Abstract
Total white blood cell count (TWBCC) and percentage (%) composition of lymphocytes (PL) or neutrophils (PN) are linked to mid- and late-life depression, though sex-specific temporal relationships between those inflammatory markers and depressive symptoms remain unclear. The association between inflammation and depressive symptoms in longitudinal data on ethnically and socioeconomically diverse urban adults was examined with two hypotheses. In hypothesis 1, we examined the relationship between TWBCC, PL and PN with change in level of depressive symptoms from baseline to follow-up, stratifying by sex. In hypothesis 2, we examined reverse causality, by testing the relationship of depressive symptoms with change in TWBCC, PL and PN. Multiple linear mixed-effects regression models were performed to examine both the hypotheses. The sample sizes of participants (n) and repeated observations (n’) were: Hypothesis 1 (n=2009; n’=3501); Hypothesis 2 (n=2081; n’=3560). Among key findings (Hypothesis 1), in women, higher TWBCC was linked to a faster increase in depressive symptom total score (γ1112±s.e.: +0.81±0.28, P=0.003), with a slower increase over time in the positive affect subdomain coupled with faster increases in depressed affect and somatic complaints. Among women, baseline score on somatic complaints was positively associated with low PN (γ01a=+1.61±0.48, P<0.001) and high PL (γ01a=+1.16±0.45, P=0.011), whereas baseline score on positive affect was inversely related to higher PL (γ01a=−0.69±0.28, P=0.017). Results among men indicated that there was a positive cross-sectional relationship between low TWBCC and depressive symptoms, depressed affect and an inverse cross-sectional relationship with positive affect. However, over time, a low TWBCC in men was linked to a higher score on positive affect. There was no evidence of a bi-directional relationship between WBC parameters and depressive symptoms (Hypothesis 2). In sum, TWBCC and related markers were linked to depressive symptoms, mostly among women. Further longitudinal studies are needed to replicate this sex-specific association.
Similar content being viewed by others
Introduction
Unipolar depression is a chronic condition1, 2 accounting for a major part of the health care system,3 with a lifetime prevalence among American adults of ~12% in men and ~21% in women.3 Depression and elevated depressive symptoms in general were linked to altered immunity.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 Several studies included in meta-analyses found an association of elevated depressive symptoms with WBC-related markers of inflammation including increased absolute leukocyte levels (that is, total white blood cell count (TWBCC)), reduced percentage lymphocytes (PL) out of total leukocytes or lymphopenia, and increased percentage neutrophils (PN) or neutrophilia. However, the results of these meta-analyses38, 39, 40 had distinctive conclusions mainly owing to heterogeneity in study design, depressive symptoms measures, potential confounders considered, measured inflammatory markers and sample characteristics (for example, age, sex, ethnic background and health behaviors including diet). Nevertheless, as reverse causality cannot be ruled out, bi-directional associations between white blood cell (WBC)-related markers (TWBCC, PN and PL) and depressive symptoms in populations are under-studied, particularly in terms of sex-specific longitudinal changes in one as predicted by the baseline value of the other.24, 28
Using longitudinal data on a large and ethnically diverse urban sample, our study had three separate objectives and two hypotheses. The first objective (Objective 1) was to assess the cross-sectional (that is, baseline vs baseline) and bi-directional longitudinal (that is, baseline vs rate of change) relationships of WBC-related markers (that is, high/low TWBCC, high/low PN, high/low PL; high: >90th percentile, low: <10th percentile) with depressive symptoms, while the second objective (Objective 2) was to assess the cross-sectional and longitudinal relationships of WBC-related markers with specific domain of depressive symptomatology. Finally, the third objective (Objective 3) was to examine whether the association in the above two objectives are sex-specific. Two longitudinal relationships were tested: Hypothesis 1: baseline WBC-related markers predict rate of change in depressive symptoms and Hypothesis 2: baseline depressive symptoms predict WBC-related markers.
