Abstract
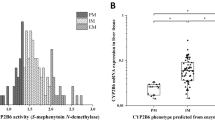
Efavirenz increases CYP2C19- and CYP3A-mediated omeprazole metabolism. We hypothesized that CYP2C19 and CYP2B6 genetic polymorphisms influence the extent of induction of omeprazole metabolism by efavirenz. Healthy subjects (n=57) were administered a single 20 mg oral dose of omeprazole on two occasions: with a single 600 mg efavirenz dose; and after a 17-day treatment with efavirenz (600 mg per day). DNA was genotyped for CYP2C19*2, *3 and *17 alleles and CYP2B6*6, *4 and *9 alleles using Taqman assays. Omeprazole, its enantiomers and metabolites were measured by liquid chromatography/tandem mass spectrometry. Our results showed that efavirenz increased omeprazole clearances in all CYP2C19 genotypes in non-stereoselective manner, but the magnitude of induction was genotype dependent. Metabolic ratios of 5-hydroxylation of omeprazole were reduced in extensive and intermediate metabolizers of CYP2C19 (P<0.05). No significant associations were observed between CYP2B6 genotypes and induction by efavirenz on omeprazole metabolism. Our data indicate how interplays between drug interactions and CYP2C19 genetic variations may influence systemic exposure of CYP2C19 substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z . The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 2003; 306: 287–300.
Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos 1999; 27: 1319–1333.
Belanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C . Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos 2009; 37: 1793–1796.
Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007; 8: 547–558.
Bae SK, Jeong YJ, Lee C, Liu KH . Identification of human UGT isoforms responsible for glucuronidation of efavirenz and its three hydroxy metabolites. Xenobiotica 41: 437–444.
Zhu M, Kaul S, Nandy P, Grasela DM, Pfister M . Model-based approach to characterize efavirenz autoinduction and concurrent enzyme induction with carbamazepine. Antimicrob Agents Chemother 2009; 53: 2346–2353.
Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A et al. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther 88: 676–684.
Michaud V, Ogburn E, Thong N, Aregbe AO, Quigg TC, Flockhart DA et al. Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers. Clin Pharmacol Ther 2012; 91: 475–482.
von Moltke LL, Greenblatt DJ, Granda BW, Giancarlo GM, Duan SX, Daily JP et al. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol 2001; 41: 85–91.
Andersson T, Weidolf L . Stereoselective disposition of proton pump inhibitors. Clin Drug Investig 2008; 28: 263–279.
Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L . Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos 2000; 28: 966–972.
Desta Z, Zhao X, Shin JG, Flockhart DA . Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002; 41: 913–958.
Wang G, Lei HP, Li Z, Tan ZR, Guo D, Fan L et al. The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol 2009; 65: 281–285.
Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E . Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther 2008; 83: 322–327.
Baldwin RM, Ohlsson S, Pedersen RS, Mwinyi J, Ingelman-Sundberg M, Eliasson E et al. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol 2008; 65: 767–774.
Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J . Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol 2010; 69: 222–230.
Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010; 121: 512–518.
Lee LS, Nafziger AN, Bertino Jr. JS . Evaluation of inhibitory drug interactions during drug development: genetic polymorphisms must be considered. Clin Pharmacol Ther 2005; 78: 1–6.
Wang LS, Zhu B, Abd El-Aty AM, Zhou G, Li Z, Wu J et al. The influence of St John's Wort on CYP2C19 activity with respect to genotype. J Clin Pharmacol 2004; 44: 577–581.
Kanebratt KP, Diczfalusy U, Backstrom T, Sparve E, Bredberg E, Bottiger Y et al. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin Pharmacol Ther 2008; 84: 589–594.
Mihara K, Svensson US, Tybring G, Hai TN, Bertilsson L, Ashton M . Stereospecific analysis of omeprazole supports artemisinin as a potent inducer of CYP2C19. Fundam Clin Pharmacol 1999; 13: 671–675.
Liu P, Foster G, Gandelman K, LaBadie RR, Allison MJ, Gutierrez MJ et al. Steady-state pharmacokinetic and safety profiles of voriconazole and ritonavir in healthy male subjects. Antimicrob Agents Chemother 2007; 51: 3617–3626.
Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun 2004; 319: 1322–1326.
Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics 2005; 15: 1–5.
Cabrera SE, Santos D, Valverde MP, Dominguez-Gil A, Gonzalez F, Luna G et al. Influence of the cytochrome P450 2B6 genotype on population pharmacokinetics of efavirenz in human immunodeficiency virus patients. Antimicrob Agents Chemother 2009; 53: 2791–2798.
