Abstract
The catechol-o-methyltransferase (COMT) genetic variations produce pleiotropic behavioral/neuroanatomical effects. Some of these effects may vary among sexes. However, the developmental trajectories of COMT-by-sex interactions are unclear. Here we found that extreme COMT reduction, in both humans (22q11.2 deletion syndrome COMT Met) and mice (COMT−/−), was associated to cortical thinning only after puberty and only in females. Molecular biomarkers, such as tyrosine hydroxylase, Akt and neuronal/cellular counting, confirmed that COMT-by-sex divergent effects started to appear at the cortical level during puberty. These biochemical differences were absent in infancy. Finally, developmental cognitive assessment in 22q11DS and COMT knockout mice established that COMT-by-sex-dichotomous effects in executive functions were already apparent in adolescence. These findings uncover that genetic variations severely reducing COMT result in detrimental cortical and cognitive development selectively in females after their sexual maturity. This highlights the importance of taking into account the combined effect of genetics, sex and developmental stage.
Similar content being viewed by others
Introduction
The catechol-o-methyltransferase (COMT) is a gene located in band q11.2 of chromosome 22,1 which encodes for the COMT enzyme, involved in the methylation of catechol molecules including dopamine, norepinephrine and catechol estrogens.2 In particular, COMT has a pivotal role in the degradation of dopamine in the prefrontal cortex (PFC).3, 4, 5 The Val/Met common and extensively studied single nucleotide polymorphism, hereafter referred to as ValMet, has been consistently reported to substantially change the stability and activity of COMT: the Met form leads to 40% lower protein levels and reduced enzymatic activity compared with the Val variant.5
Functional genetic variations in the COMT gene modulate multiple spheres of mammalian behavior with similar findings from adult humans and mice.6, 7, 8, 9, 10, 11, 12 However, if and how the effects of COMT genetic polymorphisms might change during the course of development is still unclear, with mixed and inconsistent findings. For example, two studies reported that MetMet homozygote children perform better than ValVal in PFC-dependent cognitive tests.13, 14 Conversely, other studies showed an advantage of ValVal or ValMet on PFC-dependent cognitive functions.15, 16 Moreover, including a cross-sectional and longitudinal study on a sample of healthy participants of 6–20 years old, the ValVal advantage observed in children was completely reverted in adolescence.15 Similarly, brain morphology investigations reported that 10-year-old ValVal children have reduced cortical thickness compared with MetMet subjects.17 This effect was completely reverted by the age of 20. The heterogeneity in these findings might indicate a different impact of COMT genetics throughout brain maturation, even if also other factors such as sample size, age distribution, experimental design and the effect of polymorphisms in other genes might have an influence. Indeed, COMT activity increases from childhood to adulthood;18 dopamine concentration/turnover in the PFC changes during lifetime, with a peak in adolescence;19, 20, 21, 22 the PFC morphology has a prolonged postnatal maturation.23, 24, 25, 26
Human and mouse studies have shown that behavior, brain morphology and vulnerability to psychiatric disorders modulated by COMT genetic variations are sex-dependent.9, 27, 28, 29, 30, 31 Moreover, estrogens inhibit COMT expression and enhance cortical dopamine, and COMT activity is lower in females than in males.5, 29, 32, 33 Only one study in healthy children from 8 to 10 years old suggested that the COMT ValMet polymorphism might impact cognitive function changes in relation to the pubertal status, but selectively in boys.13 Despite this, little is known about how COMT genetics might impact brain and behavioral maturational trajectories depending on the sex of the subjects.
Patients with 22q11.2 deletion syndrome (22q11DS) carry a hemizygous deletion of 1.5 (atypical form) or 3 (most frequent) megabases in the 22q11.2 locus, which include approximately 35 to 60 genes.34 22q11DS individuals have only one copy of the COMT gene and are supposedly exposed to abnormal cortical dopamine levels throughout brain maturation.10, 12, 35 Furthermore, 22q11DS Met carriers have even lower COMT levels.36 22q11DS is characterized by developmental cognitive impairments, high prevalence of attention deficit hyperactivity disorder (≈37%) from childhood and psychotic symptoms (≈30–40%) from adolescence/early adulthood.37, 38, 39, 40 In parallel, progressive brain morphological alterations are present, including greater gray matter loss in the parietal and occipital lobes with a relative preservation of the frontal lobe during childhood,41, 42, 43, 44, 45, 46, 47 and widespread alterations mainly involving temporal and frontal brain regions in adulthood.46, 47 Some studies suggested that the COMT ValMet variant might modulate brain and cognitive development,48, 49, 50, 51, 52 as well as risk for psychosis35, 53 in individuals with 22q11DS. However, a number of studies failed to detect any significant association of the ValMet variation with behavioral, neurocognitive, psychiatric and neural abnormalities.36, 39, 51, 54, 55 Thus, findings have been inconsistent and controversial.
The aim of this study was to clarify the developmental trajectories of COMT-by-sex interactions in the context of excessive changes of COMT activity. In particular, we conducted a translational study in patients affected by 22q11DS and COMT knockout mice. We first examined the influence of COMT ValMet polymorphism on cortical thickness in 22q11DS patients at different stages of sexual maturation, namely before and after puberty onset. Indeed, puberty has been indicated as a turning point in cortical development,56, 57, 58 in maturation of higher order cognitive functions59 and in the appearance of psychiatric disorders.60 Next, to dissect the effects of selective and drastic reductions in COMT, we took advantage of COMT knockout mice. These mutant mice allow to overcome the complexity of human polymorphisms, genetic and clinical heterogeneity and the potential confounding effects of other hemideleted genes in 22q11DS and other uncontrollable gene–gene and gene–environment interactions. We conducted a human-analogous magnetic resonance imaging (MRI) assessment comparing COMT null mutant −/− mice (an extreme situation approximating 22q11DS Met subjects) with their wild-type (+/+) and heterozygous (+/−) counterparts. Our results demonstrate that COMT genetics influence post-pubertal cortical brain anatomy in a sex-dependent manner in mice, consistent with what we found in 22q11DS. To strengthen our findings, we obtained molecular confirmation of altered developmental trajectories brought by COMT-by-sex interaction by measuring two key molecules implicated in dopamine pathways (TH and Akt) and neuronal/cellular density in the PFC of COMT male and female knockout mice at different developmental stages (that is, pre-pubertal, pubertal and post-pubertal). Finally, the repercussion of COMT genetics on mice and 22q11DS cognitive functions was investigated. At both neuroanatomical and behavioral levels, the interaction between COMT genetics and the sex of the subject appeared from puberty onwards.
Materials and methods
Human studies
22q11DS cohorts: 109 patients with 22q11DS were recruited and assessed (MRI, IQ, Stroop, CPT, digit span tests) in the context of the Geneva 22q11DS study,38 while other 83 patients with 22q11DS were recruited and assessed (Wisconsin Card Sorting task, IQ, Stroop, digit span tests) by the Bambino Gesù Hospital in Rome. The presence of the 22q11.2 microdeletion was confirmed by quantitative fluorescent polymerase chain reaction and hemizygosity for the COMT Met or the COMT Val allele was determined by PCR using a tetra-primer amplification refractory mutation system.61 In addition, sexual maturity was assessed using a self-questionnaire (Tanner maturational scale:62, 63 patients were classified at pre-pubertal stage (Tanner stage=1) or pubertal/post-pubertal stage (Tanner stage ⩾2)). For simplicity, we defined ‘post-pubertal’ all the subjects at a Tanner stage ⩾2. Eighteen subjects had both a data point in pre- and in post-puberty. Semi-structured interviews were used to assess the psychiatric diagnosis of the patients.64, 65, 66 See Supplementary Results for details on psychiatric diagnosis. Written informed consent was received from all the participants, and from their parents for participants younger than 18 years old, under protocols approved by the Institutional Review Board of Geneva University School of Medicine and Bambino Gesù Hospital.
