neutropenia: an independent replication study. Pharmacogenom J 2015; 16: 54–59.
Innocenti F, Schilsky RL, Ramírez J, Janisch L, Undevia S, House LK et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 2014; 32: 2328–2334.
Marcuello E, Altés A, Menoyo A, Del Rio E, Gómez-Pardo M, Baiget M . UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer 2004; 91: 678–682.
Braun MS, Richman SD, Thompson L, Daly CL, Meade AM, Adlard JW et al. Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J Clin Oncol 2009; 27: 5519–5528.
Schulz C, Heinemann V, Schalhorn A, Moosmann N, Zwingers T, Boeck S et al. UGT1A1 gene polymorphism: impact on toxicity and efficacy of irinotecan-based regimens in metastatic colorectal cancer. World J Gastroenterol 2009; 15: 5058–5066.
Mathijssen RH, Marsh S, Karlsson MO, Xie R, Baker SD, Verweij J et al. Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin Cancer Res 2003; 9: 3246–3253.
Han JY, Lim HS, Yoo YK, Shin ES, Park YH, Lee SY et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer 2007; 110: 138–147.
Han JY, Lim HS, Park YH, Lee SY, Lee JS . Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 2009; 63: 115–120.
Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD . Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat 2003; 6: 71–84.
Sai K, Kaniwa N, Itoda M, Saito Y, Hasegawa R, Komamura K et al. Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype-dependent renal clearance of irinotecan. Pharmacogenetics 2003; 13: 741–757.
Glimelius B, Garmo H, Berglund A, Fredriksson LA, Berglund M, Kohnke H et al. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenom J 2011; 11: 61–71.
Sai K, Saito Y, Maekawa K, Kim SR, Kaniwa N, Nishimaki-Mogami T et al. Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother Pharmacol 2010; 66: 95–105.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2015.
Harrell FE . rms: Regression Modeling Strategies. R package version 4.4-0, 2015. Available at: http://CRAN.R-project.org/package=rms. Accessed on November 9, 2015.
Ward LD, Kellis M . HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40: D930–D934.
Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 2012; 22: 1790–1797.
Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 2011; 473: 43–49.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74.
Roadmap Epigenomics Consortium Roadmap Epigenomics Consortium Kundaje A Roadmap Epigenomics Consortium Meuleman W Roadmap Epigenomics Consortium Ernst J Roadmap Epigenomics Consortium Bilenky M Roadmap Epigenomics Consortium Yen A et al. Integrative analysis of 111 reference human epigenomes. Nature 2015; 518: 317–330.
Kel AE, Gössling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E . MATCH: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res 2003; 31: 3576–3579.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M et al. Primer3—new capabilities and interfaces. Nucleic Acids Res 2012; 40: e115.
Koressaar T, Remm M . Enhancements and modifications of primer design program Primer3. Bioinformatics 2007; 23: 1289–1291.
Simonet WS, Bucay N, Lauer SJ, Taylor JM . A far-downstream hepatocyte-specific control region directs expression of the linked human apolipoprotein E and C-I genes in transgenic mice. J Biol Chem 1993; 268: 8221–8229.
Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genom 2011; 21: 152–161.
Létourneau IJ, Deeley RG, Cole SP . Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1). Pharmacogenet Genom 2005; 15: 647–657.
Conseil G, Cole SP . Two polymorphic variants of ABCC1 selectively alter drug resistance and inhibitor sensitivity of the multidrug and organic anion transporter multidrug resistance protein 1. Drug Metab Dispos 2013; 41: 2187–2196.
Yee SW, Chen L, Giacomini KM . Pharmacogenomics of membrane transporters: past, present and future. Pharmacogenomics 2010; 11: 475–479.
Hesselson SE, Matsson P, Shima JE, Fukushima H, Yee SW, Kobayashi Y et al. Genetic variation in the proximal promoter of ABC and SLC superfamilies: liver and kidney specific expression and promoter activity predict variation. PLoS One 2009; 4: e6942.
Choi JH, Yee SW, Kim MJ, Nguyen L, Lee JH, Kang JO et al. Identification and characterization of novel polymorphisms in the basal promoter of the human transporter, MATE1. Pharmacogenet Genom 2009; 19: 770–780.
Tahara H, Yee SW, Urban TJ, Hesselson S, Castro RA, Kawamoto M et al. Functional genetic variation in the basal promoter of the organic cation/carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5). J Pharmacol Exp Ther 2009; 329: 262–271.
Yee SW, Shima JE, Hesselson S, Nguyen L, De Val S, Lafond RJ et al. Identification and characterization of proximal promoter polymorphisms in the human concentrative nucleoside transporter 2 (SLC28A2). J Pharmacol Exp Ther 2009; 328: 699–707.
To KK, Zhan Z, Litman T, Bates SE . Regulation of ABCG2 expression at the 3' untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol 2008; 28: 5147–5161.
Pan YZ, Morris ME, Yu AM . MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol 2009; 75: 1374–1379.