Materials and methods
Database and study sample
The healthy aging in neighborhoods of diversity across the lifespan (HANDLS) study is an ongoing prospective cohort study initiated in 2004 that recruited a representative sample of African Americans and whites aged 30–64 years old and living in Baltimore, MD, USA.41 The HANDLS design consisted of an area probability sample of 13 neighborhoods (groups of contiguous census tracts), with a first phase at baseline that screened and recruited participants and administered a household interview. At phase II, in-depth examinations in mobile medical research vehicles were conducted. Written informed consent was obtained after access to a protocol booklet in layman's terms along with a video describing all the procedures and future re-contacts. The MedStar Institutional Review Board approved all the materials. Longitudinal data from baseline (visit 1, also known as wave 1, ended in 2009) and the first follow-up examination (visit 2, also known as wave 3, ended in 2013, mean±s.d. follow-up=4.65±0.93 years) were used in this study.
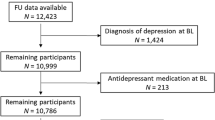
Initially, 3720 participants were recruited (Sample 1), Center for Epidemiologic Studies-Depression (CES-D) data were available for N=2725 participants at baseline and N=2258 at wave 3; while wave 1 TWBCC, PN and PL were available for N=2744–2745 participants and wave 3 TWBCC, PN and PL were available for N=2254–2266. We restricted our sample to those with 2 days of dietary recall at wave 1 and CES-D data at either wave, while further limiting the analyses to those with baseline TWBCC, PN and PL for Hypothesis 1, along with non-missing covariates (n=2009–2011; Sample 2A, Hypothesis 1; repeated observations n=3496). Participants in Sample 2A compared with remaining HANDLS participants in Sample 1 had a lower percentage with poverty income ratio >125% (56.4% vs 62.1%, P=0.005), and a higher percentage of women (56.5% vs 52.0%, P=0.009) with no significant differences by age and race distributions.
In addition, for Hypothesis 2, the final sample size consisted of participants with complete WBC variables at either wave and complete data on CES-D total score at wave 1 (Sample 2B, n=2081; 908 men and 1173 women; repeated observations: n’=3560), with similar selectivity patterns as for Sample 2A.
Depressive symptoms
Depressive symptoms were operationalized using the CES-D, at both baseline and follow-up. The 20-item CES-D is a self-reported symptom rating scale assessing affective and depressed mood.42 A score of ⩾16 on the CES-D is reflective of elevated depressive symptoms (EDS),43 and predicts clinical depression based on the Diagnostic and Statistical Manual, fourth edition (DSM-IV) criteria.44 Four CES-D sub-domains exhibiting an invariant factor structure between The National Health and Nutrition Examination Survey I and pilot HANDLS data45 were computed. We tested our hypotheses using total and domain-specific CES-D scores: (1) somatic complaints; (2) depressive affect; (3) positive affect and (4) interpersonal problems.45
White blood cell inflammatory markers
Fasting blood samples were collected from participants at baseline and follow-up to determine TWBCC, (K mm−3) and percentage WBC subtypes, including PN and PL, using electronic cell sizing, counting, cytometry and microscopy (http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=7064). Blood samples were transported to Quest diagnostics for analysis in which technicians were blinded to other study parameters, including depressive symptoms levels.
Covariates
Socio-demographic, lifestyle and health-related potential confounders
Potential confounders in our analytic models included age, sex, race (white vs African American), educational attainment (0⩽high school (HS); 1=HS and 2⩾HS), poverty status (below vs above 125% the federal poverty line), measured body mass index (BMI; kg/m2), current use of drugs ('opiates, marijuana or cocaine'=1 vs not=0), and current smoking status (0: 'never or former smoker' and 1 'current smoker').
