Abstract
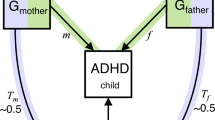
Little is known about the causes of individual differences in reward sensitivity. We investigated gene–environment interactions (GxE) on behavioral and neural measures of reward sensitivity, in light of the differential susceptibility theory. This theory states that individuals carrying plasticity gene variants will be more disadvantaged in negative, but more advantaged in positive environments. Reward responses were assessed during a monetary incentive delay task in 178 participants with and 265 without attention-deficit/hyperactivity disorder (ADHD), from N=261 families. We examined interactions between variants in candidate plasticity genes (DAT1, 5-HTT and DRD4) and social environments (maternal expressed emotion and peer affiliation). HTTLPR short allele carriers showed the least reward speeding when exposed to high positive peer affiliation, but the most when faced with low positive peer affiliation or low maternal warmth. DAT1 10-repeat homozygotes displayed similar GxE patterns toward maternal warmth on general task performance. At the neural level, DRD4 7-repeat carriers showed the least striatal activation during reward anticipation when exposed to high maternal warmth, but the most when exposed to low warmth. Findings were independent of ADHD severity. Our results partially confirm the differential susceptibility theory and indicate the importance of positive social environments in reward sensitivity and general task performance for persons with specific genotypes.
Similar content being viewed by others
Introduction
Reward sensitivity is an evolutionary important construct; rewards bring about positive feelings, and thereby reinforce the behavior associated with them, enabling learning.1 However, under certain circumstances, high reward sensitivity can lead to maladaptive behavior such as increased risk taking in daily life (for example, reckless driving and unprotected sex), substance use disorder and behavioral addictions such as gambling. During adolescence, reward sensitivity is heightened and this may have a key role in the emergence of maladaptive behavior, especially in high-risk groups such as adolescents with attention-deficit/hyperactivity disorder (ADHD).2
In the literature reward sensitivity is used as a broad construct referring to the degree in which a person reacts to the mere presence or manipulation of rewards. Indeed, different methods have been used to capture reward-related behavior. For example, by measuring the preference of smaller-sooner rewards over delayed-larger rewards in studies of temporal/delay-discounting,3 studying the willingness to perform risky actions in order to obtain rewards,4 or by comparing reaction times on rewarded and non-rewarded trials.5 Studies focusing on the neural correlates of reward sensitivity/processing have identified various brain regions, in particular the orbitofrontal cortex and striatum, which are activated when receiving or anticipating rewards.6, 7, 8, 9, 10 Current evidence suggests that a heightened-responsive neural reward system predisposes to greater reward seeking, whereby increased dopaminergic release in response to rewarding events strengthens reward-related behavior through dopamine-based learning processes.11, 12, 13
Reward sensitivity is subject to genetic influences. Studies of delay-discounting have found heritability estimates of 30–51%.14, 15 In addition, effects of genes linked to neurotransmitters involved in reward sensitivity, including dopamine, have been reported on several reward-related measures.16, 17, 18 Social environmental experiences such as interactions with parents and peers have been associated with behavioral and neural sensitivity to rewards as well. For instance, compared with adolescents with authoritarian parents who make decisions for them, adolescents involved in mutual decision-making with their parents improved on affective decision-making during the Iowa Gambling Task 1 year later.19 Similarly, functional magnetic resonance imaging (MRI) studies have revealed associations between low parental warmth and increased responses of prefrontal cortex and striatum to reward anticipation.20, 21 In contrast, maternal interpersonal affiliation has been related to increased striatal responses to reward receipt,22 and decreased responding of the prefrontal cortex and globus pallidus during reward anticipation has been associated with peer victimization20 and childhood adversity.23 Although these studies are heterogeneous with regard to how reward sensitivity was operationalized and the environmental variables that were studied, and findings are not consistent in terms of anatomical location and direction of neural activation, they indicate the importance of the social environment in determining the sensitivity to rewards. This is in line with the idea that the social environment has a key role in the development of reward learning. Across development, children’s behavior is initially shaped by external rewards such as positive social interactions with parents at first and later with peers as well. Gradually, through these interactions children learn to regulate their own behavior, a process that lasts well into the mid-20s.4, 24 Normal development of these skills then leads to the ability to perform well (on tasks) independently, with no direct need of external rewards.4
The interaction between genetic and environmental factors (GxE) also regulates behavioral reward sensitivity, as demonstrated by two earlier studies.25, 26 The first reported that parental warmth and stressful life events interacted with a catechol O-methyltransferase gene (COMT) polymorphism to influence affective decision-making. COMT Met allele carriers displayed higher reward sensitivity if they experienced more stressful events, whereas carriers of the Val/Val genotype showed better task performance if they experienced more parental warmth.25 The second study observed that adults carrying the dopamine receptor D4 (DRD4) 7-repeat allele of the variable number of tandem repeat polymorphism in exon 3 preferred immediate smaller over delayed larger rewards sooner when raised in low socioeconomic status families, but far less when not.26 The fact that in both studies genetic variants moderated the sensitivity toward positive and negative environmental influences is in line with the differential susceptibility theory.27 This theory states that individuals differ in their susceptibility to environmental experiences (partially) due to genes called ‘plasticity genes’, for better and for worse. Thus, individuals carrying specific variants in such genes will not only be most disadvantaged in negative environments but also benefit most from positive environments.28 This view extends the commonly applied diathesis-stress or dual-risk models, which focus only on the vulnerability to adverse effects of negative environments, referring to genes involved in GxE as ‘vulnerability genes’.29, 30 Support for the differential susceptibility theory comes from various studies in other contexts, although there have also been negative findings see for a recent review Belsky et al.31
The current study applied the differential susceptibility theory framework to improve our understanding of interindividual differences in reward sensitivity. We aimed to advance prior studies by examining GxE effects on both reward-related behavior and neural activation in children, adolescents and young adults with and without an ADHD diagnosis. As has become clear from the above, reward sensitivity is used in the literature to refer to heterogeneous behavioral measures and neural processes. Here we studied reward sensitivity in the context of behavioral and neural responses to obtain monetary rewards in a modified version of the monetary incentive delay (MID) task.32 Functional MRI was used to investigate responses in the ventral striatum and orbitofrontal cortex during reward anticipation and receipt. Using the MID task, our group has shown increased behavioral reward sensitivity and increased activations in the anterior cingulate and anterior frontal cortex during reward anticipation, and in the orbitofrontal cortex and nucleus accumbens during reward receipt in adolescents with ADHD compared with controls.33 This is in line with previous studies demonstrating increased behavioral reward sensitivity34 and reward-related activations in ADHD,35, 36, 37, 38 and studies associating increased striatal activation with impulsivity in healthy subjects, a related concept.39, 40 However, findings are not yet consistent as studies in adolescents and (young) adults with ADHD have also reported less striatal activation during reward anticipation compared with controls.35, 40 This inconsistency may related to how reward sensitivity was operationalized, as well as to general methodological differences such as small to moderate sample sizes. Nevertheless, our main focus was not on studying differences in reward processing between participants with and without ADHD (as this has been done elsewhere32), but rather on investigating GxE effects in an ADHD-enriched sample.
