Abstract
The role of telomerase reverse transcriptase (TERT) has been extensively investigated in the contexts of aging and cancer. Interestingly, Tert−/− mice exhibit additional but unexpected aggressive and depressive behaviors, implying the potential involvement of TERT function in mood control. Our conditional rescue experiments revealed that the depressive and aggressive behaviors of Tert−/− mice originate from Tert deficiency in two distinct brain structures. Reactivation of Tert in the hippocampus was sufficient to normalize the depressive but not the aggressive behaviors of Tert−/− mice. Conversely, re-expression of Tert in the medial prefrontal cortex (mPFC) reversed the aggressive but not the depressive behavior of Tert−/− mice. Mechanistically, decreased serotonergic signaling and increased nitric oxide (NO) transmission in the hippocampus transduced Tert deficiency into depression as evidenced by our observation that the infusion of a pharmacological agonist for serotonin receptor 1a (5-HTR1A) and a selective antagonist for neuronal NO synthase into the hippocampus successfully normalized the depressive behavior of Tert−/− mice. In addition, increased serotonergic transmission by the 5-HTR1A agonist in the mPFC was sufficient to rescue the aggressive behavior of Tert−/− mice. Thus, our studies revealed a novel function of TERT in the pathology of depression and aggression in a brain structure-specific manner, providing direct evidence for the contribution of TERT to emotional control.
Similar content being viewed by others
Introduction
Telomerase is a ribonucleoprotein complex that adds 6-bp DNA repeat sequences to chromosome ends, which are referred to as telomeres, and thereby prevents the telomeres from shortening during mitosis.1, 2 Telomerase consists of two highly conserved protein and RNA components: telomerase reverse transcriptase (TERT) is the core catalytic protein component that contains a cellular reverse transcriptase domain, and the RNA component, Terc, serves as a template for telomere synthesis.3 Because DNA polymerase cannot replicate chromosomes completely to the DNA termini, telomeres shorten as cell division proceeds. Most post-mitotic cells have low to undetectable telomerase activity. This observation raised the possibility that telomere length functions as a 'molecular clock', where shortened telomeres accumulated over successive cell divisions limit the proliferative capacity of progenitors, progressively leading to cellular senescence.2, 4
Consistent with this hypothesis, Tert-deficient mice exhibited accelerated aging processes, and reactivation of telomerase was sufficient to reverse the age-related decline in many organs of Tert−/− mice.5, 6 Upregulated telomerase activity, however, has been found in many cancers, and overexpression of telomerase extended the lifespan of cells.7, 8, 9 These observations emphasize that telomerase activity is critical for the function of actively proliferating cells and thus should be tightly regulated;10 high levels of telomerase activity may lead to the unregulated proliferation observed in cancer, while low levels of telomerase activity may accelerate the cellular aging process.
In addition to the role of telomerase in cancer and aging, telomerase abnormalities in the hematological system have also been reported in patients with mood-related disorders. Decreased telomerase activity and accelerated telomere shortening in leukocytes has been identified in individuals with chronic stress,11 major depressive disorders12 and schizophrenia.13 Although animal studies are thus far limited, a potential involvement of telomerase in mood disorders has also been suggested. Elevated anxiety-like behaviors were observed in Tert-deficient mice.14 Our previous study also showed that systematic inhibition of telomerase activity induced depressive behavior in the mouse.15 However, the anatomical regions involved and molecular mechanisms that link Tert deficiency to psychiatric deficits have remained undetermined.
Here, using Tert−/− mice,16 we investigated how TERT regulates mood-related behavior. Our behavioral analyses uncovered a novel role of TERT in the control of aggressive and depressive behavior. Using a tissue-specific, conditional rescue strategy, we revealed that re-expression of Tert in the medial prefrontal cortex (mPFC) reversed the aggressive behaviors of Tert−/− mice, whereas reactivation of Tert in the hippocampus was sufficient to rescue the depressive behaviors of these mice. Molecularly, dysregulated transmission of serotonergic and nitric oxide (NO) signals in the hippocampus and mPFC was evident in Tert−/− mice. In our rescue experiments, re-expression of Tert in the hippocampus and mPFC normalized the altered expression levels of serotonin receptor 1a (5-HTR1A) and neuronal NO synthase (nNOS), which correlated with the recovery of the respective depressive and aggressive behaviors of Tert−/− mice. Moreover, pharmacological activation of 5-HTR1A or inhibition of neuronal nNOS in the hippocampus successfully normalized the depressive behavior of Tert−/− mice, while treatment with a 5-HTR1A agonist in the mPFC rescued the aggressive behavior of Tert−/− mice. Thus, our study clearly demonstrated that the mPFC and hippocampus are two distinct brain areas that transduce Tert deficiency into aggression and depression and that 5-HTR1A and nNOS mediate the effect of Tert on emotional stability.
Materials and methods
Mice
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and Nanjing Medical University. Mice were housed in a temperature- and humidity-controlled environment with an alternating 12-h light/dark cycle.
The production of mTERT knockout mice (Tert−/−) and the lack of telomerase activity in these mice were previously described.16 Tert−/−mice were backcrossed to FVB/N mice to produce mice heterozygous for Tert. Tert+/− mice were intercrossed to produce the first generation of Tert−/− mice (F1). All subjects in the experiments were from the fourth or fifth generation after intercrossing F1 Tert−/− homozygotes. Two- to 3-month-old male mice were used for behavioral tests.