Structural MRI in humans
Structural MRI scans were acquired in the Geneva cohort only using either a Philips 1.5 Tesla Intera scanner or a Siemens Trio 3 Tesla scanner at the Center of Biomedical Imaging in Geneva (Supplementary Table 1 for groups assessed with each machine). Sequence parameters for the Philips 1.5 Tesla scanner were: TR=35 ms, TE=6 ms, flip angle=45°, NEX=1, matrix=256 × 192, field of view=24 cm2, slice thickness=1.5 mm, 124 slices; parameters for the Trio 3 Tesla scanner were: TR=2500 ms, TE=3 ms, flip angle=8°, acquisition matrix=224 × 256, field of view=220 mm, slice thickness=1.1 mm, 192 slices. Excellent cross-scanner vertex-wise consistency in cortical thickness estimates was previously confirmed using 20 participants who underwent cerebral MRI acquisitions with the two scanners on the same day.67 Structural images were processed using the software FreeSurfer version 5.1 to reconstruct accurate cortical surface models. Fully automated pre-processing included resampling into cubic voxels, intensity normalization and skull stripping. Three-dimensional surface reconstruction was used to determine the border between white and gray matter (white surface) and between gray matter and cerebrospinal fluid (pial surface).68 Cortical thickness was measured in the native space of each individual as the shortest distance between the white and the pial surfaces.69 To compare cortical thickness values between groups, cortical surfaces of each subject were registered to the fsaverage template and smoothed using a fill-width at half-maximum kernel of 10 mm.
Statistical analyses in the human sample
Demographic and behavioral data in the human cohort of patients with 22q11DS were analyzed using general linear multivariate analyses of variance with ‘genotype’ (Val or Met) and ‘sex’ (male, female) as main factors and chi-square (χ2) tests. The softwares STATISTICA (StaSoft, 12) and SPSS (Version 22.0, IBM, Armonk, NY, USA) were used. Based on the available data, in the neuroimaging and CPT analyses only the Geneva cohort was included, while for the WCST only the Rome cohort. MRI data were analyzed with the Query, Design, Estimate, Contrast tool in FreeSurfer, including age, sex and scan type as covariates. This tool provides comparisons of cortical thickness for each vertex of the cortical surface. Therefore, we reported in the results section the higher values of F and the smaller values of P for the nonsignificant results and the opposite for the significant ones. Three different analysis were conducted using a general linear model. First, we tested on the entire group of patients (pre- and post-pubertal subjects) the presence of a main effect of genotype and the interaction between genotype–sex and genotype–puberty. We could not conduct a longitudinal analysis because only 18 subjects had a time point in both pre- and post-puberty. For these 18 subjects only the pre-pubertal time point was kept for this first analysis because there we had less subjects. Second, we split the subjects into a pre-pubertal and a post-pubertal group and tested the effect of the COMT genotype, using the genotype as factor, as well as the interaction between genotype and sex. The third analysis was conducted in the group of post-pubertal subjects only. In this analysis, males and females were considered separately and, as before, the effect of the COMT genotype was investigated using the genotype as factor. As we considered males and females separately, we did not include sex as covariate in this analysis. A Monte Carlo simulation with a P-value threshold at P<0.05 was used to correct for multiple comparisons.70
Mouse studies
Subjects
All procedures were approved by the Italian Ministry of Health (permit no. 230/2009-B) and local Animal Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the European Community Council Directives. COMT null mutant mice (COMT−/−), their heterozygous (COMT+/−), and wild-type (COMT+/+) littermates with a C57BL6J background were bred by heterozygous (+/−) mating and were identified by PCR analysis of tail samples. The mice were weaned at postnatal day (P) 28 and they were group-housed in a climate-controlled animal facility (22±2 °C) and maintained on a 12 h light/dark cycle, with free access to food and water in individually ventilated cages. The testing was conducted during the light phase. The experimenter was blind to the genotype during testing. In the current work, P2 to P14 was considered as infancy in mice, whereas adolescent mice were tested at 35±1 days, mice from 3 to 6 months were considered adults. The day of birth was defined as P0. Different cohorts of naive mice were used for each single experiment.
Structural magnetic resonance imaging in mice
High-resolution morpho-anatomical T2-weighted (T2W) structural MRI scans were acquired using a 7.0 Tesla scanner at the IIT center in Rovereto, Italy. We adopted sample preparation and MRI acquisition procedure that permits to obtain artifact-free high-resolution images devoid of physiological or motion artifacts as we recently described.31, 71 Extensive details are reported in Supplementary Methods.
Voxel-based morphometry
Intergroup differences in local gray matter volume were mapped with voxel-based morphometry (VBM)72 using ANTs.73 In recent work we showed that foci of increased gray matter as detected with VBM correspond to increased cortical thickness as measured postmortem in histopathological assessments.31 This correspondence supports the use of VBM as a surrogate for cortical thickness in lissencephalic species like the laboratory mouse (more details in Supplementary Methods). To provide an illustrative description of inter-group effect size between genotypes, mean gray matter volume was quantified in representative symmetric 7x7x7 voxel regions centered over foci exhibiting significant inter-group differences.74
Antibodies and western blot analyses
The anti-phosphoAkt (Thr308, #2965; Ser473, #9271) and anti-Akt (#2920) were purchased from Cell Signaling Technology (Beverly, MA, USA); while the anti-tyrosine hydroxylase (TH, sc-25269) was purchased from Santa Cruz Biotechnology (Heidelberg, Germany), and the anti-actin antibody (A2066) from Sigma Aldrich (Milan, Italy). The mice were killed by decapitation. The PFC was rapidly dissected on an ice-cold surface and frozen in dry ice before protein extraction. More details are reported in the Supplementary Methods.
Stereological developmental analyses
For stereological counts, the number of NeuN positive cells in the inner layers of the pregenual medial PFC (mPFC), consisting of cingulate, prelimbic and infralimbic regions (Supplementary Figure 1 and Supplementary Methods for more details), was evaluated using a stereological fractionator sampling design with the optical fractionator probe of the Stereoinvestigator software as previously described.75 The number of NeuN positive cells was counted by an experimenter blind to the experimental group. Sections collection and preparation followed standard protocols as reported in ref. 31.
Behavioral procedures
A detailed description is reported in the Supplementary Methods. Developmental milestones were assessed every 2 days from P2 to P14 according to Scattoni’s protocol.76 The locomotor activity and temporal order object recognition memory tasks were performed as previously described.77, 78
Statistical analysis in the mice sample
Results are expressed as mean±standard error of the mean (s.e.m.) throughout. Mice MRI, indices of pups’ growth and development, locomotor activity, object exploration, markers of cellular (Hoechst+), neuronal (NeuN+), and dopaminergic system (TH and Akt) were all examined using general linear multivariate analyses of variance with ‘genotype’ (COMT +/+, +/−, −/−) and ‘sex’ (male, female) as main factors. Temporal order object recognition discrimination index testing whether the time investigating the fewer recent object differed from chance level was assessed using one sample t-tests. Post hoc analyses for individual group comparisons were carried out with Newman–Keuls post hoc test with multiple comparisons corrections, when statistical significance emerged in the main effects or interactions. The accepted value for significance was P<0.05. The software STATISTICA (StaSoft, 12) was used.
Sample size
For animal studies, the target number of samples in each group was determined based on equivalent tests previously published. For both humans and animals experiments, no statistical methods were used to predetermine sample size.