Given its relevance in child development and previous associations with reward sensitivity19, 21, 22, 23 we focused on the social environment, which was studied through maternal expressed emotions (EEs) and peer affiliation. Although it has been suggested that the effects of peers are stronger than parental influences in adolescence (see, for example, Harris et al.41), previous studies have mainly investigated associations between reward sensitivity and parental measures.19, 21, 22, 25 As for the candidate genes, we included those variants that have been shown to act as plasticity gene variants in previous studies:28 the short allele of the serotonin transporter (SLC6A4/5-HTT) HTTLPR polymorphism, the 7-repeat allele of the DRD4 exon 3 variable number of tandem repeat and the 9-repeat allele of the variable number of tandem repeat in the 3′-untranslated region (3′-UTR) of the dopamine transporter gene (SLC6A3/ DAT1). These genes have been frequently linked to ADHD42 and shown to act as plasticity genes in children with and without ADHD.43
On the basis of the findings that increased reward sensitivity is related to ADHD and impulsivity,33, 34, 39, 40, 44 and adverse environments,19, 20, 21, 25, 26 we hypothesized that—if differential susceptibility is applicable—participants with a plasticity variant would show increased reward sensitivity when faced with negative EE or peer affiliation and less reward sensitivity when exposed to positive EE or peer affiliation.
Materials and methods
Participants
Participants were selected from a follow-up (2009–2012) of the Dutch part of the International Multicenter ADHD Genetics (IMAGE) study, performed between 2003 and 2006.45 At first enrollment in IMAGE, inclusion criteria for children were an age between 5 and 18 years, European Caucasian descent, intelligence quotient ⩾70 and no diagnosis of autism, epilepsy, general learning difficulties, brain disorders or known genetic disorders (such as Fragile X syndrome or Down syndrome). All families were reinvited for a follow-up assessment in NeuroIMAGE at the VU University Amsterdam or Donders Centre for Cognitive Neuroimaging Nijmegen with a mean follow-up period of 5.9 years (s.d.=0.74). A comprehensive assessment protocol was administered, encompassing behavioral questionnaires, a diagnostic interview (for example, of ADHD, oppositional defiance disorder and conduct disorder), several neurocognitive measures from all family members and an extensive MRI scanning protocol in participating children. Participants were asked to withhold the use of psychoactive drugs for 48 h before measurement. To determine ADHD diagnoses at the follow-up measurement, a standardized algorithm was applied to a combination of questionnaires and a semi-structured diagnostic interview (an in-depth description is provided elsewhere46). The study was approved by the local ethics committees (Centrale Commissie Mensgebonden Onderzoek), and informed consent was signed by all participants (parents provided consent for participants under 12 years of age).
In the current analyses, participants were included when the reward task was administered and information was available on EE or peer affiliation; N=178 participants with ADHD, N=44 with subthreshold ADHD (that is, elevated symptoms of ADHD without meeting the full criteria for an ADHD diagnosis) and N=221 without ADHD, from N=261 families. A flowchart of participant inclusion can be found in Supplementary Figure S1. Sample size depended in particular on the availability of EE and peer affiliation (N⩽193 vs N⩽429) as EE could only be assessed when the diagnostic interview was administered. This led to an unequal distribution of participants with or without an ADHD diagnosis in the EE vs Peer affiliation selection. Therefore, participant characteristics in Table 1 are displayed separately for EE and peer affiliation.
Measures
Parental expressed emotion
EE was assessed during the semi-structured diagnostic interview, using codings derived from the Camberwell Family Interview.50 Only ratings of mothers were used in our study, as the data of fathers were far less complete. Warmth was assessed by the tone of voice, spontaneity, sympathy and/or empathy toward the child (range 0–3). Criticism was assessed by statements, which criticized or found fault with the child based on tone of voice and critical phrases (range 0–4).51, 52 Previous studies using similar codings for warmth and criticism have revealed an adequate inter-rater reliability (range 0.71–1.00).53, 54
Peer affiliation
Peer affiliation refers to the type of friends or peer characteristics a child or adolescent spends time with and was measured with the Friends Inventory.55 Participants assessed their peers’ behavior on 18 items rated on a 4-point Likert scale (for example, ‘my friends get good grades’, ‘my friends break the rules’; range 1=‘none of my friends are like that’ to 4=‘all of my friends are like that’). Scores were summed to yield either a positive or deviant peer affiliation score (each nine items). Both have demonstrated good internal consistency reliability range (0.88–0.92),56, 57 and moderate inter-rater reliability has been reported between self-reports, teacher reports (α=0.71 and r=0.34–0.43)55, 58 and parental reports (r=0.38).56 Several studies have used peer affiliation as a proxy of the social environment, see for example, Gifford-Smith et al.,59 Vitaro et al.60 and Fabes et al.61
ADHD severity
The Dutch Conners Parent Rating scale (CPRS-R:L) was used to assess ADHD severity (that is, the raw scores of scale N: DSM-IV: total).62 We used the CPRS-R:L as it was assessed in all participants (regardless of diagnostic status). Moreover, using a continuous measure of ADHD severity allowed us to retain as much information as possible, including the variation of scores among unaffected participants.