Construction of LV-mTert–EGFP
mTert complementary DNA was prepared by reverse transcription–PCR from an mRNA library of mouse embryonic stem cells. The primer sequences were as follows: forward, 5′-GTAGAACGCAGATCGAATTCATGACCCGCGCTCCTCG-3′; reverse, 5′-CCCTTGCTCACCATGAATTCGTCCAAAATGGTCTGAAAGTC-3′. The PCR fragment corresponding to Tert complementary DNA was digested with BamHI and AgeI and ligated into downstream of a ubiquitous Ubi promoter in a lentiviral vector (Genechem company, Shanghai, China). A reporter gene expressing a enhanced green fluorescent protein (EGFP) was also expressed under the control of the SV40 promoter (pUbi-mTert-pSV40–EGFP). The production of lentivirus (LV) was previously described.17 Briefly, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), 20 μg of pUbi-mTert-pSV40–EGFP, 10 μg of pCMV-VSVG (vesicular stomatitis virus glycoprotein), 7.5 μg of pRSV-REV (lentiviral packaging plasmid) and 3.5 μg of pMDLg/pRRE (lentiviral packaging plasmid) were cotransfected into 293 T cells. After 48 h, the supernatant was harvested from the 293 T cells, filtered at 0.45 μm and pelleted by ultracentrifugation at 18 000g for 2 h at 4 °C (~2 × 109 transducing units per ml). A LV expressing EGFP alone (LV–EGFP) was also produced and used as a control.
Stereotactic injection
The detailed procedures regarding stereotactic surgery and injection were previously described.17 Briefly, adult mice were anesthetized with a mixture of ketamine (100 mg kg−1, ACE Surgical Supply, Brockton, MA, USA) and xylazine (10 mg kg−1, Sigma-Aldrich, St Louis, MO, USA) and placed in a stereotactic apparatus (David Kopf Instruments, Tujunga, CA, USA). LVs or drugs such as 7-nitroindazole (7-NI, 10 μm, Sigma-Aldrich) or 8-hydroxy-2-dipropylaminotetralin hydrobromide (8-OH-DPAT, 5 μm, Sigma-Aldrich) were stereotactically delivered into both sides of the dentate gyrus (DG) of the hippocampus (2 μl; coordinates: 2.3 mm posterior to the bregma, 1.35 mm lateral to the midline and 2.3 mm below the dura)15 or the mPFC region (2 μl; coordinates: 1.8 mm anterior to the bregma, 0.8 mm lateral to the midline and 1 mm below the dura).18 The mice were recovered on a hot pad (37 °C) and returned back to their home cages.
Western analysis
Procedures for western analysis were previously described.19 The hippocampus and mPFC were homogenized in sample buffer containing 200 mm Tris-buffered saline, 4% SDS, 20% glycerol and 10% 2-mercaptoethanol, and denatured by boiling for 5 min. Primary antibodies used were as follows: nNOS (rabbit, 1:200; Zymed Laboratories, San Francisco, CA, USA), 5-HTR1A (rabbit, 1:100; Bioss, Bejing, China), GAPDH (rabbit, 1:2000; Sigma, St. Louis, MO, USA) and FLAG (rabbit, 1:500; Enzo, Farmingdale, NY, USA). Horseradish peroxidase-conjugated goat anti-rabbit antibody was used as a secondary antibody. A volume of 10 μl lysate containing 10 μg protein was loaded for each sample. Enhanced chemiluminescence (Pierce, Carlsbad, CA, USA) was used to detect the signals.
Behavioral measures
Resident–intruder paradigm
An adult male nNOS−/− mouse, a strain that has been reported to show elevated aggressive behavior, was used as an intruder.20 An intruder mouse was transferred into the residents’ cage (four males per cage), and attack episodes by the resident mice were recorded for 15 min. The intruder was typically attacked and defeated by the residents and showed freezing submissive behavior. Aggressive behaviors of the resident mice were scored when they bit, attacked laterally and displayed piloerection. The latency to the first attack bite, the frequency of bites and the duration of attack episodes were manually quantified. Data collected from four wild-type (WT) or Tert −/− mice in the same resident cage were analyzed as a unit.
Offensive aggressive behavior test
Four adult WT or Tert−/− mice were simultaneously introduced into a clear glass aquarium (38.5 × 26.5 × 30.7 cm). The latency to the first biting attack, the total number of attack bites and the total duration of attack episodes during 15 min were recorded and analyzed. Data collected from four WT or Tert −/− mice in the same resident cage were analyzed as a unit.20
Tail suspension test
A mouse was suspended from its tail for 6 min in a tail suspension chamber (Hamilton Kinder TS100, Kinder Scientific, Poway, CA, USA).19 The frequency and duration of escape behaviors were recorded during tail suspendsion test (TST). Immobility, which was defined as the cessation of all movements during a 6-min test period, was calculated to measure depressive status. Data from the mice that dropped from the hook, climbed up to the hook and struggled dramatically the entire time were excluded.
Forced swim test
Mice were individually forced to swim in an open cylindrical container (diameter, 28 cm; height, 33 cm) containing 20 cm of water (25±1 °C), and the duration of immobility during a 6-min period was measured.21 Immobility was scored when a mouse ceased struggling and remained floating motionlessly. Water was replaced between trials. Following swim sessions, mice were placed underneath a heating lamp for ~30 min before they were returned to their home cages. Forced swim test (FST) sessions were videotaped (Motor-Monitor System SF16R, Kinder Scientific), and immobility was measured in a double-blinded manner. Data from the mice that sunk into the water, climbed up to the container or floated the entire time were excluded.