Dietary potential confounders
We entered dietary potential confounders that were linked to depression based on previous studies and included vitamins B-6, folate and B-12, total carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene), vitamin C and α-tocopherol46, 47, 48, 49, 50, 51, 52, 53, 54 (all divided by total energy intake and expressed per 1000 kcal) and the ratio of n-3 polyunsaturated fatty acid (PUFA):n-6 PUFA.55 Total energy intake was entered as a covariate to emulate a multivariate nutrient density model.56 Models also included overall dietary quality as measured by the Healthy Eating Index (HEI-2010) total score, with details provided by the National Cancer Institute’s Applied Research (http://appliedresearch.cancer.gov/tools/hei/tools.html) and the HANDLS (http://handls.nih.gov/06Coll-dataDoc.htm) websites.
Statistical analysis
Stata 14.0 (StataCorp, College Station, TX, USA) was used in all the analyses.57 First, baseline characteristics were examined for men vs women, further differentiated by EDS status (CES-D score ⩾16 vs <16, based on mean score across waves), using t-tests and analysis of variance for continuous variables and χ2 tests for categorical variables. Second, several mixed-effects regression models on continuous CES-D total or on domain-specific score(s) were conducted to test associations with three alternative WBC exposures, controlling for potential confounders. Sex-specific associations were tested by adding interaction terms to the multivariable mixed-effects regressions and stratifying by sex. Supplementary Appendix I outlines methodology used to run mixed-effects regression models, which were previously conducted.58, 59, 60
Adjustment for non-random participant selection bias was done with a two-stage Heckman selection process.60, 61, 62 An α=0.05 was used for all the analyses, and P-values >0.05 and <0.10 were considered borderline significant for main effects, whereas a P-value <0.10 was considered significant for interaction terms63 before multiple testing correction. The process of correcting for multiple testing was done using the family-wise Bonferroni correction,64 in which we assume that each of total CES-D and sub-domains of CES-D are distinctive outcomes for which we test the association with the three WBC-related exposures that conceptually related. A similar approach was used in other previous studies.60, 65 Accounting for three exposures, type I error was reduced to 0.05/3=0.017 for main effects and 0.10/3=0.033 for interaction terms.
Results
Across-visit EDS+ was more prevalent among women vs men (46.6% vs 36.5%, P<0.001, χ2 test; Table 1). EDS+ participants had a lower prevalence of poverty income ratio ⩾125%, a higher likelihood of unemployment and lower educational attainment, a higher proportion of current smokers overall and current illicit drug users (in women), compared with EDS− participants. Despite women having a higher mean BMI compared with men (31.3 vs 28.0), BMI was not linked to EDS status. There were also consistently lower prevalence rates of current smoking and drug use among women compared with men. Poorer dietary quality (HEI-2010) was observed among men vs women, and among EDS+ (vs EDS−) participants, for both sexes. Mean micronutrient intakes per 1000 kcal at baseline were lower among EDS+ vs EDS−, specifically vitamin E, vitamin B-6, folate and vitamin C (women). In terms of WBC exposures, TWBCC was higher among EDS+ vs EDS− women (P=0.02), with women overall having higher TWBCC vs men (P=0.03). PN was higher in women who were also more likely to be in the <10th percentile of the distribution (9.9% vs 5.9%, P=0.002), when EDS+ vs EDS−. Similarly, EDS+ vs EDS− women were more likely to be above the 90th percentile of PL.
For Hypothesis 1 and Objective 1, we first tested cross-sectional and longitudinal relationships between WBC-related markers and depressive symptoms. This was examined with mixed-effects linear regression models (Supplementary Appendix I), with results presented in Table 2. The TWBCC main effect parameter reflects the cross-sectional association of TWBCC with depressive symptoms, whereas TWBCC × time interaction can be interpreted as the effect of TWBCC on the annual rate of change in depressive symptoms. Two exposure levels were compared with the 10th–90th percentile range, namely 'Low' (<10th percentile) and 'High' (>10th percentile). We found that for men there was a cross-sectional association between the <10 percentile of TWBCC and depressive symptoms (γ0111=+2.51±1.05, P=0.016) but no cross-sectional association between PN or PL and depressive symptoms, nor was there an association for any of the WBC-related markers and depressive symptoms longitudinally. For woman, high TWBCC was linked to an accelerated increase in CES-D score over time (γ1112=+0.81±0.28, P=0.003; Figure 1). In a separate model, also among women, baseline PN<10th percentile (<44.1%) was associated with higher baseline CES-D total scores compared with 10th–90th percentile range, adjusting for multiple covariates (γ0121=+2.70±1.26, P=0.031). This result, however, did not survive multiple testing.