Reward paradigm
A modified version of the MID task32, 33 was used. Participants were instructed to react as quickly as possible to a target (a circle) by pressing a button. A colored square was presented before the target indicating whether a reward could be won or not (green=reward, red=no reward). In the reward condition participants were rewarded with 20 cents if they responded within the presentation time of the target. Trials ended with the presentation of feedback indicating whether the reward was earned or not plus the total amount gained (Supplementary Figure S2). With 25 trials per condition, monetary rewards could add up to a theoretical total of 5 Euros, to be paid at the end of the experiment. However, target presentation time was adapted to the participants’ performance (shortened by 20 ms after hits and prolonged 10 ms after misses), resulting in a hit rate of ~33%. This adaptation was done for the reward and non-reward conditions separately to balance the amount of hits on both trial types. As a consequence, hit rate became non-informative as a behavioral measure. The task instruction was followed by a practice trial after which the task began. In order to maximize the design efficiency, the 50 experimental (25 rewarded and 25 non-rewarded) trials were presented in randomized sequence and interleaved with 25 trials without events resulting in a 12-min long experiment.
Behavioral outcome measures of reward sensitivity were reward speeding (mean reaction time (MRT) non-reward-MRT reward) and reward variability (s.d./MRT non-reward-s.d./MRT reward). Neural activation was assessed using the blood-oxygen-level-dependent (BOLD) response during performance on the MID task. After preprocessing of MRI data (details on the image acquisition and preprocessing can be found in the SI) we calculated first-level contrasts for reward anticipation (contrast of the parameter estimates of rewarded cue vs non-rewarded cue; mean number of trials: M=22.35, s.d.=2.57) and reward receipt (contrast of parameter estimates of rewarded vs non-rewarded accuracy (hit events vs miss events); mean number of trials: Mhits=7.33, s.d.=1.40; Mmisses=14.53, s.d.=2.32). For these two contrasts we extracted the mean BOLD response from two a priori defined regions of interest: the ventral striatum (VS) and the ventral medial prefrontal cortex. Both regions of interest are considered core regions of the reward system8 and related to ADHD (as described in the introduction). The VS was defined anatomically by segmenting each subject’s anatomical MRI scan (FSL FIRST v1.2;63 regions labels: 28/56). Because cortical regions cannot be defined anatomically as precise as subcortical regions, the ventral medial prefrontal cortex was defined on the basis of Montreal Neurological Institute (MNI) coordinates derived from a meta-analysis8 (0, 52 and −8), with a 10-mm sphere around the coordinates (as in Furukawa et al.35).
Genotyping
A description of the genotyping procedure can be found in the Supplementary Information.64
Data analyses
Gene–environment correlations
The presence of gene–environment correlations (rGE) could bias potential GxE by providing an alternative explanation for the relationship between environmental measures and genes.43, 65 Therefore, Pearson and Spearman correlation analyses (two-sided) were performed to test for rGE between maternal or adolescent candidate plasticity genes and the environmental predictors.
Main analyses
Linear mixed model analyses investigated the effects of EE, peer affiliation, genotype and GxE on each reward outcome measure (described above). In addition, we were interested in whether differential susceptibility would be present in general task performance, therefore we focused on the reaction times and variability during reward and non-reward as well. Models were run with and without the interaction term. For both EE and peer affiliation, the positive and negative scales were not sufficiently correlated to create one variable (r=−0.50 and −0.16, respectively), therefore maternal warmth, criticism, and positive and deviant peer affiliation were analyzed separately. Likewise, separate models were run for each potential plasticity gene (DAT1, 5-HTT and DRD4). Consequently, there were 4 environmental predictors, 3 genes and 10 outcome measures, resulting 3 × 4 × 10 tests.
To correct for familial dependency, as a number of participants belonged to the same families, we estimated a random intercept for family in each model. A random intercept accounts for familial dependency by estimating the correlations between cases within families. Age, gender and collection site were included as potential confounders. All continuous predictors were centered around the mean, and the outcome measures were transformed into normal scores with the use of rank scores via Van der Waerden’s formula.66
Multiple testing correction
A multiple comparisons correction was used, which adjusts for correlated tests based on the effective number (Meff) of independent comparisons.67 The Meff was derived from the Eigenvalues of a correlation matrix between the outcome measures adjusted for covariates (age, gender and collection site). In the case of zero correlations between the outcome measures, the Meff-adjusted P-value would be equivalent to a Bonferroni correction. Thus, the Meff procedure is particularly suited for correlated comparisons (such as reaction times during reward and non-reward, or neural activation in regions of interest during reward anticipation) and corrects for multiple testing balancing between being overly lenient or conservative. The Meff was calculated separately for the behavioral and neural data because of the different nature of the two the types of measures. The effective number of comparisons for both was determined to be 4, and the adjusted P-value threshold P=0.05/4=0.013.
Sensitivity analyses
Sensitivity analyses were performed when significant GxE effects were found (that survived the multiple correction threshold). First, regions of significance (RoS) and simple slope tests were performed with an online application designed for probing interactions in differential susceptibility research (http://www.yourpersonality.net/interaction/, see Roisman et al.68). Second, to investigate the role of ADHD severity, analyses were rerun including main and interaction effects of ADHD severity. Furthermore, separate sensitivity analyses checked whether significant effects were present in participants while controlling for nonlinear effects of age (age2), medication history, intelligence quotient and comorbid oppositional defiance disorder or conduct disorder diagnosis.