Sucrose preference test
The sucrose preference test was performed as previously described.22 Food and water were removed from cages for 20 h. Two bottles containing water or 1% (W/V) sucrose water were presented in the cage simultaneously, and animals had free access to these bottles for the next 10 h. Sucrose preference was scored as the ratio of the intake of 1% sucrose water to total water intake, which included that of both 1% sucrose water and regular water.
Elevated maze test
The apparatus for the elevated maze test (EMT) consisted of two open arms (30 × 5 cm), two enclosed arms (30 × 5 cm, with the end and side walls 15 cm high) and a connecting central platform (5 × 5 cm). The maze was raised 38.5 cm above the floor. Each mouse was placed on the central platform facing an open arm and allowed to freely explore for 5 min. An arm entry was scored when all four legs completely entered the maze arm. Data from the mice that dropped from the maze and stayed in one place the entire time were excluded.
Light–dark test
The dark–light box contained two plastic chambers connected by a small tunnel. A dark chamber (20 × 15 cm) was covered, and the walls of the light chamber (30 cm × 15 cm) were transparent and illuminated by a light located above (600 lx). A mouse was placed in the dark compartment, and the latency to the first exit, the number of exits from the dark chamber and the total time spent in the light compartment were recorded for 5 min.
Open field test
The test arena consisted of a bottom plastic plate (56.13–56.13 cm) and four surrounding plastic walls (35.18 cm high). The bottom plate was divided into 256 squares, and each square was marked with visible lines. A mouse was placed on a corner square of the arena, facing the corner and allowed to freely explore the open field for 5 min. The arena was cleaned with 70% EtOH between trials. Mobility was scored when a mouse crossed a border with both hind limbs. The number of square crossings and the frequency of standing during 5 min were measured.
NO measurement
NO content in the hippocampus or prefrontal cortex was determined as previously described.22 The samples were homogenized in 10 vol of deionized water and centrifuged at 1000g for 15 min at 4 °C. NOx content was measured in the supernatants using a commercially available kit (Jiancheng Bioengineering, Nanjing, China) and is expressed as nmol per mg protein.
5-HT ELISA
The concentration of serotonin in the plasma was determined following the manufacturer’s instructions (Serotonin ELISA Kit, Abcam, Cambridge, UK). Each test measured samples in triplicate, and a standard curve was plotted each time to ensure the validity of the assay.
Immunohistochemistry
The mice were anesthetized with a mixture of ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and perfused transcardially with saline followed by 4% paraformaldehyde. Brains were removed and postfixed overnight in the same solution. To identify the cell types, labeling was carried out on 40-μm free-floating sections as described. Primary antibodies were as follows: nNOS (rabbit, 1:200), 5-HTR1A (rabbit 1:100), NeuN (mice, 1:200; Millipore, Billerica, MA, USA) and GFAP (chicken, 1:1000; Abcam, Cambridge, MA, USA). They were prepared in 0.1 m PBS with 3% goat serum and 0.3% Triton X-100, and visualized with a Cy3-conjugated secondary antibody (1:200; Thermo Fisher Scientific, Waltham, MA, USA). Nuclei were visualized with 4′-6-diaminodino-2-phenylindole (DAPI, Sigma-Aldrich). Every twelfth section throughout the hippocampus was processed for nNOS or 5-HTR1A immunohistochemistry and counting. Two sections containing mPFC were processed for analysis of nNOS or 5-HTR1A in the mPFC.
Statistics
Comparisons among multiple groups were performed using one-way analysis of variance followed by Scheffe’s post hoc test. Comparisons between two groups were performed with the two-tailed Student’s t-test. Data are presented as mean±s.e.m.; P<0.05 was considered statistically significant. Detailed statistical analyses regarding the experiments performed in Figures 2 and 4 are described in Supplementary Tables 1 and 2. This study complies with randomization. The investigator was blinded to the group allocation during the experiment and/or when assessing the outcome.
Results
Aggressive and depressive behaviors in Tert−/− mice
To investigate the potential relationship between telomerase and mood disorders, Tert−/− mice in which telomerase activity is deficient were examined.16 Tert−/− mice exhibited bite wounds and scruffy coats, suggesting the presence of aggressive behavior and depression in these mice (Figure 1a).23
Aggressive and depressive phenotype of Tert−/− mice. (a) A representative photo of subordinate Tert−/− mice. The blue arrow indicates areas of the coat where the animal has been bitten. The red arrow indicates scruffy areas of poor coat quality. (b) Top panel (offensive aggressive behavior): the latency to the first biting attack (left), the total number of attacks (middle) and the total duration of attack episodes (right) are presented. Bottom panel (resident–intruder paradigm): the latency to the first biting attack (left), the total number of attacks (middle) and the total duration of attack episodes initiated by the resident (right) are presented. (c) Immobility time in the TST and the FST and sucrose preference in the SPT showed the depressive behavior of Tert−/− mice. In addition, the total time of mouse entry into the open arms in the EMT and the time spent in the light box in the LDT were examined. The locomotion of mice was recorded in the OPT. n=20. Data represent the mean±s.e.m. *P<0.05; **P<0.01; and ***P<0.001 by two-way t-test compared with WT mice. EMT, elevated maze test; FST, forced swim test; LDT, light–dark test; SPT, sucrose preference test; TST, tail suspension test; WT, wild type.