Pertaining to our second objective, we examined whether WBC-related markers are associated with specific domains of depressive symptomatology. To this end, Table 3 (Hypothesis 1, Objective 2) shows a series of comparable linear mixed-effect regression models for women, adjusting baseline CES-D subdomain scores and rate of change over time for the same covariates and entering each WBC exposure into a separate model (Supplementary Appendix I). After adjustment for multiple testing (α: 0.10 → 0.033), high TWBCC (>90th vs 10th–90th percentile) was associated with faster increase in somatic complaints and depressed affect and a slower increase in positive affect (higher positive affect → less depressive symptoms). Baseline scores on somatic complaints were also positively associated to low PN (γ01a=+1.61±0.48, P<0.001) and high PL (γ01a=+1.16±0.45, P=0.011) among women; whereas baseline score on positive affect was inversely related to higher PL (γ01a=−0.69±0.28, P=0.017).
Table 4 (Hypothesis 1, Objective 2) shows results among men for associations between WBC-related markers and CES-D component scores at baseline and rates of change over time. After adjustment for multiple testing, several key findings emerged. First, higher baseline TWBCC was linked to higher depressed affect and lower positive affect. Nevertheless, the longitudinal effects indicated that low TWBCC was associated with a slower increase in depressed affect and faster increase in positive affect. PL and PN were not associated with cross-sectional or longitudinal change in CES-D sub-domains among men.
Objective 3 of Hypothesis 1 was to examine whether cross-sectional and longitudinal associations between WBC exposures and depressive symptoms (total score and domains) differed significantly between men and women. This was done by adding relevant interaction terms in the mixed-effects regression models. First, the effect of PN<10th percentile (<44.1%) on baseline CES-D total score compared with 10th–90th percentile differed significantly between men and women. Second, the cross-sectional association between somatic complaints and low PN (γ01a=+1.61±0.48, P<0.001) as well as high PL (γ01a=+1.16±0.45, P=0.011) among women was found to be significantly different between men and women.
Finally, we examined all three objectives pertaining to Hypothesis 2 to test sex-specific associations between baseline depressive symptoms and rate of change in WBC-related markers (Supplementary Table S1), using a similar series of multiple mixed-effects regression models, stratifying by sex. Baseline CES-D total score was not associated with any of the WBC-related outcomes. The same finding pertained to depressive symptom domains (data not shown) and the associations did not differ between men and women.
Discussion
This study is among few to examine bi-directional relationships between WBC-related inflammatory markers and depressive symptoms, and we believe is the first to examine those relationships differentially between men and women in an urban population of adults. Higher TWBCC was linked to a faster increase in depressive symptoms among women, with a slower increase over time in the positive affect sub-domain, coupled with faster increases in depressed affect and somatic complaints. Results among men indicated that there was a positive cross-sectional relationship between low TWBCC and depressive symptoms, depressed affect and an inverse cross-sectional relationship with positive affect. However, over time, a low TWBCC in men was linked to a higher score on positive affect (See Supplementary Table S2 for summary of key findings).
TWBCC is an under-studied inflammatory marker of depression, with studies limited to small case–control design,6, 16, 31 and a handful of cross-sectional and longitudinal studies.21, 24, 34, 35, 36 Bi-directional relationships were examined only in one study.24 In the largest study to date (N=11 367–11 857, EPIC-Norfolk, age:40–80 years), after multivariate adjustment for age and cigarette smoking, an association between major depressive disorder and TWBCC count was reported in men, but not women.34 Our study shows that the longitudinal association between TWBCC and depressive symptoms and sub-domains was restricted to women and in the expected direction (that is, higher TWBCC increases the rate of depression over time). In contrast, significant findings among men were mostly cross-sectional indicating that lower TWBCC may be associated with elevated depressive symptoms at baseline, with the possible exception of positive affect rate of change, which was directly linked to lower TWBCC among men.