Code availability
All analyses (except for RoS and simple slope tests) were performed with the Statistical Package for the Social Sciences, version 20.0 (IBM, Armonk, NY, USA). All computer codes used to compute the results are available on www.neuroimage.nl.
Results
A significant rGE was found between adolescent DRD4 genotype and deviant peer affiliation (r=0.11, P=0.028; Supplementary Table S1). Furthermore, maternal DAT1 was negatively correlated with maternal warmth (r=−0.18, P=0.015), and maternal 5-HTT associated with deviant peer affiliation and maternal warmth (r=0.10, P=0.045; r=−0.20, P=0.005, respectively). Significant rGEs, however, were relatively small, and are unlikely to have biased possible GxE interactions. In describing the outcomes of the mixed model analyses we restricted ourselves to the results that survived correction for multiple testing. Nominally significant effects can be found in Supplementary Table S2–S5.
Reward speeding
Our linear mixed model showed that both maternal warmth and criticism were significantly associated with adolescent reward speeding (Bwarmth=−0.19, P=0.013; Bcriticism=0.20, P=0.008; Supplementary Table S2). For maternal warmth this effect was moderated by 5-HTT genotype (B=−0.45, P=0.005; Supplementary Table S3). A similar GxE interaction was found between 5-HTT and positive peer affiliation (B=−0.07, P=0.012). As can be seen in Figures 1a and b, participants with the HTTLPR short allele showed a significant negative association between reward speeding and maternal warmth and positive peer affiliation. Simple slope analyses revealed both slopes were significant (ppositive=0.049, pwarmth<0.001), whereas slopes for participants with the HTTLPR L/L genotype were not (ppositive=0.994 and pwarmth=0.558). However, inspection of the RoS with respect to the environmental predictors revealed the difference between the two genotypes was not significant for high warmth (that is, no values fell above the upper RoS threshold X=1.64). Thus, HTTLPR short-allele carriers showed the most reward speeding when exposed to low maternal warmth (RoS threshold X=−0.34) or low positive peer affiliation (RoS threshold X=−5.61), but the least when exposed to high positive peer affiliation (RoS threshold X=3.71) when compared with adolescents with the HTTLPR L/L genotype.
(a) Interaction between 5-HTT and maternal warmth on reward speeding (B=−0.45, P=0.005; normal score (0)=27.71 ms). The shaded areas indicate the regions of significance (RoS), lower threshold X=−0.34; upper threshold X= 1.64. (b) Interaction between 5-HTT and positive peer affiliation on reward speeding (B= −0.07, P=0.012; normal score (0)=25.52 ms). The shaded areas indicate the RoS, lower threshold X=−5.61; upper threshold X= 3.71. (c) Interaction between DAT1 and maternal warmth on the mean reaction time during non-reward (B=0.40, P=0.012; normal score (0)=324.90 ms). The shaded areas indicate the RoS, lower threshold X=−0.80; upper threshold X=1.40. (d) Interaction between DAT1 and maternal warmth on the mean reaction time during reward (B=0.41, P=0.013; normal score (0)=296.31 ms). The shaded areas indicate the RoS, lower threshold X=−1.51; upper threshold X=0.82. Values in the RoS are significant. MRT, mean reaction time.
Subsequent analyses of the reaction times in each condition separately (reward vs non-reward) revealed a significant interaction between DAT1 and maternal warmth. Opposite to our predictions, the MRT was negatively associated with maternal warmth in participants with the DAT1 10/10 genotype, regardless of reward condition, see Figures 1c and d (Breward=0.41, P=0.013; Bnon-reward=0.40, P=0.012). Simple slope analyses revealed the slopes were significant for participants with the DAT1 10/10 genotype (preward=0.044, pnon-reward=0.001), but not for 9-repeat carriers (preward=0.103, pnon-reward=0.567). Hence, DAT1 10/10 homozygotes had the longest reaction times when exposed to low warmth, but the shortest when exposed to high warmth, compared with DAT1 9-repeat carriers. However, no values of the non-reward reaction times fell within the upper RoS threshold for maternal warmth (X=1.40). Therefore, here DAT1 10-repeat homozygotes only differed significantly from each other when exposed to low maternal warmth (RoS threshold X=−0.80). For reward reaction times, values fell within both the lower (X=−1.51) and upper RoS threshold (X=0.82).
Reward variability
Analyses of reward variability showed no effects that survived correction for multiple testing (all P-values >0.018; Supplementary Table S2). Looking at the conditions separately, no effects were present in the reward or non-rewarded condition either (all P-values >0.030).
Neural activation
A significant interaction between DRD4 and maternal warmth was found for VS activation during reward anticipation, shown in Figure 2 (B=−0.55, P=0.004; Supplementary Table S5). Simple slope analyses revealed only the slope of DRD4 7-repeat carriers was significant (carriers: P=0.014; non-carriers: P=0.140). Adolescents with the 7-repeat allele showed the highest activation when exposed to low maternal warmth (RoS threshold X=−1.20), but lowest when exposed to high warmth (RoS threshold X=0.51), compared with those without the 7-repeat. Furthermore, separate main effect analyses indicated that maternal criticism was positively associated with the VS BOLD response during reward receipt (B=0.21, P=0.009; Supplementary Table S4). No interactions were found during reward receipt in the VS or for the ventral medial prefrontal cortex activation (all P-values >0.109).
Interaction between DRD4 and maternal warmth on the activation in the ventral striatum during reward anticipation (B=−0.55, P=0.004; normal score (0)=blood-oxygen-level-dependent (BOLD) signal change 197.42). The shaded areas indicate the regions of significance (RoS), lower threshold X=−1.20; upper threshold X= 0.51. Values in the RoS are significant.