In an offensive aggressive behavior test,20 Tert−/− mice exhibited a significantly shorter latency to the first biting attack, increased attack frequency and a longer total duration of attack episodes compared with WT mice (P=0.0035, P=0.0048 and P=0.0018, respectively, by t-test; n=20 for each group; Figure 1b). The resident–intruder paradigm test, a standard test used to measure aggressive behavior in rodents, also confirmed the aggressive phenotype of Tert−/− mice.20, 24 Consistent with the results of the offensive aggression test, in the resident–intruder paradigm test Tert−/− mice showed aggressive behavior, as supported by a significantly shorter latency to the first biting attack, a higher number of attacks and a longer total attack time (P=0.0094, P=0.0002 and P=0.0001, respectively, by t-test; n=30 for each group; Figure 1b).
We used a series of behavioral tests to measure different endophenotypes of depression: behavioral despair, anhedonia and anxiety-related behaviors. To evaluate behavioral despair, the TST and the FST were used.15, 22 Compared with WT mice, Tert−/− mice exhibited a significantly prolonged immobility time in the TST (P=0.0096, t-test; WT, n=20; Tert−/− mice, n=17) and the FST (P=0.0027, t-test; WT, n=20; Tert−/− mice, n=18; Figure 1c), revealing that Tert−/− mice quickly abandoned attempts to escape from an adverse stimulus and thus exhibited depressive-like symptoms. Tert−/− mice also showed decreased sucrose preference, suggesting a reduced ability to experience pleasure (P=0.0162, t-test; WT, n=18; Tert−/− mice, n=13) (Figure 1c). We also measured anxiety-related behavior by performing an EMT and a light–dark test.25, 26 Tert−/− mice showed decreased entry time in the open arm in the EMT (P=0.0177, t-test; WT, n=19; Tert−/− mice, n=17) and in the light box in the light-dark test (P=0.0003, t-test; WT, n=18; Tert−/− mice, n=16), indicating that Tert−/− mice displayed elevated anxiety compared with WT mice (Figure 1c). However, the locomotor ability of Tert−/− mice did not change (Figure 1c, P=0.2783, t-test; WT, n=20; Tert−/− mice, n=20), suggesting that behavioral changes were not due to altered motor functions.
Re-expression of Tert in the hippocampus and mPFC rescued depression and aggression in Tert−/− mice
Both the hippocampus and mPFC play a critical role in depressive and aggressive behaviors.27, 28 We tested whether re-expression of Tert in the hippocampus and mPFC was able to rescue the depression and aggression of Tert−/− mice (Figure 2a). To achieve this goal, LV expressing a full-length mouse Tert complementary DNA and an EGFP reporter gene (LV-mTert–EGFP) was injected into both sides of either the DG of the hippocampus or the mPFC of Tert−/− mice (Tert−/−/LV-mTert–EGFP). As a control, LV expressing only EGFP (but not mTert) was injected into WT (WT/LV–EGFP) and Tert−/− (Tert−/−/LV–EGFP) mice. Twenty eight days later, the mice were subjected to behavioral tests (Figure 2) and molecular analyses (Figure 3).
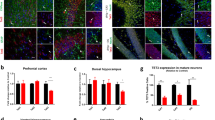
Rescue of aggression and depression in Tert−/− mice by re-expression of Tert in the mPFC and hippocampus. (a) Experimental scheme. (b) Resident–intruder paradigm: the latency to the first attack bite (left), the total number of attacks (middle) and the total duration of attack episodes initiated by the resident (right) showed that aggressive behaviors were not affected by Tert re-expression in the DG. (c) Immobility time in the TST and the FST and the total time spent in the open arms in the EMT showed the rescue of the depressive phenotype of Tert−/− mice by re-expression of Tert in the DG of the hippocampus. (d) Resident–intruder paradigm: the latency to the first attack bite (left), the total number of attacks (middle) and the total duration of attack episodes initiated by the resident (right) showed the reversal of aggressive behavior in Tert−/− mice by re-expression of Tert in the mPFC. (e) Immobility time in the TST and the FST and the total time spent in the open arms of the EMT showed no effect of Tert re-expression in the mPFC on the depressive behavior of Tert−/− mice. (f, g) The efficient transgene expression and viral coverage in the hippocampus and mPFC are shown by EGFP IHC (left), counting of EGFP-positive cells (middle) and western blot (right). ''n'' in each group is listed in Supplementary Table 1. Data represent the mean±s.e.m. One-way ANOVA, *P<0.05; **P<0.01; and ***P<0.001 compared with WT mice; #P<0.05; ##P<0.01; and ###P<0.01 compared with Tert −/−/LV–GFP mice; NS indicates no significance compared with Tert −/−/LV–GFP. ANOVA, analysis of variance; DG, dentate gyrus; EGFP, enhanced green fluorescent protein; EMT, elevated maze test; FST, forced swim test; IHC, immunohistochemistry; mPFC, medial prefrontal cortex.
TERT regulates the expression of 5-HTR1A and nNOS in the mPFC and hippocampus. (a) Western blot showing the re-expression of TERT normalized the expression levels of 5-HTR1A and nNOS in the hippocampus of Tert−/− mice. n=4. (b) IHC showed that the expression patterns of 5-HTR1A and nNOS in the hippocampus of Tert−/− mice were rescued by re-expression of TERT. (c, d) Measurement of signal intensity and quantitative analyses show the normalization of 5-HTR1A and nNOS expression in the hippocampus of Tert−/− mice by TERT re-expression. n=3. (e) Western blot showing that the re-expression of TERT normalized the expression level of 5-HTR1A in the mPFC of Tert−/− mice. n=4. (f) IHC showed that the expression pattern of 5-HTR1A in the mPFC of Tert−/− mice was rescued by re-expression of TERT. (g, h) Measurement of signal intensity and quantitative analyses show the normalization of 5-HTR1A and nNOS expression in the mPFC of Tert−/− mice by TERT re-expression. n=3. Data represent the mean±s.e.m. One-way ANOVA, **P<0.01 compared with WT; LV–EGFP mice; ##P<0.01 compared with Tert−/−; LV–EGFP mice. ANOVA, analysis of variance; DG, dentate gyrus; EGFP, enhanced green fluorescent protein; IHC, immunohistochemistry; LV, lentivirus; mPFC, medial prefrontal cortex; TERT, telomerase reverse transcriptase.