Replication
Results from western blot experiments were replicated three times. Behavioral experiments and cell counting in mice were replicated four to five times.
Randomization
For behavioral and histological analysis, age- and sex-matched animals were randomly chosen from our colony. No explicit randomization algorithm was used.
Exclusion criteria
For behavioral studies, only one adolescent female COMT+/− mouse was excluded as it died before the day of temporal order object recognition experiment for unknown reasons. For human studies, no data points were excluded.
Results
In 22q11DS, female COMT Met carriers show reduced cortical thickness after puberty
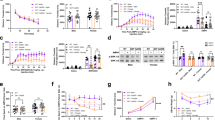
When considering the entire group of patients with 22q11DS, no significant effects of the COMT genotype (F1,89<23.79, P>0.05) or significant interactions between genotype–sex (F1,89<20.65, P>0.05) and genotype–puberty (F1,89<19.33, P>0.05) were observed. However, the relatively small sample size of the experimental groups, the different pharmacological status between pre- and post-pubertal subjects (Supplementary Tables 1 and 2), as well as the cross-sectional nature of our assessment limited the power of this analysis. Indeed, separate analyses conducted in the groups of pre- and post-pubertal patients revealed that cortical thickness did not differ between COMT Met and Val carriers before puberty (Supplementary Figures 2A and B; F1,37<16.04, P>0.05). Conversely, cortical thickness values were significantly reduced in Met patients compared to Val after the puberty onset in the left precentral cortex, spanning to the inferior and middle frontal cortices and, in the right hemisphere, in the superior frontal cortex (lateral and medial), precuneus and posterior fusiform gyrus (Figures 1a and b; F1,68>9.84, P<0.04).
22q11DS COMT Met carriers have reduced cortical thickness compared with Val carriers only after puberty and especially in females. (a) A significant reduction of cortical thickness in 22q11DS Met carriers was observed compared with Val carriers in post-puberty. There were no clusters of greater thickness in Met compared with Val carriers. (b) Mean cortical thickness values for the clusters 1 to 6 are reported in the plots. (1=left precentral/inferior middle frontal cortices, 2=left superior frontal cortex, 3=right superior frontal cortex, 4=right medial superior frontal cortex, 5=precuneus, 6=posterior fusiform gyrus). Post-pubertal: COMT Val N=34; COMT Met N=36. *P<0.05 and **P<0.01 versus 22q11DS Val carriers. (c) When considering only females post-pubertal patients, a significant reduction of cortical thickness in 22q11DS Met carriers compared with Val carriers was similarly observed. Cortical thickness did not significantly differ in male patients carrying the Met and Val COMT allele. (d) The plots represent mean cortical thickness values in female patients for each cluster. (1=left superior frontal cortex, 2=left inferior frontal/insula/superior temporal cortices, 3=left occipital cortex, 4=left supramarginal gyrus, 5= left medial superior frontal cortex, 6=right inferior frontal cortex, 7=right medial superior frontal/anterior cingulate cortices, 8=right precuneus, 9=right posterior fusiform gyrus/lateral occipital cortex).*P<0.05 and **P<0.01 versus 22q11DS Val carriers.
To explore the presence of sex-related differences in the cortical thickness reduction in Met post-pubertal subjects, we repeated the analysis in males and females. No differences were observed between Met and Val males (Supplementary Figures 2C and D; F1,30<18.05, P>0.05). In contrast, a significant difference was observed in females. In particular, female patients carrying the Met allele showed regions of reduced thickness bilaterally in the superior frontal cortex spanning medially to the right anterior cingulate gyrus, in the inferior frontal cortex comprising the left insula and left superior temporal cortex, in the lateral occipital cortex, in the left supramarginal gyrus and in the right posterior fusiform gyrus and precuneus (Figures 1c and d; F1,37>12.76, P<0.039). These results start to suggest that cortical thinning associated with reduced COMT activity in post-pubertal subjects might be prominently driven by females.
Demographic characteristics for the patients with 22q11DS revealed no significant differences in sex, age, handedness, psychiatric diagnosis and medications between COMT Met and Val subjects (all P>0.23; Supplementary Tables 1 and 2). However, in the group of post-pubertal subjects included in the Geneva cohort there was a significant effect of the genotype (P=0.046), with less Val subjects meeting the criteria for a psychiatric diagnosis compared with Met subjects.
Adult female COMT knockout mice show decreased cortical gray matter volume
To directly and more selectively examine the effects of genetic-driven COMT reduction, we next used COMT knockout mice (+/− and −/−) and their wild-type (+/+) littermates. Indeed, our human data might be biased by the unbalance between the numbers of pre- and post-pubertal patients as well as the presence of pharmacological treatments in post-pubertal but not in pre-pubertal patients.
Our previous MRI, VBM analysis of gray matter in mice showed that COMT genetic reduction resulted in increased fronto-cortical and postero-parieto-temporal gray matter in males, an effect that was tightly associated with local cortical thickness increases as measured with histopathological assessments.31 This supports the use of VBM as a surrogate for cortical thickness in lissencephalic species like the mouse, while in human imaging genetics thickness measurements are preferred.79
In the present work, we re-analyzed the same cohort of mice that underwent VBM analysis in our previous study31 to corroborate the role of COMT genetic reduction on brain aberrations observed in female patients carrying the low-activity variant. In agreement with the results from 22q11DS human patients (Figure 1), and conversely to male humans and mice,31 VBM gray matter mapping revealed bilateral foci of decreased cortical volume in female COMT−/− compared with COMT+/+ (P<0.05, threshold free cluster enhancement (TFCE) corrected; Figure 2a), and COMT+/− littermates (P<0.05, TFCE corrected; Figure 2b).
Decreased cortical gray matter volume is evident only in COMT−/− female mice. Structural magnetic resonance imaging (MRI) in COMT knockout female mice. (a and b) 3D volumetric reconstruction and representative coronal slices of the areas showing statistically significant decreased gray matter (GM) volume. (a) Comparisons between female COMT−/− versus +/+. (b) Comparisons between GM volume in female COMT+/− versus −/−. (c) GM effect size estimation in representative bilateral region of interest. Red asterisks indicate significant F-statistics across genotypes as measured with analysis of variance (ANOVA). Black asterisks indicate significant t-statistics between genotypes. *P<0.05 and **P<0.01. (FrA=frontal association cortex; M1=primary motor cortex; Cg=cingulate cortex; RS=retrosplenial cortex, Thal=thalamus, V1=primary visual cortex). COMT+/+ females N=6; COMT+/− females N=8; COMT−/− females N=5.
No differences were evident between COMT+/+ and +/− mice (P>0.06; Figure 2c), indicating that only extreme reduction of COMT can bring female mice to these consistent cortical thinning. Regions particularly affected were frontal associations, primary motor, visual cingulate and retrosplenial cortices, plus additional foci of gray matter decrease in the thalamus (P<0.05, TFCE corrected; Figure 2). No foci of significant gray matter volume increase were observed. When these data sets were analyzed on a regional basis using analysis of variance, we found significant F-statistics across genotypes in frontal association and primary visual cortex (Figure 2c).
The new results of cortical thinning in females with 22q11DS and COMT−/− mice are inverse to the cortical thickening we previously observed in post-pubertal healthy men Met carriers as well as in COMT knockout adult males.31 Notably, we provide initial biological validation that genetic modifications resulting in excessive reduced COMT activity lead to decreased cortical gray matter selectively in females.