Sensitivity analyses
Sensitivity analyses were performed to check whether the above-described significant GxE interactions were affected by ADHD severity, as measured by the CPRS. These revealed no significant three-way interactions (all P-values >0.175). Moreover, including ADHD severity as a main effect did not change significant GxE effects. Finally, accounting for nonlinear age effects, intelligence quotient, oppositional defiance disorder, conduct disorder or medication history by rerunning the analyses for significant GxE effects while separately including these measures in the model did not affect GxE interactions.
Discussion
We found evidence for differential genetic susceptibility toward positive social environments for behavioral-related and striatal sensitivity to rewards in a large sample of adolescents, independent of ADHD severity. Up to now, authors have speculated about the role of the brain when investigating GxE effects on reward sensitivity. We believe we showed here for the first time that DRD4 genotypes moderate the association between warmth and neural responses to the anticipation of rewards in the VS.
Several explanations have been outlined to understand the relationship between reward-seeking behavior in daily life, as observed in adolescence, and neural activation during reward processing in imaging paradigms.11 Current evidence suggests that a hyper-responsive neural reward system predisposes to greater reward seeking, whereby increased dopaminergic release in response to rewarding events strengthens reward-related behavior through dopamine-based learning processes.11, 12, 13 In agreement with this perspective, the genetic moderation of both behavioral and neural responsiveness found in this paper could be explained by altered transcriptional activity, which affects the amount of dopamine released. For example, the DRD4 7-repeat polymorphism is associated with decreased postsynaptic inhibition of dopamine, which in turn leads to increased levels of dopamine.69 In addition, animal studies have demonstrated an association between maternal deprivation and increased dopamine levels.70 Further, studies in humans have revealed protective effects of positive parenting,19, 21 as well as detrimental effects of low warmth on behavioral and neural measures of reward sensitivity. Differential effects toward the environment then might be caused by exacerbation of dopamine increase in negative environments, but compensation when exposed to positive influences. This idea is supported by our finding of DRD4 7-repeat carriers showing the most striatal activation during reward anticipation when exposed to low maternal warmth, but the least when exposed to high levels of warmth.
Similar processes might occur for the 5-HTT gene, as participants carrying the HTTLPR short allele showed the least reward speeding when exposed to high positive peer affiliation, but the most when faced with low positive peer affiliation or low maternal warmth. Similar to the DRD4 7-repeat variant, decreased transcriptional activity has been associated with the HTTLPR short allele, resulting in an excess of serotonin levels.71 Besides dopamine, serotonin is also relevant for reward processing, and it is suggested that the interaction between dopamine and serotonin controls the behavioral response to rewards.72
For DAT1, in contrast to what we expected, we found DAT1 10/10 homozygotes displayed a similar differential pattern toward warmth for general task performance in both rewarded and non-rewarded conditions. On the basis of a previous GxE study in children with ADHD51 we had hypothesized that the 9-repeat would be the plasticity variant. However, evidence for the 10-repeat as candidate plasticity variant has been found in a community study,73 although neither study focused on reward sensitivity. Similar mixed results have been reported as to whether the 9- or 10-repeat shows increased or decreased expression.74 Finally, it is important to note that other variants and epigenetic factors not included in this study may influence the functional levels of the genes we investigated.75, 76 Considering how much is still unknown about the exact workings of dopamine and serotonin variation as a consequence of gene variants, especially in relation to environmental effects, more research is needed before we can truly state which and how gene variants enhance susceptibility.
Taken together, our findings partially support the differential susceptibility theory. Yet, this theory states individuals carrying plasticity alleles are sensitive to both positive and negative environments,43 while our results almost exclusively involved positive environments. However, besides less reward sensitivity when exposed to positive environments, the present study revealed increased reward sensitivity when faced with low positive environments as well. Although seemingly not the best way to operationalize an adverse environment, the absence of a positive environment is often associated with negative effects in child development, for example, Newman et al.77 and Yap et al.89 Therefore, viewing low warmth or low positive peer affiliation as adverse experiences seems valid, thereby placing the results in line with the differential susceptibility theory.
Two GxE interactions were found that do not fit the criteria of differential susceptibility: the interactions of 5-HTT and DAT1 with warmth on reward speeding and non-rewarded reactions times, respectively. Here carriers of the candidate susceptibility variants only differed from non-carriers when exposed to low maternal warmth. When viewing low warmth as a form of adversity (as argued above), these findings are more in line with the diathesis-stress model.29, 30 This theory states that genes moderate a person’s vulnerability to adverse effects only, while making no differences in positive environments.43 Thus, focusing on the same candidate plasticity gene and reward outcome measure (5-HTT and reward speeding), but different environmental measures (warmth vs positive peer affiliation), or focusing on the same gene and environmental predictor (DAT1 and warmth), but different outcome measures (rewarded vs non-rewarded MRT) led to the support for either differential susceptibility or diathesis-stress. These results demonstrate the complexity of how and in which situations individuals differ in their susceptibility toward environmental experiences.
The absence of significant interactions with the negative social environment in this study could indicate that positive social environments are more important for reward sensitivity. Indeed, the positive social environment has a key role in the development of reward learning (as described in Introduction). Our findings are in line with the idea that positive social influences promote optimal reward learning, and more so for adolescents with particular genotypes. We did, however, find main effects of criticism on reward receipt activation and reward speeding, as well as nominally significant interactions effects with both negative environmental measures. Therefore, investigation of both positive and negative effects in larger samples and from different populations is warranted before further conclusions as to which social environment has a stronger role in the development of reward learning.