In another large cross-sectional study (n=3769 participants (EPESE)), TWBCC count was higher among EDS+ compared with the EDS− group,21 though results were not sex-specific. This result was replicated in a cohort study of cardiovascular disease-free adults (453 men (19–89 years old) and 400 women (18–84 years old)), in which the Zung Self-Rating Depression Scale (range 0–100) total score was positively correlated with TWBCC, after multivariate adjustment.35 Baseline depressive symptoms independently predicted higher subsequent TWBCC, but not vice versa, which is at odds with our results.35 Our study design differed from the latter’s by including both healthy and CHD participants and participants with other comorbidities and selecting both whites and African American adults.35
In our study, among women, baseline somatic complaint score was positively associated with low PN and high PL, whereas baseline positive affect score was inversely related to higher PL. Similarly, WBC subtypes (including PL and PN) or WBC activity (for example, natural killer cell activity) in relation to depressive outcomes were examined in mostly small case–control studies.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 17, 18, 20, 30, 31, 37 Notwithstanding these controversial findings, a recent meta-analysis indicates that depressed individuals tend to have a state of neutrophilia (that is, high PN) coupled with lymphopenia (that is, low PL).40 In studies of depression with a higher percentage of females, depressed participants have greater proportional increases in B- and T-cell numbers compared with the non-depressed, indicating lymphocytosis predominates.40 These findings agree with our results for female participants of HANDLS. A study by Nakata et al.28 investigated the putative temporal bi-directionality between depressive symptoms and counts of lymphocyte subsets and found that depressive symptoms preceded variation in follow-up lymphocyte counts, particularly NK cells. In contrast, baseline NK cells were not predictive of follow-up depressive symptoms.28 Our study indicates that baseline depressive symptoms are associated with baseline PL and PN, specifically among women, without any indication of baseline depressive symptoms affecting or being affected by those parameters over time.
Biological mechanisms behind baseline leukocytosis and increase in depressive symptoms among women are largely speculative. One potential mechanism is chronic stress stimulating hematopoietic stem cells in the bone marrow, increasing blood cortisol (hypercortisolemia) and reducing vagal nerve activity, possibly leading to the accelerated production of WBCs.66, 67 Our findings, however, indicate that although TWBCC can result from chronic stress, depressive symptoms among women worsened over time with pre-existing elevated TWBCC. In contrast, elevated baseline depressive symptoms did not alter the rate of change in TWBCC in both sexes. Another possibility is that chronic stress could lead to poor health behaviors, including smoking, sedentary behavior and poor diet, which could lead to central adiposity and inflammation, independent of BMI.68, 69 Those factors themselves could influence immunity by increasing levels of inflammation, partly manifested by a higher TWBCC. Consequently, TWBCC becomes an adverse exposure increasing depressive symptoms over time, or reducing the rate of increase in positive affect. Many of those adverse health behaviors, excluding physical activity and central adiposity, were adjusted for in this model. It is possible that other mediators may have a role, including poor sleep which was found to mediate this relationship in at least one other study.24 Residual confounding by those factors included cannot be ruled out, given that those measures were self-reported and liable to measurement error.24 Moreover, when injected in non-depressed individuals, monokines, specifically interleukin-1, can trigger symptoms suggestive of depression based on the DSM-III-R by provoking hormonal changes.70 Estrogen can activate macrophages and produce monokines, which explains the 3:1 female/male incidence of depression ratio in many adult populations.70 Our findings indicated that higher TWBCC in females can lead to a faster increase in depressive symptoms, potentially due to a synergistic effect between estrogen and stress hormones in the presence of chronic stress.
