Abstract
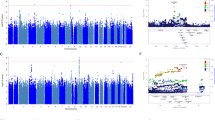
Suicidal thoughts during antidepressant treatment have been the focus of several candidate gene association studies. The aim of the present genome-wide association study was to identify additional genetic variants involved in increasing suicidal ideation during escitalopram and nortriptyline treatment. A total of 706 adult participants of European ancestry, treated for major depression with escitalopram or nortriptyline over 12 weeks in the Genome-Based Therapeutic Drugs for Depression (GENDEP) study were genotyped with Illumina Human 610-Quad Beadchips (Illumina, San Diego, CA, USA). A total of 244 subjects experienced an increase in suicidal ideation during follow-up. The genetic marker most significantly associated with increasing suicidality (8.28 × 10−7) was a single-nucleotide polymorphism (SNP; rs11143230) located 30 kb downstream of a gene encoding guanine deaminase (GDA) on chromosome 9q21.13. Two suggestive drug-specific associations within KCNIP4 (Kv channel-interacting protein 4; chromosome 4p15.31) and near ELP3 (elongation protein 3 homolog; chromosome 8p21.1) were found in subjects treated with escitalopram. Suggestive drug by gene interactions for two SNPs near structural variants on chromosome 4q12, one SNP in the apolipoprotein O (APOO) gene on chromosome Xp22.11 and one on chromosome 11q24.3 were found. The most significant association within a set of 33 candidate genes was in the neurotrophic tyrosine kinase receptor type 2 (NTRK2) gene. Finally, we also found trend for an association within genes previously associated with psychiatric phenotypes indirectly linked to suicidal behavior, that is, GRIP1, NXPH1 and ANK3. The results suggest novel pathways involved in increasing suicidal ideation during antidepressant treatment and should help to target treatment to reduce the risk of this dramatic adverse event. Limited power precludes definitive conclusions and replication in larger sample is warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
US Food Drug Administration. Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults. US Food Drug Administration, http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-fda.pdf, 2006.
World Health Organization. Suicide prevention in Europe. The WHO European Monitoring Survey on National Suicide Prevention Programmes and Strategies. World Health Organization: Geneva, 2002.
Perroud N, Uher R, Marusic A, Rietschel M, Mors O, Henigsberg N et al. Suicidal ideation during treatment of depression with escitalopram and nortriptyline in genome-based therapeutic drugs for depression (GENDEP): a clinical trial. BMC Med 2009; 7: 60.
Perlis RH, Beasley Jr CM, Wines Jr JD, Tamura RN, Cusin C, Shear D et al. Treatment-associated suicidal ideation and adverse effects in an open, multicenter trial of fluoxetine for major depressive episodes. Psychother Psychosom 2007; 76: 40–46.
Szanto K, Mulsant BH, Houck PR, Dew MA, Dombrovski A, Pollock BG et al. Emergence, persistence, and resolution of suicidal ideation during treatment of depression in old age. J Affect Disord 2007; 98: 153–161.
Laje G, Allen AS, Akula N, Manji H, John Rush A, McMahon FJ . Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics 2009; 19: 666–674.
Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164: 1530–1538.
Perlis RH, Purcell S, Fava M, Fagerness J, Rush AJ, Trivedi MH et al. Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D study. Arch Gen Psychiatry 2007; 64: 689–697.
Perroud N, Aitchison KJ, Uher R, Smith R, Huezo-Diaz P, Marusic A et al. Genetic predictors of increase in suicidal ideation during antidepressant treatment in the GENDEP project. Neuropsychopharmacology 2009; 34: 2517–2528.
Wing J, Sartorius N, Ustin T . Diagnosis and Clinical Measurement in Psychiatry. A Reference Manual for SCAN. World Health Organization: Geneva, 1998.
Uher R, Maier W, Hauser J, Marusic A, Schmael C, Mors O et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry 2009; 194: 252–259.
Uher R, Perroud N, Mandy YN, Hauser J, Henigsberg N, Maier W et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010; 167: 555–564.
Sanchez C, Bergqvist PB, Brennum LT, Gupta S, Hogg S, Larsen A et al. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology (Berl) 2003; 167: 353–362.
Sanchez C, Hyttel J . Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 1999; 19: 467–489.
Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med 2008; 38: 289–300.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Price AL, Weale ME, Patterson N, Myers SR, Need AC, Shianna KV et al. Long-range LD can confound genome scans in admixed populations. Am J Hum Genet 2008; 83: 132–135, author reply 135-139.
Baca-Garcia E, Diaz-Sastre C, Ceverino A, Perez-Rodriguez MM, Navarro-Jimenez R, Lopez-Castroman J et al. Suicide attempts among women during low estradiol/low progesterone states. J Psychiatr Res 2010; 44: 209–214.
Baca-Garcia E, Vaquero-Lorenzo C, Perez-Rodriguez MM, Gratacos M, Bayes M, Santiago-Mozos R et al. Nucleotide variation in central nervous system genes among male suicide attempters. Am J Med Genet B Neuropsychiatr Genet 2010; 153B: 208–213.
Dudbridge F, Gusnanto A . Estimation of significance thresholds for genomewide association scans. Genet Epidemiol 2008; 32: 227–234.
Gauderman W, Morrison J . Quanto 1 1 A computer program for power and sample size calculations for genetic-epigemiology studies. http://hydra.usc.edu/GxE, 2006.
Li J, Ji L . Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 2005; 95: 221–227.
Nyholt DR . A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 2004; 74: 765–769.
Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL . Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci 2004; 7: 145–152.
Kubo K, Honda H, Honda H, Sannomiya K, Aying Y, Mei W et al. Histochemical and immunohistochemical investigation of guanase and nedasin in human tissues. J Med Invest 2006; 53: 264–270.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B . Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 70–75.
Firestein BL, Firestein BL, Brenman JE, Aoki C, Sanchez-Perez AM, El-Husseini AE et al. Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron 1999; 24: 659–672.
Tannu NS, Howell LL, Hemby SE . Integrative proteomic analysis of the nucleus accumbens in rhesus monkeys following cocaine self-administration. Mol Psychiatry 2010; 15: 185–203.
Reines A, Cereseto M, Ferrero A, Sifonios L, Podesta MF, Wikinski S . Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioral response. Neuropsychopharmacology 2008; 33: 1896–1908.
Sifonios L, Trinchero M, Cereseto M, Ferrero A, Cladouchos ML, Macedo GF et al. An enriched environment restores normal behavior while providing cytoskeletal restoration and synaptic changes in the hippocampus of rats exposed to an experimental model of depression. Neuroscience 2009; 164: 929–940.
Kristiansen LV, Meador-Woodruff JH . Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr Res 2005; 78: 87–93.
Gratacos M, Costas J, de Cid R, Bayes M, Gonzalez JR, Baca-Garcia E et al. Identification of new putative susceptibility genes for several psychiatric disorders by association analysis of regulatory and non-synonymous SNPs of 306 genes involved in neurotransmission and neurodevelopment. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 808–816.
Choi KH, Zepp ME, Higgs BW, Weickert CS, Webster MJ . Expression profiles of schizophrenia susceptibility genes during human prefrontal cortical development. J Psychiatry Neurosci 2009; 34: 450–458.
Bladt F, Tafuri A, Gelkop S, Langille L, Pawson T . Epidermolysis bullosa and embryonic lethality in mice lacking the multi-PDZ domain protein GRIP1. Proc Natl Acad Sci USA 2002; 99: 6816–6821.
van den Oord EJ, Kuo PH, Hartmann AM, Webb BT, Moller HJ, Hettema JM et al. Genomewide association analysis followed by a replication study implicates a novel candidate gene for neuroticism. Arch Gen Psychiatry 2008; 65: 1062–1071.
Brezo J, Paris J, Turecki G . Personality traits as correlates of suicidal ideation, suicide attempts, and suicide completions: a systematic review. Acta Psychiatr Scand 2006; 113: 180–206.
Fiori LM, Zouk H, Himmelman C, Turecki G . X chromosome and suicide. Mol Psychiatry; epub ahead of print 15 December 2009, doi:10.1038/mp.2009.132.
Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, Vessaz M et al. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet 2009; 150B: 799–807.
Huezo-Diaz P, Uher R, Smith R, Rietschel M, Henigsberg N, Marusic A et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry 2009; 195: 30–38.
Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry 2009; 14: 755–763.
Chen YW, Dilsaver SC . Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biol Psychiatry 1996; 39: 896–899.