Lindfelt T, O'Brien J, Song JC, Patel R, Winslow DL . Efavirenz plasma concentrations and cytochrome 2B6 polymorphisms. Ann Pharmacother 2010; 44: 1572–1578.
Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18: 2391–2400.
Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 2007; 320: 72–80.
Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther 2002; 72: 1–9.
Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A et al. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther 2010; 88: 676–684.
Gonzalez HM, Romero EM, Peregrina AA, de J Chávez T, Escobar-Islas E, Lozano F et al. CYP2C19- and CYP3A4-dependent omeprazole metabolism in West Mexicans. J Clin Pharmacol 2003; 43: 1211–1215.
Isaza C, Henao J, Martinez JH, Sepulveda Arias JC, Beltran L . Phenotype-genotype analysis of CYP2C19 in Colombian mestizo individuals. BMC Clin Pharmacol 2007; 7: 6.
Uno T, Niioka T, Hayakari M, Yasui-Furukori N, Sugawara K, Tateishi T . Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol 2007; 63: 143–149.
Yin OQ, Tomlinson B, Chow AH, Waye MM, Chow MS . Omeprazole as a CYP2C19 marker in Chinese subjects: assessment of its gene-dose effect and intrasubject variability. J Clin Pharmacol 2004; 44: 582–589.
Bottiger Y . Use of omeprazole sulfone in a single plasma sample as a probe for CYP3A4. Eur J Clin Pharmacol 2006; 62: 621–625.
Wang LS, Zhou G, Zhu B, Wu J, Wang JG, Abd El-Aty AM et al. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther 2004; 75: 191–197.
Svensson US, Ashton M, Trinh NH, Bertilsson L, Dinh XH, Nguyen VH et al. Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther 1998; 64: 160–167.
Feng HJ, Huang SL, Wang W, Zhou HH . The induction effect of rifampicin on activity of mephenytoin 4'-hydroxylase related to M1 mutation of CYP2C19 and gene dose. Br J Clin Pharmacol 1998; 45: 27–29.
Zhou HH, Anthony LB, Wood AJ, Wilkinson GR . Induction of polymorphic 4'-hydroxylation of S-mephenytoin by rifampicin. Br J Clin Pharmacol 1990; 30: 471–475.
Rengelshausen J, Banfield M, Riedel KD, Burhenne J, Weiss J, Thomsen T et al. Opposite effects of short-term and long-term St John’s Wort intake on voriconazole pharmacokinetics. Clin Pharmacol Ther 2005; 78: 25–33.
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 2006; 79: 103–113.
Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 2000; 68: 598–604.
Matheny CJ, Ali RY, Yang X, Pollack GM . Effect of prototypical inducing agents on P-glycoprotein and CYP3A expression in mouse tissues. Drug Metab Dispos 2004; 32: 1008–1014.
Tannergren C, Engman H, Knutson L, Hedeland M, Bondesson U, Lennernas H . St John’s wort decreases the bioavailability of R- and S-verapamil through induction of the first-pass metabolism. Clin Pharmacol Ther 2004; 75: 298–309.
Habtewold A, Amogne W, Makonnen E, Yimer G, Nylen H, Riedel KD et al. Pharmacogenetic and pharmacokinetic aspects of CYP3A induction by efavirenz in HIV patients. Pharmacogenomics J, advance online publication, 23 October 2012; doi:10.1038/tpj.2012.46 (e-pub ahead of print).
Sugimoto K, Uno T, Tateishi T . Effects of the CYP3A5 genotype on omeprazole sulfoxidation in CYP2C19 PMs. Eur J Clin Pharmacol 2008; 64: 583–587.
Acknowledgements
The project described here was supported by Award Number R01GM078501, 3R01GM078501-04S1, R56 grant (2R56GM067308-09A1) from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, MD), and by Award Number M01-RR00750 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Veronique Michaud is the recipient of a fellowship from the Canadian Institutes of Health Research and winner of the Bisby award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Pharmacogenomics Journal website
Rights and permissions
About this article
Cite this article
Michaud, V., Kreutz, Y., Skaar, T. et al. Efavirenz-mediated induction of omeprazole metabolism is CYP2C19 genotype dependent. Pharmacogenomics J 14, 151–159 (2014). https://doi.org/10.1038/tpj.2013.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2013.17