Our study has several notable strengths. First, being longitudinal in design, this study unveiled temporality of associations between WBC-related markers and depressive symptoms. The relationship was in the direction of baseline WBC markers→ depressive symptoms but not vice versa. Second, the analytic sample size permitted stratification by sex without significant loss in statistical power. Third, a large number of covariates were entered into the mixed-effects regression models to control for confounding effects at the level of the intercept as well as the slope. Nevertheless, our study findings should be interpreted with caution in light of several limitations, including potential selection bias owing to non-participation, which was minimized using a two-stage Heckman selection model. Moreover, despite the direct measures of WBC markers, other variables including the CES-D were self-reported, leading to measurement error and residual confounding in the main associations of interest.
In sum, WBC-related markers were linked to depressive symptoms, mostly among women. Further longitudinal studies are needed to replicate this sex-specific association. In addition, adding a third wave of data would allow examining potential mediating factors between those markers of inflammation and depressive symptoms, particularly TWBCC. Finally, studies are needed to distinguish between behavioral and genetic factors behind those associations.
References
Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry 1998; 55: 694–700.
Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM et al. Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry 1992; 49: 809–816.
Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51: 8–19.
Kronfol Z, Nasrallah HA, Chapman S, House JD . Depression, cortisol metabolism and lymphocytopenia. J Affect Disord 1985; 9: 169–173.
Murphy D, Gardner R, Greden JF, Carroll BJ . Lymphocyte numbers in endogenous depression. Psychol Med 1987; 17: 381–385.
Darko DF, Rose J, Gillin JC, Golshan S, Baird SM . Neutrophilia and lymphopenia in major mood disorders. Psychiatry Res 1988; 25: 243–251.
Nerozzi D, Santoni A, Bersani G, Magnani A, Bressan A, Pasini A et al. Reduced natural killer cell activity in major depression: neuroendocrine implications. Psychoneuroendocrinology 1989; 14: 295–301.
Schleifer SJ, Keller SE, Bond RN, Cohen J, Stein M . Major depressive disorder and immunity. Role of age, sex, severity, and hospitalization. Arch Gen Psychiatry 1989; 46: 81–87.
Targum SD, Marshall LE, Fischman P, Martin D . Lymphocyte subpopulations in depressed elderly women. Biol Psychiatry 1989; 26: 581–589.
Irwin M, Caldwell C, Smith TL, Brown S, Schuckit MA, Gillin JC . Major depressive disorder, alcoholism, and reduced natural killer cell cytotoxicity. Role of severity of depressive symptoms and alcohol consumption. Arch Gen Psychiatry 1990; 47: 713–719.
Wilson SN, Surman OS, Colvin R, Ozonoff D, Gelenberg AJ, Jenike MA et al. Unusual lymphocyte subset distribution in some depressed patients. J Clin Psychiatry 1990; 51: 51–52.
Bartoloni C, Guidi L, Frasca D, Antico L, Pili R, Cursi F et al. Immune parameters in a population of institutionalized elderly subjects: influence of depressive disorders and endocrinological correlations. Mech Ageing Dev 1991; 60: 1–12.
Caldwell CL, Irwin M, Lohr J . Reduced natural killer cell cytotoxicity in depression but not in schizophrenia. Biol Psychiatry 1991; 30: 1131–1138.
Guidi L, Bartoloni C, Frasca D, Antico L, Pili R, Cursi F et al. Impairment of lymphocyte activities in depressed aged subjects. Mech Ageing Dev 1991; 60: 13–24.
Miller AH, Asnis GM, Lackner C, Halbreich U, Norin AJ . Depression, natural killer cell activity, and cortisol secretion. Biol Psychiatry 1991; 29: 878–886.
Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C et al. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med 1992; 22: 45–53.
Marazziti D, Ambrogi F, Vanacore R, Mignani V, Savino M, Palego L et al. Immune cell imbalance in major depressive and panic disorders. Neuropsychobiology 1992; 26: 23–26.