Tyrosine hydroxylase levels revealed puberty as a turning point in the COMT-by-sex interactive effects
Increased dopaminergic tone might act as a neurotrophic factor influencing cortical development,80, 81 and COMT finely modulates dopamine levels in PFC in adulthood.5, 82 To examine how reduced COMT might differentially modulate the development of the cortical dopaminergic system in a sex-specific manner, we first analyzed in the PFC the protein levels of the rate limiting step in the dopamine synthesis: TH.83
In the pre-pubertal period (P15), when the sexual hormones levels are lower than post-puberty, we did not see any sex difference in the TH protein level (F1,26=0.14, P=0.7). During this time window, only a genotype effect was evident (F2,26=6.49, P<0.005). In particular, COMT−/− mice showed increased TH levels compared with wild-type mice (P<0.005; Figure 3a), independently from the sex of the subject.
Dopamine-related biochemical markers indicate puberty as the starting point of the appearance of COMT-by-sex developmental dichotomy. Densitometric analysis of tyrosine hydroxylase (TH) in the PFC of COMT +/+, +/− and −/− male and female mice at three different stage of development, (a) pre-puberty: P15, (e) puberty: P35 and (i) post-puberty: 5–6 months old. Actin was used as a loading control and did not differ between groups in each developmental stage. Expression levels were normalized to the male wild-type group. Pre-pubertal COMT+/+ males N=6, females, N=6; COMT+/− males N=8, females N=7; COMT−/− males N=3, females N=3. Pubertal COMT+/+ males N=8, females, N=8; COMT+/− males N=13, females N=12; COMT−/− males N=5, females N=9. Post-pubertal COMT+/+ males N=5, females, N=6; COMT+/− males N=5, females N=9; COMT−/− males N=6, females N=5. *P<0.05 versus COMT +/+ within the same sex, #P<0.05 versus male within the same genotype. Densitometric analysis of total Akt levels (b, f and j), phosphoAkt (pAkt, THr308) (c, g and k), and pAkt (Ser473) (d, h and l) in PFC of COMT +/+, +/− and −/− male and female mice at three different stage of development, (b–d) pre-puberty: P15, (f–h) puberty: P35, and (j–l) post-puberty: 5–6 months old. For the measurement of total protein levels, actin was used as loading controls; whereas, for the measurement of phospho-protein levels, total protein levels were used as loading controls. Expression levels were normalized to the male wild-type group. Pre-pubertal COMT+/+ males N=6, females, N=6; COMT+/− males N=6, females N=6; COMT−/− males N=4, females N=3. Pubertal COMT+/+ males N=7, females, N=5; COMT+/− males N=5, females N=6; COMT−/− males N=6, females N=7. Post-pubertal COMT+/+ males N=4, females, N=5; COMT+/− males N=3, females N=3; COMT−/− males N=4, females N=6. *P<0.05 versus COMT +/+ within the same sex, #P<0.05 versus male within the same genotype, $P<0.05 versus males. PFC, prefrontal cortex.
During puberty (P35), when the sexual maturity is reached,84 we observed a different pattern of TH protein levels. Indeed, a strong COMT-by-sex interaction was now evident (F2,70=7.92, P<0.005). Although COMT genetic reduction decreased the TH levels in males (P<0.05; Figure 3e), the opposite was true in females (P<0.05; Figure 3e). Thus, females kept having the same ‘pre-pubertal’ phenotype, whereas males showed a completely opposite phenotype. Furthermore, wild-type females presented reduced TH levels compared with the wild-type males (P=0.05; Figure 3e).
In post-pubertal mice (90<P<180), an effect of COMT-by-sex interaction was still present (F2,70=7.92, P=<0.001, Figure 3i). In particular, as in puberty, adult male COMT−/− showed lower TH levels compared with +/+ males (P<0.05). In contrast, adult females presented an equal amount of TH among the three genotypes (P=0.98). Representative western blots for all analyses are reported in Supplementary Figure 3. The results obtained in adult male mice are in line with previous findings reporting that higher COMT activity in males is associated with higher TH protein levels.7
Together, these findings indicate that COMT-by-sex divergent interactive effects in the cortical dopamine system start to appear during puberty.
Akt levels and activity confirmed puberty as a turning point in the COMT-by-sex interactive effects
To further explore the evidence that the COMT-by-sex interaction appear during puberty, we analyzed total and phosphorylated levels of Akt, an intracellular key regulatory protein related to dopamine/D2 effectors.85 Moreover, Akt regulates cell proliferation, growth, survival and metabolism, and it has been implicated in sex differences and psychiatric disorders.5, 86
Pre-pubertal female mice showed higher level of total Akt than males (F1,27=5.73, P<0.05; Figure 3b), independently from the COMT genotype (F1,27=0.03, P=0.97). In contrast, no effect of either sex or genotype was evident in the rate of phosphorylation at both the Thr308 (sex: F1,25=3.07, P=0.09; genotype: F2,25=0.33, P=0.71; Figure 3c) and Ser473 sites (sex F1,24=0.09, P=0.7, genotype: F2,24=0.49, P=0.6; Figure 3d).
In contrast, during puberty, the total Akt protein levels were unaffected by the sex (F1,35=0.08, P=0.7) and COMT genotype (F2,35=0.17, P=0.8) of the subjects (Figure 3f). However, a strong sex-by-COMT interaction was evident for the phosphorylation at the Thr308 site (F2,29=6.56, P<0.005; Figure 3g), but not at the Ser473 site (F2,25=0.16, P=0.8; 3H). In particular, although no difference was detected between males and females COMT+/+ mice (P=0.3; Figure 3g), COMT genetic reduction decreased pAkt levels exclusively in males (P<0.05; Figure 3g). Moreover, COMT+/− and −/− females had much higher levels than males within the same genotype (P<0.05; Figure 3g).
A similar sex-by-COMT interaction effect in the pAkt levels at the Thr308 site was evident also in post-pubertal mice (F2,18=3.8, P<0.05; Figure 3k). Representative western blots for all analyses are reported in Supplementary Figure 3.
Higher dopamine corresponds to lower level of Thr308pAkt.85 Thus, these data correlate well with the TH levels found in COMT developing mice, further strengthening the conclusion that COMT-by-sex interactive effects in the cortical dopamine system start to appear during adolescence.
Sex-dichotomous effects of COMT genetic mutations on PFC neuronal counting in mice arise at puberty
We previously found that COMT knockout adult males exhibited an increase in neuronal density in the mPFC layers V/VI, but not in layers II/III.31 Conversely, COMT knockout adult females exhibited a decreased neuronal density in the mPFC layers V/VI, but not in layers II/III.31 Here we asked whether COMT genetics might affect the absolute neuronal and cellular counting in the mPFC (Supplementary Figure 1) throughout the lifespan and if and when COMT sex-dependent effects start to appear. We adopted a stereological unbiased approach to estimate the total number of neurons in the mPFC in pre-pubertal, pubertal and post-pubertal mice.
In pre-pubertal mice (P15), analysis on neuronal estimate (NeuN positive cells) did not show any significant difference for sex (F1,15=1.56, P=0.23), COMT genotype (F2,15=0.22, P=0.8) or their interaction (F2,15=0.31, P=0.74; Figure 4a). Similarly, the total number of cells (Hoechst positive) was not affected by sex (F1,15=0.48, P=0.5), COMT genotype (F2,15=1.2, P=0.3), or their interactions (F2,15=0.87, P=0.4; Figure 4d).
Sexually divergent effects of COMT genetic disruption on neuronal and total cells counting in deep layers of the medial prefrontal cortex (mPFC) arise during adolescence. COMT genetic disruption does not affect neuronal counting (NeuN+) in infant mice (a), while a sex-by-genotype interaction is shown in adolescent (b) and adult (c) mice. COMT genetic deletion does not affect cellular counting (Hoechst+) in (d) infant mice and (f) adult mice, whereas it increases the total number of cells in (e) adolescent males. Pre-pubertal COMT+/+ males N=4, females, N=4; COMT+/− males N=3, females N=5; COMT−/− males N=4, females N=3. Pubertal COMT+/+ males N=5, females, N=4; COMT+/− males N=5, females N=4; COMT−/− males N=5, females N=4. Post-pubertal COMT+/+ males N=5, females, N=6; COMT+/− males N=3, females N=3; COMT−/− males N=5, females N=4. *P<0.05 versus COMT+/+ of the same sex.