Valtonen HM, Suominen K, Sokero P, Mantere O, Arvilommi P, Leppamaki S et al. How suicidal bipolar patients are depends on how suicidal ideation is defined. J Affect Disord 2009; 118: 48–54.
Wasserman D, Geijer T, Rozanov V, Wasserman J . Suicide attempt and basic mechanisms in neural conduction: relationships to the SCN8A and VAMP4 genes. Am J Med Genet B Neuropsychiatr Genet 2005; 133B: 116–119.
Shindo S, Yoshioka N . Polymorphisms of the cholecystokinin gene promoter region in suicide victims in Japan. Forensic Sci Int 2005; 150: 85–90.
Yanagi M, Shirakawa O, Kitamura N, Okamura K, Sakurai K, Nishiguchi N et al. Association of 14-3-3 epsilon gene haplotype with completed suicide in Japanese. J Hum Genet 2005; 50: 210–216.
De Luca V, Tharmalingam S, Kennedy JL . Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur Psychiatry 2007; 22: 282–287.
Acknowledgements
We acknowledge the contribution of Maja Bajs, Mara Barreto, Cristian Bonvicini, Desmond Campbell, Elzbieta Cegielska, Monika Dmitrzak-Weglarz, Amanda Elkin, Caterina Giovannini, Joanna M Gray, Cerisse Gunasinghe, Bhanu Gupta, Sudhir Kumar, Susanne Höfels, Petra Kalember, Paweł Kapelski, Zrnka Kovacic, Dejan Kozel, Anna Leszczynska-Rodziewicz, Sylvie Linotte, Andrej Marusic, Julien Mendlewicz, Metka Paragi, Laura Pedrini, Jorge Perez, Ute Pfeiffer, Aleksandra Rajewska-Rager, Luciana Rillosi, Christine Schmäl, Anna Schuhmacher, Maria Skibiñska, Rebecca Smith, Farzana Hoda, Jana Strohmaier, Jerneja Sveticic, Aleksandra Szczepankiewicz, Alenka Tancic, Sandra Weber, Thomas Schulze, Piotr Czerski and Richard J Williamson. The GENDEP study was funded by a European Commission Framework 6 grant, EC Contract Ref: LSHB-CT-2003-503428. Lundbeck provided both nortriptyline and escitalopram free of charge for the GENDEP study. GlaxoSmithKline, the Medical Research Council and the Biomedical Research Centre for Mental Health at the Institute of Psychiatry, King's College London and South London and Maudsley NHS Foundation Trust (funded by the National Institute for Health Research, Department of Health, UK) contributed by funding add-on projects in the London center. The genotyping was funded by joint grant from the Medical Research Council, UK and GlaxoSmithKline (G0701420). The fund providers had no role in the design and conduct of the study, in data collection, analysis, interpretation or writing the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Perroud, Uher, Ng, Hauser, Guipponi, Maier, Mors, Gennarelli, Rietschel, Dernovsek, Stamp, Lathrop, Breen, Craig and Lewis declare no competing interests. Henigsberg and Souery participated in clinical trials sponsored by pharmaceutical companies including GlaxoSmithKline and Lundbeck. Henigsberg has received honoraria for participating in expert panels for pharmaceutical companies. Aitchison, Farmer and McGuffin have received consultancy fees and honoraria for participating in expert panels for pharmaceutical companies including Lundbeck and GlaxoSmithKline.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Perroud, N., Uher, R., Ng, M. et al. Genome-wide association study of increasing suicidal ideation during antidepressant treatment in the GENDEP project. Pharmacogenomics J 12, 68–77 (2012). https://doi.org/10.1038/tpj.2010.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2010.70
Keywords
This article is cited by
-
Investigating genetic variants for treatment response to selective serotonin reuptake inhibitors in syndromal factors and side effects among patients with depression in Taiwanese Han population
The Pharmacogenomics Journal (2023)
-
Saliva microbiome, dietary, and genetic markers are associated with suicidal ideation in university students
Scientific Reports (2022)
-
The mechanism of electroacupuncture for depression on basic research: a systematic review
Chinese Medicine (2021)
-
Polymorphisms of stress pathway genes and emergence of suicidal ideation at antidepressant treatment onset
Translational Psychiatry (2020)
-
Polymorphism A118G of opioid receptor mu 1 (OPRM1) is associated with emergence of suicidal ideation at antidepressant onset in a large naturalistic cohort of depressed outpatients
Scientific Reports (2019)