This study had a number of strengths and limitations. Strengths were the use of a well-characterized sample, inclusion of both positive and negative environments, with both parental- and peer influences assessed, and the analysis of both behavioral and neural measures of reward processing. A limitation is the cross-sectional study design; longitudinal studies are needed to establish a direction of causality. Establishing the direction of effects is particularly difficult when focusing on parental and peer factors. Indeed, both maternal EE and peer affiliations have not only been suggested to influence child behavior, but in turn be influenced by child behavior as well.78, 79, 80, 81, 82, 83, 84, 85 Another possible limitation is that peer affiliation was measured by self-report and therefore reflects perception rather than an objective measure. Direct observations of peer affiliations would have eliminated the possible bias of self-observation and may have been the ideal measure. However, this is very difficult, if not impossible, to achieve in adolescence and young adulthood and was not feasible within our study as it was not solely aimed at investigating peer affiliations. Furthermore, not all participants included had an EE measurement, as it was assessed when a full diagnostic interview was administered. This led to loss of power, unequal numbers and an unequal distribution of ADHD and controls in the EE vs peer affiliation analyses. Nevertheless, the GxE effect on reward speeding was present in both social environments and most interaction effects were found for EE only, suggesting that it is a powerful moderator. Moreover, sensitivity analyses revealed no effect of ADHD severity on significant GxE interactions. Previous power calculations with a sample size of ~350 indicated that we had adequate power to detect GxE effects with an explained variance of 3–5% or higher,86 but this amount of variance is considered to be quite large in the GxE literature87 and smaller but still relevant effects may go undetected. We therefore emphasize the need of replication studies. Finally, as we used a modified version of the MID task with a lower hit rate compared with the original version of the task (33% vs 66%), it could be suggested that this might have led to participants finding the task too difficult and to feelings of frustration. However, the value of rewards has been suggested to depend on the context.88 Consequently, the lower hit rate would only have been experienced as frustrating when participants had been able to compare it with higher hit rates (see also von Rhein et al.33). Still, here too replication studies are a necessity.
In conclusion, these results indicate GxE interplay is relevant for an improved understanding of interindividual differences in behavioral and neural measures of reward sensitivity and general task performance. Our findings may ultimately also have implications for clinical settings, as targeting parents or peers of at-risk adolescents could be particularly helpful for carriers of the HTTLPR short allele and the DRD4 7-repeat. Importantly, our results were not modified by ADHD severity. This suggests that the effects of genes, social environment and their interplay contribute in a general way to interindividual differences in striatal responses during reward anticipation, reward speeding and general task performance, and are not specific for ADHD. Considering the new research questions and novel findings, more research on GxE interactions and reward sensitivity is needed, in particular replication of our findings in independent large data sets with additional types of positive and negative social environments.
References
Blaukopf CL, DiGirolamo GJ . Reward, context, and human behaviour. Sci World J 2007; 7: 626–640.
Fairchild G . The developmental psychopathology of motivation in adolescence. Dev Cogn Neurosci 2011; 1: 414–429.
Reynolds B . A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol 2006; 17: 651–667.
Steinberg L . A social neuroscience perspective on adolescent risk-taking. Dev Rev 2008; 28: 78–106.
Knutson B, Adams CM, Fong GW, Hommer D . Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21: RC159.
Haber SN, Knutson B . The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26.
Sescousse G, Caldu X, Segura B, Dreher JC . Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev 2013; 37: 681–696.
Bartra O, McGuire JT, Kable JW . The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013; 76: 412–427.
Diekhof EK, Kaps L, Falkai P, Gruber O . The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 2012; 50: 1252–1266.
Liu X, Hairston J, Schrier M, Fan J . Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2011; 35: 1219–1236.
Galvan A . Adolescent development of the reward system. Front Hum Neurosci 2010; 4: 6.
Schultz W, Tremblay L, Hollerman JR . Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci 2003; 26: 321–328.
Chambers RA, Taylor JR, Potenza MN . Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003; 160: 1041–1052.
Anokhin AP, Golosheykin S, Grant JD, Heath AC . Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet 2011; 41: 175–183.
Sparks JC, Isen JD, Iacono WG . Preference on cash-choice task predicts externalizing outcomes in 17-year-olds. Behav Genet 2014; 44: 102–112.
Paloyelis Y, Asherson P, Mehta Ma, Faraone SV, Kuntsi J . DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology 2010; 35: 2414–2426.
Bowirrat A, Chen TJ, Oscar-Berman M, Madigan M, Chen AL, Bailey JA et al. Neuropsychopharmacology and neurogenetic aspects of executive functioning: should reward gene polymorphisms constitute a diagnostic tool to identify individuals at risk for impaired judgment? Mol Neurobiol 2012; 45: 298–313.
Hahn T, Heinzel S, Dresler T, Plichta MM, Renner TJ, Markulin F et al. Association between reward-related activation in the ventral striatum and trait reward sensitivity is moderated by dopamine transporter genotype. Hum Brain Mapp 2011; 32: 1557–1565.
Xiao L, Bechara A, Palmer PH, Trinidad DR, Wei Y, Jia Y et al. Parent-child engagement in decision making and the development of adolescent affective decision capacity and binge drinking. Pers Individ Dif 2011; 51: 285–292.
Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE et al. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci 2014; 8: 18–27.
Morgan JK, Shaw DS, Forbes EE . Maternal depression and warmth during childhood predict age 20 neural response to reward. J Am Acad Child Adolesc Psychiatry 2014; 53: 108–117 e101.
Schneider S, Brassen S, Bromberg U, Banaschewski T, Conrod P, Flor H et al. Maternal interpersonal affiliation is associated with adolescents’ brain structure and reward processing. Transl Psychiatry 2012; 2: e182.
Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA . Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 2009; 66: 206–213.
Zeman J, Cassano M, Perry-Parrish C, Stegall S . Emotion regulation in children and adolescents. J Dev Behav Pediatr 2006; 27: 155–168.
He Q, Xue G, Chen C, Lu ZL, Chen C, Lei X et al. COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Sci Rep 2012; 2: 677.
Sweitzer MM, Halder I, Flory JD, Craig AE, Gianaros PJ, Ferrell RE et al. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: evidence for differential susceptibility. Soc Cogn Affect Neurosci 2013; 8: 499–508.