In contrast, a COMT-by-sex interaction effect on the number of neurons emerged at puberty (F2,20=3.8, P=0.03; Figure 4b), while no effect of sex (F1,20=2.56, P=0.13) or genotype (F2,20=0.42, P=0.66) was evident. In particular, COMT−/− and +/− male mice (P<0.04; Figure 4b), but not females (P=0.5; Figure 4b), had more neurons than COMT+/+. Similarly, for the total number of cells, we detected a significant COMT-by-sex interaction (F2,20=3.6, P=0.004). In particular, COMT−/− males (P<0.05; Figure 4e), but not females (P=0.4; Figure 4e), had a higher number of cells compared with +/+ mice.
Finally, in post-pubertal mice, as expected, we found a significant COMT-by-sex interaction effect (F2,19=6.7, P=0.006). In particular, post hoc analysis revealed that COMT+/− and −/− males had a higher number of neurons compared with COMT+/+ in the mPFC (P<0.05; Figure 4c). Furthermore, for the total amount of cells, we observed a significant effect of genotype (F2,19=5.8, P=0.01), but not of sex (F1,19=2.0, P=0.2) or genotype-by-sex interaction (F2,19=2.1, P=0.1; Figure 4f).
Taken together, these results indicate that COMT- and COMT-by-sex interaction effects start to appear during adolescence, are absent before puberty and persist in adulthood.
Genetic-driven COMT reduction in mice did not alter developmental milestones and locomotor functions from birth to adulthood
Based on the consistent molecular and anatomical data showing that COMT-by-sex interacting effects start to appear during adolescence, we next checked whether similar pattern of effects might be evident in behavioral outputs.
Genetic reduction or absence of COMT in mice did not affect developmental trajectories of somatic growth indexes, general health, physical maturation and locomotor functions in either sexes from birth up to adulthood (Supplementary Results, Supplementary Figure 4 and Supplementary Table 3).
Taken together, these results indicate that COMT genetic reduction does not alter pups’ development nor interact with the sex of the subjects for gross behavioral maturation from early postnatal periods, across puberty and up to adulthood.
Sex-dichotomous COMT genetic effects in cognitive functions are apparent from adolescence in mice
COMT genetic variants impact cortical development in a sex-dichotomous way starting from adolescence (Figures 1). COMT-by-sex-dichotomous effects in PFC-dependent cognitive abilities are evident in adults.7, 31 Thus, we addressed when these COMT-by-sex interacting effects might appear during development. The T-maze task that we previously used in adult mice7, 31 is not suited to test developing mice in a specific age point as it requires long training, dietary restraint and stressful manipulation. We then took advantage of the temporal order object recognition task, a cognitive test for mice that requires a functional PFC, perhirinal cortex and hippocampus and that depends on cortical dopamine signaling.77, 87
Pre-pubertal mice (P15) did not show any discrimination ability in the temporal order object recognition task (data not shown),88 consistent with a still immature cortical development.89
Cognitive performance of pubertal and post-pubertal mice revealed a strong sex-by-genotype interacting effect (F2,128=6.0, P=0.003). Indeed, COMT+/− and −/− females had a worse cognitive performance compared with females COMT+/+, and males +/+ and −/− (P<0.05; Figures 5a and b). More specifically, in pubertal mice, all males groups (COMT+/+, +/− and −/−) showed a positive discrimination index as they explored the object presented least recently more than chance levels (P<0.05; Figure 5a). This indicated intact recency memory abilities in COMT knockout males. If anything, COMT−/− males showed a tendency towards an improved performance compared with +/+ males (P=0.07). In contrast, in females, only the wild-type group showed a positive discrimination index (P<0.05), whereas both COMT+/− and −/− were not able to distinguish between the two objects (P>0.42). Thus, COMT genetic reduction impaired this cognitive function only in pubertal females.
COMT sexually divergent effects on cognitive functions are evident from adolescence in both mice and human patients with 22q11DS. Discrimination index displayed by (a) adolescent and (b) adult COMT+/+, +/− and −/− males and females littermates mice during the 5 min temporal order object recognition test. Pubertal COMT+/+ males N=12, females, N=14; COMT+/− males N=17, females N=12; COMT−/− males N=6, females N=6. Post-pubertal COMT+/+ males N=16, females, N=11; COMT+/− males N=11, females N=18; COMT−/− males N=9, females N=8. *P<0.05 and **P<0.005 versus chance levels. (c) Number of errors made during the Wisconsin Card Sorting test (WCST) by subjects affected by 22q11DS divided by their sex (males or females), and COMT genotype (Val or Met). COMT Val males N=22, females, N=12; COMT Met males N=20, females N=12. *P<0.05 versus all other groups.
Similarly, in adult mice, all males groups (COMT+/+, +/− and −/−) showed a positive discrimination index (P<0.003; Figure 5b), indicating that mice were able to discern between the object presented least recently than the object presented most recently. Again, COMT−/− males tended to outperform +/+ male littermates (P=0.08). Conversely, in females, only the wild-type group showed a positive discrimination index (P<0.01), while both COMT+/− and −/− were not able to distinguish between the two objects (P>0.40; Figure 5b).
In both pubertal and adult mice, the total amount of time spent exploring objects in the different experimental phases showed no effects of genotype, sample phase or sample phase-by-genotype interaction (Supplementary Figure 5). Thus, COMT genetic reduction did not alter motivation, curiosity, motor, olfactory, tactile or visual functions that might affect object recognition. These findings confirm at the behavioral cognitive level that COMT-by-sex interactive effects are already apparent during adolescence.
Sex-dichotomous COMT genetic effects in executive functions are apparent from adolescence in patients with 22q11DS
To investigate whether the COMT sex-dichotomous effects predicted by our mouse studies in cognitive functions would be observable also in patients with 22q11DS, we analyzed their performance in the Wisconsin Card Sorting task. The Wisconsin Card Sorting task is a neuropsychological test commonly used to evaluate PFC-dependent executive control.90 A significant COMT ValMet-by-sex interaction effect was evident (F1,61=3.8, P=0.05). Post hoc analyses revealed that Met females made more errors compared with all the other groups (P<0.05; Figure 5c). This is similar to the cognitive findings from COMT genetically modified mice.
In agreement to previous studies in healthy subjects, patients with schizophrenia and genetically modified mice,7, 9, 31, 32, 91, 92, 93, 94 COMT-dependent effects were selective to executive functions. Indeed, no COMT or COMT-by-sex interaction effects were evident in general intelligence (IQ), cognitive inhibition (Stroop), attention (CPT) and short-term memory (digit span test; Supplementary Figure 6).
Discussion
In the present study, we demonstrated that COMT genetic variations produce cortical alterations in a sex-specific manner starting from puberty. This was similarly evident in patients with 22q11DS and in COMT knockout genetically modified mice. Notably, COMT-dependent anatomical changes in the cortex, and correlated cognitive functions, were paralleled by altered cortical developmental trajectories of molecular markers of the dopamine system. Again, these COMT-by-sex molecular rearrangements emerged during adolescence.