Tert expression in the DG of the hippocampus efficiently rescued the depressive but not the aggressive behavior of Tert−/− mice (Figures 2b and c). Re-expression of Tert in the hippocampus reversed the immobility time of Tert−/− mice in the TST and the FST and also rescued the entry time in the open arm of the EMT in a statistically significant manner (Figure 2c, Supplementary Table 1). However, the latency to the first biting attack, the number of attack episodes and the total time of attack episodes remained unchanged when evaluated both by the offensive aggressive behavior test (Supplementary Figure 1a) and by the resident–intruder paradigm test (Figure 2b, Supplementary Table 1).
Tert expression in the mPFC successfully rescued the aggressive phenotypes of Tert−/− mice. Re-expression of Tert in the mPFC restored the latency to the first biting attack, reduced the number of total attacks and decreased the total time of attack episodes during the resident–intruder paradigm test (Figure 2d, Supplementary Table 1) and during the offensive aggressive behavior test (Supplementary Figure 1b). However, re-expressing Tert in the mPFC did not reverse the depressive phenotype of Tert−/− mice. The immobility time in the TST and FST and the entry time in the open arm in the EMT did not change in Tert−/− mice (Figure 2e, Supplementary Table 1). Together, these data indicated that Tert plays a critical role in the hippocampus in the control of depressive behavior, while Tert expression in the mPFC controls aggressive behavior.
Using an EGFP reporter gene present in both the control and mTert-expressing lentiviral vectors, the efficiency and coverage of viral infection were examined. Western analysis of virus-infected brains showed that an equivalent amount of transgene was transduced by both the control and mTert-expressing LVs and that TERT was efficiently expressed (Figures 2f and g, Supplementary Figure 2). Histological analyses of EGFP-expressing cells revealed the efficient infection of both control and mTert-expressing virus into the DG of the hippocampus or mPFC. Infected virus spread throughout the target structures, covering a large volume of the DG or mPFC (Supplementary Figure 3). Importantly, the number of cells infected by control or mTert-expressing LV was comparable among the experimental groups, suggesting that the quality and injection of virus into the target areas were consistent across animal groups (Figures 2f and g).
Expression of 5-HTR1A and nNOS is regulated by TERT
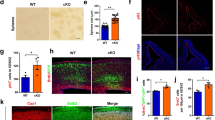
It has been shown that serotonin (5-hydroxytryptamine (5-HT)) and NO play a critical role in depression and aggression.19, 22, 29, 30 Several key components necessary for 5-HT and NO signaling are expressed in the hippocampus, as well as in the mPFC. Among the different types of 5-HT receptors, 5-HTR1A has been implicated in depression and aggression.31, 32, 33, 34 5-HTR1A is expressed in neurons located in the DG of the hippocampus, as well as in the mPFC (Supplementary Figures 4a and b; Figures 3b and g). nNOS, a key enzyme essential for the production of NO, is also expressed in the hippocampus and mPFC (Supplementary Figures 4c–d). Interestingly, the expression of nNOS in the hippocampus is restricted to neurons in the hilus (Supplementary Figure 4c; Figure 3b). To determine whether serotonin or NO signaling is altered in Tert−/− mice and whether tissue-specific reactivation of TERT restores altered serotonin or NO transmission, we examined the level of 5-HT and its cognate receptor, 5-HTR1A, as well as NO and nNOS in the hippocampus and mPFC of WT (WT/LV–EGFP), Tert knockout (Tert−/−/LV–EGFP) and Tert rescue (Tert−/−/LV -mTert–EGFP) mice as described in Figure 2a.
In the hippocampus, transmission of both serotonergic and NO was disrupted in Tert−/− mice. While the concentrations of 5-HT did not change (Supplementary Figure 5a), the amount of 5-HTR1A expressed in Tert−/− mice was significantly reduced (Figure 3a). Subsequent immunohistochemistry confirmed the decreased expression of 5-HTR1A in neurons located in the DG of the hippocampus in Tert−/− mice (Figures 3b and c). In contrast to the decreased serotonergic transmission, NO transmission increased in Tert−/− mice. In Tert−/− mice, the level of NO (Supplementary Figure 5b), the expression of nNOS protein, and the number of nNOS-expressing neurons significantly increased in the hilus of the hippocampus (Figures 3a, b and d). Surprisingly, re-expressing Tert in the hippocampus of Tert−/− mice (Tert−/−/LV-mTert–EGFP) reversed the altered expression of 5-HTR1A and nNOS to normal levels compared with WT mice (WT/LV–EGFP; Figures 3a–d).
In the mPFC, only serotonergic transmission was impaired in Tert−/− mice. Consistent with unchanged concentrations of both 5-HT and NO (Supplementary Figure 5b), the level of nNOS protein and the number of nNOS-expressing neurons was not altered in the mPFC of Tert−/− mice (Figures 3e, f and h). However, the expression of 5-HTR1A was significantly reduced, as determined by western and immunohistochemistry analyses (Figures 3e, f and g). In the brains of Tert−/− mice that received LV-mTert–EGFP, reactivation of TERT in the mPFC normalized the altered expression of 5-HTR1A, while the number of nNOS-expressing cells remained unchanged. These data collectively showed that the expression of 5-HTR1A and nNOS was disrupted in the brains of Tert−/− mice and that reactivation of TERT in the brains of Tert−/− mice normalized this altered expression, with concomitant recovery of the aggressive and depressive behavioral phenotype of Tert−/− mice.