Belsky J . Variation in susceptibility to environmental influence: an evolutionary argument. Psychol Inq 1997; 8: 182–186.
Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R . Vulnerability genes or plasticity genes? Mol Psychiatry 2009; 14: 746–754.
Zubin J, Spring B . Vulnerability—a new view of schizophrenia. J Abnorm Psychol 1977; 86: 103–126.
Monroe SM, Simons AD . Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull 1991; 110: 406–425.
Belsky J, Pluess M . Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev Psychopathol 2013; 25: 1243–1261.
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D . Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001; 12: 3683–3687.
von Rhein D, Cools R, Zwiers MP, van der Schaaf M, Franke B, Luman M et al. Increased neural responses to reward in adolescents and young adults with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry 2015; 54: 394–402.
Luman M, Tripp G, Scheres A . Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev 2010; 34: 744–754.
Furukawa E, Bado P, Tripp G, Mattos P, Wickens JR, Bramati IE et al. Abnormal striatal BOLD responses to reward anticipation and reward delivery in ADHD. PLoS One 2014; 9: e89129.
Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J . Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51: 722–732.e9.
Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2009; 65: 7–14.
Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 2008; 39: 966–972.
Kennis M, Rademaker AR, Geuze E . Neural correlates of personality: an integrative review. Neurosci Biobehav Rev 2013; 37: 73–95.
Plichta MM, Scheres A . Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 2014; 38: 125–134.
Harris KM, Halpern CT, Hussey J, Whitsel EA, Killeya-Jones L, Tabor J et al. Social, behavioral, and genetic linkages from adolescence into adulthood. Am J Public Health 2013; 103 (Suppl 1): S25–S32.
Gizer IR, Ficks C, Waldman ID . Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 2009; 126: 51–90.
Belsky J, Pluess M . Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009; 135: 885–908.
Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, Arcuri V et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat 2008; 4: 893–918.
Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 2006; 11: 934–953.
von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J et al. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. Eur Child Adolesc Psychiatry 2015; 24: 265–281.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
Wechsler D . WAIS-III Nederlandstalige bewerking. Technische handleiding. The Psychological Corperation: London, UK, 2000.
Wechsler D . WISC-III Handleiding. The Psychological Corperation: London, UK, 2002.
Brown GW . The measurement of family activities and relationships: a methodological study. Hum Relations 1966; 19: 241–263.
Sonuga-Barke EJ, Oades RD, Psychogiou L, Chen W, Franke B, Buitelaar J et al. Dopamine and serotonin transporter genotypes moderate sensitivity to maternal expressed emotion: the case of conduct and emotional problems in attention deficit/hyperactivity disorder. J Child Psychol Psychiatry 2009; 50: 1052–1063.
Richards JS, Arias Vásquez A, Rommelse NNJ, Oosterlaan J, Hoekstra PJ, Franke B et al. A follow-up study of maternal expressed emotion toward children with attention-deficit/hyperactivity disorder: relation with severity and persistence of ADHD and comorbidity. J Am Acad Child Adolesc Psychiatry 2014; 53: 311–319.
Schachar R, Taylor E, Wieselberg M, Thorley G, Rutter M . Changes in family function and relationships in children who respond to methylphenidate. J Am Acad Child Adolesc Psychiatry 1987; 26: 728–732.
Chen W . PACS interview and genetic research in ADHD. In: Oades R, Taylor E (eds). Attention-Deficit/Hyperactivity Disorder HKS: Current ideas and ways forward. 1st edn. Nova Science Publishers Inc.: New York, NY, USA, 2006, pp 3–20.
Walden B, McGue M, Lacono WG, Burt SA, Elkins I . Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J Abnorm Psychol 2004; 113: 440–450.
Burt SA, Klump KL . Prosocial peer affiliation suppresses genetic influences on non-aggressive antisocial behaviors during childhood. Psychol Med 2014; 44: 821–830.
Burt SA, McGue M, Iacono WG . Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: results from a cross-lagged monozygotic twin differences design. Dev Psychol 2009; 45: 1752–1760.
Hicks BM, South SC, Dirago AC, Iacono WG, McGue M . Environmental adversity and increasing genetic risk for externalizing disorders. Arch Gen Psychiatry 2009; 66: 640–648.
Gifford-Smith M, Dodge KA, Dishion TJ, McCord J . Peer influence in children and adolescents: crossing the bridge from developmental to intervention science. J Abnorm Child Psychol 2005; 33: 255–265.
Vitaro F, Brendgen M, Boivin M, Cantin S, Dionne G, Tremblay RE et al. A monozygotic twin difference study of friends’ aggression and children’s adjustment problems. Child Dev 2011; 82: 617–632.
Fabes RA, Hanish LD, Martin CL, Moss A, Reesing A . The effects of young children’s affiliations with prosocial peers on subsequent emotionality in peer interactions. Br J Dev Psychol 2012; 30: 569–585.
Conners CK, Sitarenios G, Parker JD, Epstein JN . The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998; 26: 257–268.
Patenaude B, Smith SM, Kennedy DN, Jenkinson M . A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011; 56: 907–922.
Landaas ET, Johansson S, Jacobsen KK, Ribases M, Bosch R, Sanchez-Mora C et al. An international multicenter association study of the serotonin transporter gene in persistent ADHD. Genes Brain Behav 2010; 9: 449–458.
Knafo A, Jaffee SR . Gene-environment correlation in developmental psychopathology. Dev Psychopathol 2013; 25: 1–6.
Lehman EL . Nonparametrics: Statistical Methods Based on Ranks. Holden-Day: San Fransisco, CA, USA, 1975.
Li J, Ji L . Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005; 95: 221–227.
Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC . Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Dev Psychopathol 2012; 24: 389–409.
Congdon E, Canli T . A neurogenetic approach to impulsivity. J Pers 2008; 76: 1447–1484.
Hall FS, Perona MT . Have studies of the developmental regulation of behavioral phenotypes revealed the mechanisms of gene-environment interactions? Physiol Behav 2012; 107: 623–640.