Studies in humans and genetically modified mice have shown COMT-by-sex interacting effects on cortical morphology31 and cognitive functions.9, 29, 31 However, the developmental period during which this interaction becomes evident remained unclear. Only few studies investigated the effect of COMT genetics on working memory and brain morphology during development,13, 15, 17 showing increased cognitive performance with age15 and faster rates of cortical thinning17 in MetMet subjects, as well as potential COMT-by-puberty interaction in Met homozygous males.13 In our translational study combining results from patients with 22q11DS and COMT genetically modified mice, we demonstrated that puberty is the developmental period when COMT-by-sex interactions start to appear, influencing cortical morphology as well as related cognitive functions. Indeed, we found several clusters of reduced gray matter volume in adult COMT−/− female mice, in cingulate, retrosplenial, dorso-frontal cortical areas, recapitulating anatomical features of some of the clusters of reduced cortical thickness in female 22q11DS Met carriers. Similarly, in both adolescent and adult mice, but not in pre-pubertal mice, we also detected sex-dimorphic effects of COMT in the PFC in terms of number of neurons and molecular markers involved in the postnatal maturation and function of the dopaminergic system. The same pattern of effects was evident in cortical-dependent cognitive tasks. Overall, these data show that excessive reduction of COMT, as can be found in Met 22q11DS patients and COMT−/− mice, was deleterious after puberty onset in females but not in males.
Previous studies addressing COMT-related differences in 22q11DS reported heterogeneous and fragmented findings. The Met allele has been associated with better cognitive performance and no cortical alterations in children.50, 95 In contrast, in adolescent and adult patients, the Met allele has been associated with poorer cognitive abilities,48, 51, 52 reduced cortical volume35, 96 and increased vulnerability for psychiatric symptoms.35, 96, 97 Also, COMT-by-sex interactions have previously been showed in 22q11DS.27, 28, 30 In particular, Kates et al.30 have shown that Met girls and Val boys with 22q11DS have increased volume in the dorsal PFC, but reduced volume in the orbital PFC. Coman et al.28 showed that female patients carrying the Val allele and male patients carrying the Met allele had increased activation in frontal brain regions during the processing of pleasant stimuli, whereas Met females and Val males had an increased activation in limbic regions during the processing of unpleasant stimuli. Furthermore, Boot et al.27 reported evidence suggesting an effect of sex-by-COMT interactions on dopaminergic markers. In the current study, the use of genetically modified mice was important in suggesting which effects were selectively dependent on the extreme COMT genetic disruption present in 22q11DS Met female subjects. In particular, avoiding the interacting effects of other genetic variations in and outside the 22q11 deleted region, overcoming the relatively small human samples size, the different pharmacological status between pre- and post-pubertal patients, and the cross-sectional nature of the measurements instead of longitudinal. Furthermore, taking into account the different developmental stages of mice and humans, our findings support the conclusion that the worsening of brain and cognitive alterations observed in post-pubertal patients with 22q11DS are caused, at least in part, by COMT genetic reduction in female subjects.
The molecular mechanisms underlying these developmental sex-dependent COMT effects might be complex. We report initial evidence from molecular indicators of the dopaminergic system suggesting that reduced COMT modulates in a very selective age- and sex-dependent way the development and maintenance of the cortical dopamine system. In infancy, the genetic reduction of COMT enhanced TH protein levels independently from the sex of the subjects. This suggests that low levels of COMT, reasonably via cortical dopamine increase, might support the postnatal development of the dopamine system by inducing upregulation of TH enzyme, required for dopamine biosynthesis, and Akt signaling, involved in neuronal survival. Also in the PFC of humans, it has been shown a peak of very high levels of TH during infancy and toddlerhood,98 highlighting the large synthetic demand for dopamine early in life when cortical pyramidal neurons are still maturing26 and inhibitory interneurons are still migrating and differentiating.99 Conversely, from adolescence to adulthood, we found a completely opposite COMT-dependent pattern of TH expression in males while females kept on showing the same phenotype as during infancy. This pattern of changes in TH and Akt in male mice from infancy to adolescence was similar to the switch we found from pre- to post-puberty in cortical thickness in male patients with 22q11DS. Future studies will be required to address the functional relevance of these abrupt changes and how they are related to sexual maturity. However, the negative feedback that might aim to balance the increase of synaptic dopamine and cortical thickness in COMT knockout males is consistent with previous findings reporting compensatory mechanisms in PFC TH levels following COMT-dependent dopamine modulation.7, 82 Instead, this compensatory effect in adolescent COMT knockout females might be prevented by the synaptic overdrive of dopamine due to pubertal increase of estrogen levels.100 The pAkt data further support this view. In particular, COMT-by-sex interactive effects in adolescence were selectively evident in the level of phosphorylation of Akt at the thr309 site, which is directly correlated with the activity of D2 postsynaptic receptors.85 This evidence suggests an implication of dopamine/D2 pathways. Of note, Akt pathways are altered in patients with schizophrenia and haloperidol, a D2 antagonist with antipsychotic properties increase the phosphorilation of Akt in wild-type mice.86 Overall, these molecular findings strength our morphological and behavioral evidence that COMT genetics effects appear during puberty. Puberty is a critical time in relationship to sexual hormones.101, 102 Thus, the mechanism underlying COMT-by-sex interaction might depend on estrogen and testosterone hormonal levels. Although we cannot ignore the possible implication of testosterone in the phenotypes of males, here we speculated on the possible involvement of estrogens in females phenotypes. Indeed, we recently showed that sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in women.92 Moreover, endogenous physiological fluctuation of estradiol in healthy women differentially modulated working memory performance based on the COMT ValMet genotype.32 Estradiol enhances dopamine activity, synthesis, release and turnover.100, 103 Indeed, baseline extracellular PFC dopamine levels, mesocortical dopaminergic cells and the release of dopamine after stimulation are all higher in women than in men.104, 105, 106 Furthermore, a recent paper107 demonstrated a role for progesterone on PFC dopamine maturation and behavioral cognitive performance. Thus, our findings suggest that in the context of extremely low COMT levels, the potential dopaminergic overdrive in post-pubertal/pre-menopausal females has detrimental effects in brain morphology and cognition.
In conclusion, we demonstrated that genetic COMT effects in brain structure, behavior and cortical expression of dopamine-related proteins are not static throughout development and are influenced by sexual maturation. In particular, we clarified that COMT genetic variations modulate the maturation of brain and behavior from infancy, to adolescence and adulthood in a distinct way in males and females. This information will be crucial to elucidate previous inconsistent findings on COMT-dependent effects. Moreover, it provides the background to implement more effective and personalized therapeutic treatments targeting the dopaminergic system based on the COMT genotype, the sex and the developmental stage of each person.
References
Grossman MH, Emanuel BS, Budarf ML . Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1→q11.2. Genomics 1992; 12: 822–825.
Axelrod J, Tomchick R . Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem 1958; 233: 702–705.
Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem 2010; 114: 1745–1755.
Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT . Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci 2007; 27: 10196–10209.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al. Functional analysis of genetic variation in catechol-o-methyltransferase (COMT): effects on mRNA, protein enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75: 807–821.
O'Tuathaigh CMP, Clarke G, Walsh J, Desbonnet L, Petit E, O'Leary C et al. Genetic vs. pharmacological inactivation of COMT influences cannabinoid-induced expression of schizophrenia-related phenotypes. Int J Neuropsychopharmacol 2012; 15: 1331–1342.
Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR et al. Genetic dissection of the role of catechol-o-methyltransferase in cognition and stress reactivity in mice. J Neurosci 2008; 28: 8709–8723.
Heinz A, Smolka MN . The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci 2006; 17: 359–367.
Papaleo F, Erickson L, Liu G, Chen J, Weinberger DR . Effects of sex and COMT genotype on environmentally modulated cognitive control in mice. Proc Natl Acad Sci USA 2012; 109: 20160–20165.