McAdams C, Leonard BE . Neutrophil and monocyte phagocytosis in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 1993; 17: 971–984.
Atanackovic D, Kroger H, Serke S, Deter HC . Immune parameters in patients with anxiety or depression during psychotherapy. J Affect Disord 2004; 81: 201–209.
Birmaher B, Rabin BS, Garcia MR, Jain U, Whiteside TL, Williamson DE et al. Cellular immunity in depressed, conduct disorder, and normal adolescents: role of adverse life events. J Am Acad Child Adolesc Psychiatry 1994; 33: 671–678.
Brown SL, Salive ME, Guralnik JM, Wallace RB, Ostfeld AM, Blazer D . Depressive symptoms in the elderly: association with total white blood cell count. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 849–860.
Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK et al. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry 2014; 4: e344.
Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA . Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 2007; 21: 229–237.
Duivis HE, Kupper N, Penninx BW, Na B, de Jonge P, Whooley MA . Depressive symptoms and white blood cell count in coronary heart disease patients: prospective findings from the Heart and Soul Study. Psychoneuroendocrinology 2013; 38: 479–487.
Frank MG, Wieseler Frank JL, Hendricks SE, Burke WJ, Johnson DR . Age at onset of major depressive disorder predicts reductions in NK cell number and activity. J Affect Disord 2002; 71: 159–167.
Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord 2009; 115: 177–182.
McGuire L, Kiecolt-Glaser JK, Glaser R . Depressive symptoms and lymphocyte proliferation in older adults. J Abnorm Psychol 2002; 111: 192–197.
Nakata A, Irie M, Takahashi M . Psychological distress, depressive symptoms, and cellular immunity among healthy individuals: a 1-year prospective study. Int J Psychophysiol 2011; 81: 191–197.
Park EJ, Lee JH, Chae JH, Lee KH, Han SI, Jeon YW . Natural Killer T cells in patients with major depressive disorder. Psychiatry Res 2006; 144: 237–239.
Schleifer SJ, Bartlett JA, Keller SE, Eckholdt HM, Shiflett SC, Delaney BR . Immunity in adolescents with major depression. J Am Acad Child Adolesc Psychiatry 2002; 41: 1054–1060.
Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H . Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr Scand 1996; 94: 198–204.
Steptoe A, Kunz-Ebrecht SR, Owen N . Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med 2003; 33: 667–674.
Tsuboi H, Kawamura N, Hori R, Kobayashi F, Iwasaki Y, Takeuchi H et al. Depressive symptoms and life satisfaction in elderly women are associated with natural killer cell number and cytotoxicity. Int J Behav Med 2005; 12: 236–243.
Surtees P, Wainwright N, Day N, Luben R, Brayne C, Khaw KT . Association of depression with peripheral leukocyte counts in EPIC-Norfolk—role of sex and cigarette smoking. J Psychosom Res 2003; 54: 303–306.
Panagiotakos DB, Pitsavos C, Chrysohoou C, Tsetsekou E, Papageorgiou C, Christodoulou G et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur Heart J 2004; 25: 492–499.
Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS . Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med 2010; 72: 626–635.
Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res 1992; 26: 125–134.
Herbert TB, Cohen S . Depression and immunity: a meta-analytic review. Psychol Bull 1993; 113: 472–486.
Stein M, Miller AH, Trestman RL . Depression, the immune system, and health and illness. Findings in search of meaning. Arch Gen Psychiatry 1991; 48: 171–177.
Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain behav Immun 2001; 15: 199–226.
Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB . Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis 2010; 20: 267–275.
Radloff L . The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385–401.
Ramos MI, Allen LH, Haan MN, Green R, Miller JW . Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr 2004; 80: 1024–1028.
Myers JK, Weissman MM . Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry 1980; 137: 1081–1084.
Nguyen HT, Kitner-Triolo M, Evans MK, Zonderman AB . Factorial invariance of the CES-D in low socioeconomic status African Americans compared with a nationally representative sample. Psychiatry Res 2004; 126: 177–187.