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274: 1527–1531.
Cools R, Nakamura K, Daw ND . Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology 2011; 36: 98–113.
Belsky J, Beaver KM . Cumulative-genetic plasticity, parenting and adolescent self-regulation. J Child Psychol Psychiatry 2011; 52: 619–626.
Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J . Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol Psychiatry 2014; 19: 880–889.
Bralten J, Franke B, Waldman I, Rommelse N, Hartman C, Asherson P et al. Candidate genetic pathways for attention-deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive symptoms in children with ADHD. J Am Acad Child Adolesc Psychiatry 2013; 52: 1204–1212.e1.
Homberg JR, van den Hove DL . The serotonin transporter gene and functional and pathological adaptation to environmental variation across the life span. Prog Neurobiol 2012; 99: 117–127.
Newman K, Harrison L, Dashiff C, Davies S . Relationships between parenting styles and risk behaviors in adolescent health: an integrative literature review. Rev Lat Am Enfermagem 2008; 16: 142–150.
Asarnow JR, Tompson M, Hamilton EB, Goldstein MJ, Guthrie D . Family-expressed emotion, childhood-onset depression, and childhood-onset schizophrenia spectrum disorders: is expressed emotion a nonspecific correlate of child psychopathology or a specific risk factor for depression? J Abnorm Child Psychol 1994; 22: 129–146.
Hou J, Chen Z, Natsuaki MN, Li X, Yang X, Zhang J et al. A longitudinal investigation of the associations among parenting, deviant peer affiliation, and externalizing behaviors: a monozygotic twin differences design. Twin Res Hum Genet 2013; 16: 698–706.
Hale WW 3rd, Keijsers L, Klimstra TA, Raaijmakers QA, Hawk S, Branje SJ et al. How does longitudinally measured maternal expressed emotion affect internalizing and externalizing symptoms of adolescents from the general community? J Child Psychol Psychiatry 2011; 52: 1174–1183.
Cartwright KL, Bitsakou P, Daley D, Gramzow RH, Psychogiou L, Simonoff E et al. Disentangling child and family influences on maternal expressed emotion toward children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011; 50: 1042–1053.
Burt SA, Klump KL . Prosocial peer affiliation suppresses genetic influences on non-aggressive antisocial behaviors during childhood. Psychol Med 2014; 44: 821–830.
Hoza B . Peer functioning in children with ADHD. J Pediatr Psychol 2007; 32: 655–663.
Hirshfeld DR, Biederman J, Brody L, Faraone SV, Rosenbaum JF . Associations between expressed emotion and child behavioral inhibition and psychopathology: a pilot study. J Am Acad Child Adolesc Psychiatry 1997; 36: 205–213.
Tarantino N, Tully EC, Garcia SE, South S, Iacono WG, McGue M . Genetic and environmental influences on affiliation with deviant peers during adolescence and early adulthood. Dev Psychol 2014; 50: 663–673.
Richards JS, Hartman CA, Franke B, Hoekstra PJ, Heslenfeld DJ, Oosterlaan J et al. Differential susceptibility to maternal expressed emotion in children with ADHD and their siblings? Investigating plasticity genes, prosocial and antisocial behaviour. Eur Child Adolesc Psychiatry 2015; 24: 209–217.
Duncan LE, Keller MC . A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry 2011; 168: 1041–1049.
Tobler PN, Fiorillo CD, Schultz W . Adaptive coding of reward value by dopamine neurons. Science 2005; 307: 1642–1645.
Yap MB, Pilkington PD, Ryan SM, Jorm AF . Parental factors associated with depression and anxiety in young people: a systematic review and meta-analysis. J Affect Disord 2014; 156: 8–23.
Acknowledgements
The International Multisite ADHD Genetics (IMAGE) project is a multi-site, international effort. Funding support for the IMAGE project was provided by NIH grants R01MH62873 and R01MH081803 to SVF, and the genotyping of samples was provided through the Genetic Association Information Network (GAIN). The data set used for the analyses described in this manuscript were obtained from the database of Genotypes and Phenotypes (dbGaP) found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number #20726-2. This work was further supported by an NWO Large Investment Grant 1750102007010 and NWO Brain & Cognition an Integrative Approach grant (433-09-242) (to SVB), and grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. The research leading to these results also received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 278948 (TACTICS) and no. 602450 (IMAGEMEND), as well as from the European Community’s Horizon 2020 Programme under grant agreement no. 643051 (MiND). BF is supported by a Vici grant from NWO (grant no. 016-130-669), and she and SVB received funding from the National Institutes of Health (NIH) Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence. SVF is supported by the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 602805, and NIMH grants R13MH059126 and R01MH094469.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In the past year, SVF received income, travel expenses and/or research support from and/or has been on an Advisory Board for Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, NeuroLifeSciences, and received research support from the National Institutes of Health (NIH). His institution is seeking a patent for the use of sodium–hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. SVF receives royalties from books published by Guilford Press (Straight Talk about Your Child’s Mental Health) and Oxford University Press (Schizophrenia: The Facts). In the past 3 years, JKB has been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, Shering Plough, UCB, Shire, Novartis and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents and royalties. In the past 3 years, PJH has been a consultant to/member of advisory board of Shire and has received an unrestricted investigator-initiated research grant from Shire. JO has received an unrestricted investigator initiated research grant from Shire. BF received a speaker fee from Merz. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Richards, J., Arias Vásquez, A., von Rhein, D. et al. Adolescent behavioral and neural reward sensitivity: a test of the differential susceptibility theory. Transl Psychiatry 6, e771 (2016). https://doi.org/10.1038/tp.2016.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.37
This article is cited by
-
Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder
Translational Psychiatry (2021)
-
Gene-Environment Interactions in Psychiatry: Recent Evidence and Clinical Implications
Current Psychiatry Reports (2019)