Scheggia D, Sannino S, Scattoni M, Papaleo F . COMT as a drug target for cognitive functions and dysfunctions. CNS Neurol Disord Drug Targets 2012; 11: 209–221.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98: 6917–6922.
Mier D, Kirsch P, Meyer-Lindenberg A . Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry 2010; 15: 918–927.
Barnett J, Heron J, Ring S, Golding J, Goldman D, Xu K et al. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am J Psychiatry 2007; 164: 142–149.
Diamond A, Briand L, Fossella J, Gehlbach L . Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry 2004; 161: 125–132.
Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J et al. Influence of the COMT genotype on working memory and brain activity changes during development. Biol Psychiatry 2011; 70: 222–229.
Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ et al. Variations in the catechol O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biol Psychiatry 2007; 61: 626–632.
Raznahan A, Greenstein D, Lee Y, Long R, Clasen L, Gochman P et al. Catechol-o-methyl transferase (COMT) val158met polymorphism and adolescent cortical development in patients with childhood-onset schizophrenia, their non-psychotic siblings, and healthy controls. Neuroimage 2011; 57: 1517–1523.
Tunbridge E, Weickert C, Kleinman J, Herman M, Chen J, Kolachana B et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex 2007; 17: 1206–1212.
Andersen S, Dumont N, Teicher M . Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH- DPAT. Naunyn Schmiedebergs Arch Pharmacol 1997; 356: 173–181.
Meyer BM, Huemer J, Rabl U, Boubela RN, Kalcher K, Berger A et al. Oppositional COMT Val158Met effects on resting state functional connectivity in adolescents and adults. Brain Struct Funct 2016; 221: 103–114.
Rosenberg D, Lewis D . Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol 1995; 358: 383–400.
Wahlstrom D, Collins P, White T, Luciana M . Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn 2010; 72: 146–159.
Padmanabhan A, Luna B . Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn 2014; 89: 27–38.
Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 2001; 13: 786–793.
Luna B, Sweeney JA . Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull 2001; 27: 443–455.
Lambe EK, Krimer LS, Goldman-Rakic PS . Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci 2000; 20: 8780–8787.
Boot E, Booij J, Abeling N, Meijer J, da Silva Alves F, Zinkstok J et al. Dopamine metabolism in adults with 22q11 deletion syndrome, with and without schizophrenia – relationship with COMT Val108/158 Met polymorphism, gender and symptomatology. J Psychopharmacol 2011; 25: 888–895.
Coman IL, Gnirke MH, Middleton FA, Antshel KM, Fremont W, Higgins AM et al. The effects of gender and catechol O-methyltransferase (COMT) Val108/158Met polymorphism on emotion regulation in velo-cardio-facial syndrome (22q11.2 deletion syndrome): an fMRI study. Neuroimage 2010; 53: 1043–1050.
Harrison PJ, Tunbridge EM . Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology 2007; 33: 3037–3045.
Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W et al. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome). Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 274–280.
Sannino S, Gozzi A, Cerasa A, Piras F, Scheggia D, Manago F et al. COMT genetic reduction produces sexually divergent effects on cortical anatomy and working memory in mice and humans. Cereb Cortex 2015; 25: 2529–2541.
Jacobs E, D’Esposito M . Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci 2011; 31: 5286–5293.
Xie T, Ho S-L, Ramsden D . Characterization and implications of estrogenic downregulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol 1999; 56: 31–38.
Karayiorgou M, Simon TJ, Gogos JA . 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci 2010; 11: 402–416.
Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci 2005; 8: 1500–1502.
Gothelf D, Law AJ, Frisch A, Chen J, Zarchi O, Michaelovsky E et al. Biological effects of COMT haplotypes and psychosis risk in 22q11.2 deletion syndrome. Biol Psychiatry 2014; 75: 406–413.
Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison to youth with developmental delay and medical comorbidities. Mol Psychiatry 2014; 19: 1205–1211.
Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree MBM et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry 2014; 171: 627–639.
Murphy KC, Jones LA, Owen MJ . High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch General Psychiatry 1999; 56: 940–945.
Schneider M, Van der Linden M, Glaser B, Rizzi E, Dahoun SP, Hinard C et al. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res 2012; 196: 277–284.
Kates W, Burnette C, Bessette B, Bradley SF, Leslie S, Ethylin WJ et al. Frontal and caudate alterations in velocardio- facial syndrome (deletion at chromosome 22q11.2). J Child Neurol 2004; 19: 337–342.
Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF et al. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. Neuroimage Clin 2013; 3: 405–415.
Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M et al. Regional cortical white matter reductions in velocardiofacial syndrome: a volumetric MRI analysis. Biol Psychiatry 2001; 49: 677–684.
Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J . Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage 2005; 25: 169–180.
Eliez S, Schmitt J, White C, AL R . Children and adolescents with velocardiofacial syndrome: a volumetric MRI study. Am J Psychiatry 2000; 157: 409–415.
Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR et al. Aberrant cortical morphometry in the 22q11.2 deletion syndrome. Biol Psychiatry 2015; 78: 135–143.
Gothelf D, Schaer M, Eliez S . Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev Disabil Res Rev 2008; 14: 59–68.
Takarae Y, Schmidt L, Tassone F, Simon TJ . Catechol-O-methyltransferase polymorphism modulates cognitive control in children with chromosome 22q11.2 deletion syndrome. Cogn Affect Behav Neurosci 2009; 9: 83–90.
Shashi V, Howard TD, Keshavan MS, Kaczorowski J, Berry MN, Schoch K et al. COMT and anxiety and cognition in children with chromosome 22q11.2 deletion syndrome. Psychiatry Res 2010; 178: 433–436.
Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry 2004; 161: 1700–1702.
Bassett AS, Caluseriu O, Weksberg R, Young DA, Chow EW . Catechol-O-methyl transferase and expression of schizophrenia in 73 adults with 22q11 deletion syndrome. Biol Psychiatry 2007; 61: 1135–1140.
Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D . COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry 2005; 58: 23–31.
Gothelf D, Penniman L, Gu E, Eliez S, Reiss AL . Developmental trajectories of brain structure in adolescents with 22q11.2 deletion syndrome: a longitudinal study. Schizophr Res 2007; 96: 72–81.
Campbell LE, Azuma R, Ambery F, Stevens A, Smith A, Morris RG et al. Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust N Z J Psychiatry 2010; 44: 364–371.
Glaser B, Debbane M, Hinard C, Morris MA, Dahoun SP, Antonarakis SE et al. No evidence for an effect of COMT Val158Met genotype on executive function in patients with 22q11 deletion syndrome. Am J Psychiatry 2006; 163: 537–539.
Koolschijn PCMP, Peper JS, Crone EA . The influence of sex steroids on structural brain maturation in adolescence. PLoS ONE 2014; 9: e83929.
Sisk CL, Zehr JL . Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 2005; 26: 163–174.
Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE 2012; 7: e33850.
Spear LP . The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Reviews 2000; 24: 417–463.
Paus T, Keshavan M, Giedd JN . Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008; 9: 947–957.
Ruiz-Sanz JI, Aurrekoetxea I, Ruiz del Agua A, Ruiz-Larrea MB . Detection of catechol-O-methyltransferase Val158Met polymorphism by a simple one-step tetra-primer amplification refractory mutation system-PCR. Mol Cell Probes 2007; 21: 202–207.
Marshall WA, Tanner JM . Variations in pattern of pubertal changes in girls. Arch Dis Childh 1969; 44: 291–303.
Marshall WA, Tanner JM . Variations in the pattern of pubertal changes in boys. Arch Dis Childh 1970; 45: 13–23.