Pharmacological normalization of 5-HT and NO transmission in the DG and mPFC rescues the behavioral abnormalities of Tert−/− mice
To determine the role of the altered levels of 5-HTR1A and nNOS in the behavioral abnormalities of Tert−/− mice, we selectively increased or decreased the activity of 5-HTR1A and nNOS using the pharmacological drugs 8-OH-DPAT, a 5-HTR1A-selective agonist,25 or 7-NI, a selective nNOS antagonist.22 First, we administered 8-OH-DPAT (5 μm, 2 μl) or 7-NI (10 μm, 2 μl) into the DG bilaterally and performed behavioral tests 28 days later (Figure 4a). WT mice infused with DMSO were used as a control. The infusion of 8-OH-DPAT or 7-NI into the hippocampus significantly reduced immobility time in the TST and the FST and also increased entry time in the open arm in the EMT, indicating that either 5-HTR1A activation or nNOS inhibition in the hippocampus rescued the depressive phenotype of Tert−/− mice (Figure 4c, Supplementary Table 2). However, 8-OH-DPAT or 7-NI infusion into the hippocampus did not affect aggressive behaviors, as shown by the resident–intruder paradigm test (Figure 4b, Supplementary Table 2). In Tert−/− mice infused with 8-OH-DPAT or 7-NI, the latency to the first biting attack, the number of attacks and the total time of attack episodes did not change (Figure 4b, Supplementary Table 2).
5-HTR1A and nNOS in the mPFC and hippocampus underlie the phenotype of Tert−/− mice. (a) Diagram describing the design of the experiment. (b) Resident–intruder paradigm: the latency to the first attack bite (left), the total number of attacks (middle, and the total duration of attack episodes initiated by the resident (right) showed no effect of 7-NI or 8-OH-DPAT infusion in the DG of the hippocampus on aggression in Tert−/− mice. (c) Immobility time in the TST and FST and the total time spent in the open arms in the EMT showed the rescue of the depressive behavior of Tert−/− mice by infusion of 7-NI or 8-OH-DPAT into the DG. (d) Resident–intruder paradigm: the latency to the first attack bite (left), the total number of attacks (middle) and the total duration of attack episodes initiated by the resident (right) revealed the normalization of aggressive behavior of Tert−/− mice by the infusion of 8-OH-DPAT into the mPFC. (e) Immobility time in the TST and the FST and the total time spent in the open arms in the EMT showed no effect of 7-NI or 8-OH-DPAT in the mPFC on depression in Tert−/− mice. 'n' in each group is listed in Supplementary Table 2. Data represent the mean±s.e.m. One-way ANOVA, *P<0.05; **P<0.01; and ***P<0.001 compared with WT/DMSO; ##P<0.01; ###P<0.001 compared with Tert −/−/DMSO; NS indicates no significance compared with Tert −/−/DMSO. ANOVA, analysis of variance; DG, dentate gyrus; EMT, elevated maze test; FST, forced swim test; mPFC, medial prefrontal cortex; TST, tail suspension test.
Next, we infused 7-NI (10 μm, 2 μl) or 8-OH-DPAT (5 μm, 2 μl) into the mPFC and examined the behavioral changes of Tert−/− mice (Figures 4d and e). Interestingly, only infusion of 8-OH-DPAT, but not infusion of 7-NI, normalized the aggressive phenotype of Tert−/− mice (Figure 4d, Supplementary Table 2), showing a significantly decreased latency to the first attack, a reduced number attacks and a shortened total time of attacks (Figure 4d, Supplementary Table 2). However, infusion into the mPFC with either 8-OH-DPAT or 7-NI did not affect the depressive behaviors of Tert−/− mice (Figure 4e, Supplementary Table 2), as assessed by the TST, the FST and the EMT. These results showed that in the hippocampus, increased activation of 5-HTR1A and decreased activity of nNOS successfully restored the depressive behavior of Tert−/− mice, and that in the mPFC treatment with a 5-HTR1A agonist rescued the aggressive behavior of Tert−/− mice.
Discussion
The recent observations that leukocyte telomere shortening is associated with mental illness suggested the potential involvement of dysregulated telomerase function in the pathology of psychiatric disorders.11, 35, 36, 37, 38 However, direct evidence supporting the relation between telomerase function and mood disorders has remained absent. In this report, using mice deficient for Tert, a key enzyme for the maintenance of telomere length, we demonstrated the requirement of mouse telomerase for mood stability.
The function of TERT has been studied in the contexts of aging and cancer. However, we found that Tert−/− mice exhibited additional but unexpected aggressive and depressive behavioral phenotypes. Our LV-mediated rescue experiments revealed that Tert controls aggression and depression by acting in different areas of the brain (Figure 5). Reactivation of Tert in the hippocampus was sufficient to normalize the depressive behavior of Tert−/− mice, while the same treatment did not alter the aggressive behavior of Tert−/− mice. Conversely, re-expression of Tert in the mPFC rescued the aggressive phenotype of Tert−/− mice; however, the depressive phenotype of these mice was not affected. Thus, our study revealed a novel role of Tert in emotional control in a brain region-specific manner; the depressive and aggressive behaviors observed in Tert−/− mice are anatomically distinct modalities, and Tert plays a critical role in depressive and aggressive behavior by acting in the hippocampus and mPFC, respectively (Figure 5).