Kim MJ, Skewes-Cox P, Fukushima H, Hesselson S, Yee SW, Ramsey LB et al. Functional characterization of liver enhancers that regulate drug-associated transporters. Clin Pharmacol Ther 2011; 89: 571–578.
O'Brien VP, Bokelmann K, Ramírez J, Jobst K, Ratain MJ, Brockmöller J et al. Hepatocyte nuclear factor 1 regulates the expression of the organic cation transporter 1 via binding to an evolutionary conserved region in intron 1 of the OCT1 gene. J Pharmacol Exp Ther 2013; 347: 181–192.
Poonkuzhali B, Lamba J, Strom S, Sparreboom A, Thummel K, Watkins P et al. Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms. Drug Metab Dispos 2008; 36: 780–795.
Zhou Q, Sparreboom A, Tan EH, Cheung YB, Lee A, Poon D et al. Pharmacogenetic profiling across the irinotecan pathway in Asian patients with cancer. Br J Clin Pharmacol 2005; 59: 415–424.
Iyer L, Ramírez J, Shepard DR, Bingham CM, Hossfeld DK, Ratain MJ et al. Biliary transport of irinotecan and metabolites in normal and P-glycoprotein-deficient mice. Cancer Chemother Pharmacol 2002; 49: 336–341.
Tagen M, Zhuang Y, Zhang F, Harstead KE, Shen J, Schaiquevich P et al. P-glycoprotein, but not multidrug resistance protein 4, plays a role in the systemic clearance of irinotecan and SN-38 in mice. Drug Metab Lett 2010; 4: 195–201.
Chen ZS, Furukawa T, Sumizawa T, Ono K, Ueda K, Seto K et al. ATP-dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol Pharmacol 1999; 55: 921–928.
Roelofsen H, Vos TA, Schippers IJ, Kuipers F, Koning H, Moshage H et al. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells. Gastroenterology 1997; 112: 511–521.
Seiser EL, Xia K, Wright FA, Innocenti F . Meta-analysis of liver eQTL studies and cross-tissue eQTL comparison using GTEx data. Presented at the 64th Annual Meeting of The American Society of Human Genetics, 20 October 2014, San Diego, CA, USA, (abstract 667S).
Schellens JH, Maliepaard M, Scheper RJ, Scheffer GL, Jonker JW, Smit JW et al. Transport of topoisomerase I inhibitors by the breast cancer resistance protein. Potential clinical implications. Ann NY Acad Sci 2000; 922: 188–194.
Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer 2004; 109: 858–854.
Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 2002; 1: 611–616.
Phelps MA, Sparreboom A . Irinotecan pharmacogenetics: a finished puzzle? J Clin Oncol 2014; 32: 2287–2289.
Fujiwara Y, Minami H . An overview of the recent progress in irinotecan pharmacogenetics. Pharmacogenomics 2010; 11: 391–406.
Marsh S, Hoskins JM . Irinotecan pharmacogenomics. Pharmacogenomics 2010; 11: 1003–1010.
Hoskins JM, Rosner GL, Ratain MJ, McLeod HL, Innocenti F . Pharmacodynamic genes do not influence risk of neutropenia in cancer patients treated with moderately high-dose irinotecan. Pharmacogenomics 2009; 10: 1139–1146.
Acknowledgements
This work was supported by NIH/NIGMS U01GM061390 (Pharmacogenetics of Membrane Transporters), NIH/NIGMS U01GM0061393 (PAAR-Pharmacogenomics of Anticancer Agents Research Group), NIH/NIGMS T32 GM007175, NIH/NIDDK R21DK081157 and NIH/NCI K07CA140390-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Drs Innocenti and Ratain are patent holders on the UGT1A1 testing for irinotecan neutropenia. Dr Ratain also receives intermittent royalties on multiple patents related to irinotecan pharmacogenetics and is an inventor on a pending patent application for a genomic prescribing system. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, M., Seiser, E., Baldwin, R. et al. ABC transporter polymorphisms are associated with irinotecan pharmacokinetics and neutropenia. Pharmacogenomics J 18, 35–42 (2018). https://doi.org/10.1038/tpj.2016.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.75
This article is cited by
-
Chemotherapy-induced neutropenia as a prognostic factor in patients with extensive-stage small cell lung cancer
European Journal of Clinical Pharmacology (2023)
-
Irinotecan-Induced Toxicity: A Pharmacogenetic Study Beyond UGT1A1
Clinical Pharmacokinetics (2023)
-
Integration of DNA sequencing with population pharmacokinetics to improve the prediction of irinotecan exposure in cancer patients
British Journal of Cancer (2022)
-
Evaluation of pharmacogenomics and hepatic nuclear imaging–related covariates by population pharmacokinetic models of irinotecan and its metabolites
European Journal of Clinical Pharmacology (2022)
-
Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of 6 randomized trials
BMC Cancer (2021)