Morris DW, Trivedi MH, Rush AJ . Folate and unipolar depression. J Altern Complement Med 2008; 14: 277–285.
Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry 2004; 12: 631–638.
D'Anci KE, Rosenberg IH . Folate and brain function in the elderly. Curr Opin Clin Nutr Metab Care 2004; 7: 659–664.
Oishi J, Doi H, Kawakami N . Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama 2009; 63: 9–17.
Owen AJ, Batterham MJ, Probst YC, Grenyer BF, Tapsell LC . Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr 2005; 59: 304–306.
Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord 2000; 58: 241–246.
Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y et al. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition 2008; 24: 140–147.
Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D . Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr 2008; 62: 939–945.
Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P et al. Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol Aging 2005; 26: 987–994.
Beydoun MA, Tanaka T, Beydoun HA, Ding EL, Ferrucci L, Zonderman AB . Vitamin D receptor and megalin gene polymorphisms are associated with central adiposity status and changes among US adults. J Nutr Sci 2013; 2: e33.
Willet WC . Nutritional Epidemiology, 2nd edn. Oxford University Press: New York, NY, USA, 1998.
STATA. Statistics/Data Analysis: Release 14.0. Stata Corporation: College Station, TX, USA, 2015.
Blackwell E, de Leon CF, Miller GE . Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med 2006; 68: 870–878.
Beydoun MA, Gamaldo AA, Beydoun HA, Tanaka T, Tucker KL, Talegawkar SA et al. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. J Nutr 2014; 144: 890–901.
Beydoun MA, Beydoun HA, Dore GA, Fanelli-Kuczmarski MT, Evans MK, Zonderman AB . Total serum cholesterol, atherogenic indices and their longitudinal association with depressive symptoms among US adults. Transl Psychiatry 2015; 5: e518.
Heckman JJ . Sample selection bias as a specification error. Econometrica 1979; 47: 153–161.
Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM et al. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging 2010.
Selvin S . Statistical Analysis of Epidemiologic Data. 3rd edn. Oxford University Press: New York, NY, 2004.
Hochberg Y, Tamhane A C . Multiple Comparison Procedures. Wiley: New York, NY, USA, 1987.
Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Rostant OS, Evans MK, Zonderman AB . Associations of the ratios of n-3 to n-6 dietary fatty acids with longitudinal changes in depressive symptoms among US women. Am J Epidemiol 2015; 181: 691–705.
Gidron Y, Kupper N, Kwaijtaal M, Winter J, Denollet J . Vagus-brain communication in atherosclerosis-related inflammation: a neuroimmunomodulation perspective of CAD. Atherosclerosis 2007; 195: e1–e9.
Widmaier EP, Raff H, Strang K T . Vander's Human Physiology. McGraw-Hill: New York, NY, USA, 2011.
Ryder E, Diez-Ewald M, Mosquera J, Fernandez E, Pedreanez A, Vargas R et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab Syndr 2014; 8: 197–204.
Cavalcante-Silva LH, Galvao JG, da Silva JS, de Sales-Neto JM, Rodrigues-Mascarenhas S . Obesity-driven gut microbiota inflammatory pathways to metabolic syndrome. Front Physiol 2015; 6: 341.
Smith RS . The macrophage theory of depression. Med Hypotheses 1991; 35: 298–306.
Acknowledgements
This work was fully supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, NIA/NIH/IRP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author Contributions
MAB had full access to the data used in this manuscript and completed all the statistical analyses.
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Beydoun, M., Beydoun, H., Dore, G. et al. White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Transl Psychiatry 6, e895 (2016). https://doi.org/10.1038/tp.2016.180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.180
This article is cited by
-
Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women
BMC Women's Health (2021)
-
Inflammation predicts new onset of depression in men, but not in women within a prospective, representative community cohort
Scientific Reports (2021)
-
The BXD21/TyJ recombinant inbred strain as a model for innate inflammatory response in distinct brain regions
Scientific Reports (2020)
-
Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults
Translational Psychiatry (2017)