A model illustrating a molecular mechanism underlying the mood-related behaviors of Tert−/− mice. A schematic model indicates that decreased serotonergic and/or increased NO transmission in the hippocampus account for the depressive behaviors of Tert −/−mice and that decreased serotonergic signals in the mPFC lead to aggressive behaviors Tert −/−mice. DG, dentate gyrus; mPFC, medial prefrontal cortex; NO, nitric oxide.
In the hippocampus, the link between serotonergic and NO transmission and depression has been well-established.22, 25 Knockout mice lacking 5-HTR1A showed anxiety/depressive behaviors, while conditional expression of 5-HTR1A in the hippocampus and cortex successfully rescued the anxiety/depressive behavioral phenotypes of 5-HTR1A knockout mice.39 The requirement of hippocampal neurogenesis for the function of SSRI (selective serotonin reuptake inhibitor)-type antidepressants further supports the critical role of the hippocampus in the control of depressive behavior.40 In previous studies, we showed that increased nNOS expression in the hippocampus induced depressive behaviors, whereas a decreased level of nNOS functions as an antidepressant.19, 22 In the mPFC, serotonergic signaling has been also implicated in aggressive behaviors. Aggressive behaviors were shown to be associated with decreased 5-HT in the mPFC,41 whereas activation of 5-HTR1A in the mPFC reduced aggressive behavior.42, 43, 44 These results collectively suggested that decreased serotonergic and/or increased NO transmission in the hippocampus is sufficient to induce depressive behaviors and that decreased serotonergic signals in the mPFC lead to aggressive behaviors.
Our tissue-specific rescue experiments, as well as pharmacological epistasis studies demonstrated that 5-HTR1A and nNOS are downstream effector molecules that mediate Tert-dependent emotional behavior. In Tert−/− mice, 5-HTR1A expression was decreased in both the hippocampus and mPFC, while nNOS expression was increased in the hippocampus, raising the possibility that disrupted serotonergic and NO signals may transduce the effect of Tert deficiency on depression and aggression. Consistent with this hypothesis, our LV-mediated rescue experiments showed that re-expression of Tert in the hippocampus or mPFC normalized the altered expression of 5-HTR1A and/or nNOS, which was associated with the recovery of depressive and aggressive behaviors of Tert−/− mice. Moreover, epistatic analyses performed with pharmacological reagents that normalize the altered activity of 5-HTR1A or nNOS in Tert−/− mice strongly suggested that 5-HTR1A and/or nNOS are downstream effector molecules that transduce TERT function to emotion. This pharmacological normalization of serotonergic and NO signaling reversed the behavioral deficits of Tert−/− mice in a brain region-specific manner. Increased serotonergic transmission by infusion of a 5-HTR1A agonist, 8-OH-DPAT, or decreased NO signaling by infusion of an nNOS antagonist, 7-NI, into the hippocampus successfully reversed the depressive phenotype of Tert−/− mice. In addition, 8-OH-DPAT infusion into the mPFC was sufficient to normalize the aggressive behavior of Tert−/− mice. The combination of our tissue-specific rescue experiments and epistatic analyses using pharmacological reagents clearly demonstrated that Tert in the hippocampus and mPFC modulates depressive and aggressive behaviors through the serotonergic and NO pathways, respectively (Figure 5).
While our studies showed that serotonergic and/or NO signaling transduces the effect of TERT on mood control, whether TERT regulates emotion directly or indirectly is still speculative. It is possible that depression and aggression may be due to the accelerated cellular aging of Tert−/− mice.45 Psychiatric alterations such as depression and aggression are known to be associated with Alzheimer’s disease, an aging-dependent disorder.46 The correlation between telomere shortening and age-dependent processes has been well-established.2 Telomeres are a critical structure for the maintenance of genetic integrity by preventing chromosome ends from degrading. Because DNA polymerase fails to fully synthesize DNA terminal sequences (containing telomeres), the length of telomeres progressively shortens with successive cell divisions. To a certain extent, Tert, a cellular reverse transcriptase, has the ability to compensate for telomere loss by adding new telomere sequences onto chromosome ends; however, this is not sufficient to maintain telomere length as evidenced by the telomere shortening that occurs with age. Thus, the failure of telomere maintenance in Tert−/− mice may accelerate the aging process, which in turn induces age-associated aggressive and depressive behaviors. Thus, our results can be interpreted to suggest that reactivation of TERT in the brain rescued the emotional deficits of Tert−/− mice by reversing or slowing aging-associated processes.5, 47, 48 Alternatively, Tert may regulate emotional behavior in a telomere length- and elongation-independent manner.49 Recent studies proposed a non-canonical function for Tert as a transcriptional regulator. For example, it has been shown that Tert interacts with BRG1 in the β-catenin transcriptional complex and occupies the promoters of WNT-dependent genes, suggesting that Tert can act as a transcription factor.50 This observation raises the possibility that Tert may directly control the expression level of 5-HTR1A and/or nNOS. Future studies will dissect the direct molecular mechanism by which Tert controls emotional behavior.
References
Lundblad V, Wright WE . Telomeres and telomerase: a simple picture becomes complex. Cell 1996; 87: 369–375.
Armanios M, Blackburn EH . The telomere syndromes. Nat Rev Genet 2012; 13: 693–704.
Autexier C, Lue NF . The structure and function of telomerase reverse transcriptase. Annu Rev Biochem 2006; 75: 493–517.
Blackburn EH . Switching and signaling at the telomere. Cell 2001; 106: 661–673.
Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011; 469: 102–106.
Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 1999; 96: 701–712.
Harley CB . Telomerase and cancer therapeutics. Nat Rev Cancer 2008; 8: 167–179.
Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008; 9: 1048–1057.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–2015.
Lee HW, Blasco MA, Gottlieb GJ, Horner JW 2nd, Greider CW, DePinho RA . Essential role of mouse telomerase in highly proliferative organs. Nature 1998; 392: 569–574.
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 2004; 101: 17312–17315.
Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry 2006; 60: 432–435.
Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry 2008; 13: 118–119.
Lee J, Jo YS, Sung YH, Hwang IK, Kim H, Kim SY et al. Telomerase deficiency affects normal brain functions in mice. Neurochem Res 2010; 35: 211–218.
Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci 2011; 31: 12258–12269.
Yuan XM, Ishibashi S, Hatakeyama S, Saito M, Nakayama J, Nikaido R et al. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes Cells 1999; 4: 563–572.
Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH . In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007; 1: 515–528.
Fejgin K, Palsson E, Wass C, Svensson L, Klamer D . Nitric oxide signaling in the medial prefrontal cortex is involved in the biochemical and behavioral effects of phencyclidine. Neuropsychopharmacology 2008; 33: 1874–1883.
Zhou QG, Zhu LJ, Chen C, Wu HY, Luo CX, Chang L et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci 2011; 31: 7579–7590.
Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 1995; 378: 383–386.
Kaster MP, Ferreira PK, Santos AR, Rodrigues AL . Effects of potassium channel inhibitors in the forced swimming test: possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res 2005; 165: 204–209.
Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 2007; 103: 1843–1854.
Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P . Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 2004; 9: 278–286.
Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ . The resident–intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp 2013; 77: e4367.
Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y et al. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J Neurosci 2010; 30: 2433–2441.
Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA 2002; 99: 6370–6375.
Russo SJ, Nestler EJ . The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14: 609–625.
Drevets WC, Price JL, Furey ML . Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213: 93–118.
Dhir A, Kulkarni SK . Nitric oxide and major depression. Nitric Oxide 2011; 24: 125–131.
Berton O, Nestler EJ . New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 2006; 7: 137–151.
Savitz J, Lucki I, Drevets WC . 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol 2009; 88: 17–31.
Alekseyenko OV, Kravitz EA . Serotonin and the search for the anatomical substrate of aggression. Fly 2014; 8: 200–205.
Popova NK, Naumenko VS . 5-HT1A receptor as a key player in the brain 5-HT system. Rev Neurosci 2013; 24: 191–204.
Popova NK, Naumenko VS, Plyusnina IZ . Involvement of brain serotonin 5-HT1A receptors in genetic predisposition to aggressive behavior. Neurosci Behav Physiol 2007; 37: 631–635.
Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol 2015; 66: 17–20.
Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JYY et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry 2012; 17: 719–727.
Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci USA 2011; 108: E513–E518.
Wei YB, Backlund L, Wegener G, Mathe AA, Lavebratt C . Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol 2015; 18: pyv002.
Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 2002; 416: 396–400.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
van Erp AMM, Miczek KA . Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 2000; 20: 9320–9325.
Popova NK, Avgustinovich DF, Kolpakov VG, Plyusnina IZ . Specific [H-3]8-OH-DPAT binding in brain regions of rats genetically predisposed to various defense behavior strategies. Pharmacol Biochem Behav 1998; 59: 793–797.
Olivier B, Mos J, vanOorschot R, Hen R . Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 1995; 28: 80–90.
Pruss K, Skrebuhhova-Malmros T, Rudissaar R, Matto V, Allikmets L . 5-HT1A receptor agonists buspirone and gepirone attenuate apomorphine-induced aggressive behaviour in adult male Wistar rats. J Physiol Pharmacol 2000; 51: 833–846.
Eitan E, Hutchison ER, Mattson MP . Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci 2014; 37: 256–263.
Borroni B, Costanzi C, Padovani A . Genetic susceptibility to behavioural and psychological symptoms in Alzheimer disease. Curr Alzheimer Res 2010; 7: 158–164.
Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW . Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2014; 19: 895–901.
Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 2008; 135: 609–622.
Li Y, Tergaonkar V . Noncanonical functions of telomerase: implications in telomerase-targeted cancer therapies. Cancer Res 2014; 74: 1639–1644.
Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009; 460: 66–72.
Acknowledgements
We would like to thank Fuyuki Ishikawa in the Department of Gene Mechanisms, Graduate School of Biostudies, Kyoto University, Kyoto, Japan, for providing the Tert knockout mice. This study was supported by the National Institute of Alcohol Abuse and Alcoholism (R01AA022377 to HS), the Whitehall Foundation (to HS), and the American Federation for Aging Research (to HS). This work was also supported by grants from the National Natural Science Foundation of China (91232304 and 3153000026 to D-YZ; 81370033 and 81571269 to Q-GZ), the National Basic Research Program of China (973 Program) (2011CB504404 to D-YZ), the Natural Science Foundation of Jiangsu Province (BK2011029 to D-YZ; BK20140964 to XL) and by the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine for data collection, analysis and interpretation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, QG., Wu, HY., Zhou, H. et al. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert−/− mice. Transl Psychiatry 6, e836 (2016). https://doi.org/10.1038/tp.2016.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2016.106
This article is cited by
-
SNP rs10420324 in the AMPA receptor auxiliary subunit TARP γ-8 regulates the susceptibility to antisocial personality disorder
Scientific Reports (2021)
-
Sucrose preference test for measurement of stress-induced anhedonia in mice
Nature Protocols (2018)