Reich W . Diagnostic Interview for Children and Adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39: 59–66.
Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
First MB, Spitzer R, Miriam G, Williams JBW . Structured Clinical Interview for the DSMIV Axis I Disorders (SCID-I). American Psychiatric Press: Washington, DC, USA, 1996.
Mutlu AK, Schneider M, Debbane M, Badoud D, Eliez S, Schaer M . Sex differences in thickness, and folding developments throughout the cortex. Neuroimage 2013; 82: 200–207.
Dale AM, Fischl B, Sereno MI . Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–194.
Fischl B, Dale AM . Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97: 11050–11055.
Hagler DJ, Saygin AP, Sereno MI . Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 2006; 33: 1093–1103.
Dodero L, Damiano M, Galbusera A, Bifone A, Tsaftsaris SA, Scattoni ML et al. Neuroimaging evidence of major morpho-anatomical and functional abnormalities in the BTBR T+TF/J mouse model of autism. PLoS ONE 2013; 8: e76655.
Pagani M, Damiano M, Galbusera A, Tsaftaris SA, Gozzi A . Semi-automated registration-based anatomical labelling, voxel based morphometry and cortical thickness mapping of the mouse brain. J Neurosci Methods 2016; 267: 62–73.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC . A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011; 54: 2033–2044.
Cutuli D, Pagani M, Caporali P, Galbusera A, Laricchiuta D, Foti F et al. Effects of omega-3 fatty acid supplementation on cognitive functions and neural substrates: a voxel-based morphometry study in aged mice. Front Aging Neurosci 2016; 8: 38.
Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM et al. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci 2011; 31: 8381–8394.
Scattoni ML, Gandhy SU, Ricceri L, Crawley JN . Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE 2008; 3: e3067.
Barker GRI, Bird F, Alexander V, Warburton EC . Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 2007; 27: 2948–2957.
Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 2014; 39: 1102–1114.
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010; 53: 1135–1146.
Robinson TE, Kolb B . Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997; 17: 8491–8497.
Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang H-Y, Friedman E et al. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci 2000; 20: 4606–4614.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95: 9991–9996.
Daubner SC, Le T, Wang S . Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 2011; 508: 1–12.
Blakemore SJ, Burnett S, Dahl RE . The role of puberty in the developing adolescent brain. Hum Brain Mapp 2010; 31: 926–933.
Beaulieu J-M, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 2007; 27: 881–885.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA . Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 2004; 36: 131–137.
Barker GRI, Warburton EC . When is the hippocampus involved in recognition memory? J Neurosci 2011; 31: 10721–10731.
Managò F, Mereu M, Mastwal S, Mastrogiacomo R, Scheggia D, Emanuele M et al. Genetic Disruption of Arc/Arg3.1 in Mice Causes Alterations in Dopamine and Neurobehavioral Phenotypes Related to Schizophrenia. Cell Rep 2016; 16: 2116–2128.
Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R . Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 2012; 109 (Suppl 2): 17186–17193.
Eling P, Derckx K, Maes R . On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cogn 2008; 67: 247–253.
Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry 2005; 58: 901–907.
Papaleo F, Sannino S, Piras F, Spalletta G . Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. Eur Neuropsychopharmacol 2015; 25: 2349–2363.
Scheggia D, Bebensee A, Weinberger DR, Papaleo F . The ultimate intra-/extra-dimensional attentional set-shifting task for mice. Biol Psychiatry 2014; 75: 660–670.
White TP, Loth E, Rubia K, Krabbendam L, Whelan R, Banaschewski T et al. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology 2014; 39: 2560–2569.
Shashi V, Veerapandiyan A, Keshavan MS, Zapadka M, Schoch K, Kwapil TR et al. Altered development of the dorsolateral prefrontal cortex in chromosome 22q11.2 deletion syndrome: an in vivo proton spectroscopy study. Biol Psychiatry 2012; 72: 684–691.
Gothelf D, Hoeft F, Ueno T, Sugiura L, Lee AD, Thompson P et al. Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psychiatr Res 2011; 45: 322–331.
Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Stone EV et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry 2007; 164: 663–669.
Rothmond DA, Weickert CS, Webster MJ . Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neurosci 2012; 13: 18.
Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS . Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry 2010; 167: 1479–1488.
Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V . Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem 1995; 65: 1651–1657.
Harris GC, Levine JE . Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology 2003; 144: 163–171.
Sisk CL, Richardson HN, Chappell PE, Levine JE . In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 2001; 142: 2929–2936.
Xiao L, Becker JB . Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 1994; 180: 155–158.
Becker JB . Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 1999; 64: 803–812.
Kritzer MF, Creutz LM . Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci 2008; 28: 9525–9535.
Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ . A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology 2011; 61: 544–549.
Willing J, Wagner CK . Progesterone receptor expression in the developing mesocortical dopamine pathway: importance for complex cognitive behavior in adulthood. Neuroendocrinology 2016; 103: 207–222.
Acknowledgements
We thank Dr M Morini and R Navone for technical assistance. We thank Dr ML Scattoni for teaching SS the measurement of developmental milestones in mice and Dr S Pontis for teaching stereological methods. We thank E Ranza and M Gagnebin for the genetic analysis in the group of patients with 22q11DS. This work was supported by FP funding from the Istituto Italiano di Tecnologia, the Marie Curie Reintegration-Grant (FP7-268247), by the Italian Ministry of Health Grants for Young Researchers (GR-2010-2315883), by the 2015 NARSAD Independent Investigator Grant (23234), and by the Compagnia di San Paolo (grant n.2015-0321). The 22q11DS Geneva Cohort is supported by grants by the Swiss National Foundation (SNF) to SE (324730_121996 and 324730_144260) and by the National Center of Competence in Research (NCCR) Synapsy-The Synaptic Bases of Mental Diseases (SNF), grant number 51NF40-158776. Individual grants have also been attributed to MSchaer (#145760 and 158831) and ES (#145250). The funders had no role in the study design, data collection and analysis, decision to publish or in the preparation of the manuscript.
Author contributions
Conceptualization was done by SS, MCP, SE and FP; SS, MCP, FM, MSchneider, MSchaer, ES, FS-B, MM, GC, CC, MP, AG and FP were involved in the methodology; investigation was done by SS, MCP, FM, MSchneider, MA, MP, AG and FP; SV, SE and FP provided the resource; SS, MCP, MSchneider, MSchaer, SE and FP were involved in writing the manuscript; SS, MCP, FM, MSchaer, ES, AG and FP were involved in the visualization and analysis; supervision was done by SE and FP; funding acquisition was done by MA, SE and FP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sannino, S., Padula, M., Managò, F. et al. Adolescence is the starting point of sex-dichotomous COMT genetic effects. Transl Psychiatry 7, e1141 (2017). https://doi.org/10.1038/tp.2017.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2017.109
This article is cited by
-
Striatal dopaminergic alterations in individuals with copy number variants at the 22q11.2 genetic locus and their implications for psychosis risk: a [18F]-DOPA PET study
Molecular Psychiatry (2023)
-
Examining how and why polygenic dopamine composite levels moderate adolescents’ vulnerability to peer victimization
Child and Adolescent Psychiatry and Mental Health (2022)
-
Catechol-O-Methyltransferase Effects on Smoking: a Review and Proof of Concept of Sex-Sensitive Effects
Current Behavioral Neuroscience Reports (2022)
-
Novel, non-nitrocatechol catechol-O-methyltransferase inhibitors modulate dopamine neurotransmission in the frontal cortex and improve cognitive flexibility
Psychopharmacology (2020)
-
Remote memories are enhanced by COMT activity through dysregulation of the endocannabinoid system in the prefrontal cortex
Molecular Psychiatry (2018)