Abstract
The molecular mechanisms at the origin of eating disorders (EDs), including anorexia nervosa (AN), bulimia and binge-eating disorder (BED), are currently unknown. Previous data indicated that immunoglobulins (Igs) or autoantibodies (auto-Abs) reactive with α-melanocyte-stimulating hormone (α-MSH) are involved in regulation of feeding and emotion; however, the origin of such auto-Abs is unknown. Here, using proteomics, we identified ClpB heat-shock disaggregation chaperone protein of commensal gut bacteria Escherichia coli as a conformational antigen mimetic of α-MSH. We show that ClpB-immunized mice produce anti-ClpB IgG crossreactive with α-MSH, influencing food intake, body weight, anxiety and melanocortin receptor 4 signaling. Furthermore, chronic intragastric delivery of E. coli in mice decreased food intake and stimulated formation of ClpB- and α-MSH-reactive antibodies, while ClpB-deficient E. coli did not affect food intake or antibody levels. Finally, we show that plasma levels of anti-ClpB IgG crossreactive with α-MSH are increased in patients with AN, bulimia and BED, and that the ED Inventory-2 scores in ED patients correlate with anti-ClpB IgG and IgM, which is similar to our previous findings for α-MSH auto-Abs. In conclusion, this work shows that the bacterial ClpB protein, which is present in several commensal and pathogenic microorganisms, can be responsible for the production of auto-Abs crossreactive with α-MSH, associated with altered feeding and emotion in humans with ED. Our data suggest that ClpB-expressing gut microorganisms might be involved in the etiology of EDs.
Similar content being viewed by others
Introduction
Anorexia nervosa (AN), bulimia nervosa (BN) and binge-eating disorder (BED) are the main forms of eating disorders (EDs) with a combined prevalence of up to 5% of women and 2% of men.1 Although significant advances in understanding the neurobiological changes of ED have been achieved,2, 3, 4 the molecular mechanisms triggering the onset and maintenance of ED still remain unknown, and the specific genetic influence is uncertain.5 Accordingly, the unknown pathophysiology of ED explains the absence of specific pharmacological treatment.6
One novel line of clinical and experimental research, further developed in the present study, suggests that biological mechanisms of ED may involve immunoglobulins (Igs) or autoantibodies (auto-Abs) reactive with peptide hormones regulating appetite and emotion. In fact, after the initial identification of serum IgG from AN and BN patients binding to α-melanocyte-stimulating hormone (α-MSH) in hypothalamic neurons,7 the relevance of α-MSH-reactive auto-Abs to ED was shown by significant correlations of their plasma levels and the ED Inventory-2 (EDI-2) scores in AN and BN patients.8 Moreover, it was shown that production of α-MSH auto-Abs in rats is physiologically regulated and can be influenced by stress, food restriction and intestinal inflammation, that is, factors that often preceded ED.9,10 Furthermore, it was shown that changes in levels, Ig class and affinity properties of α-MSH auto-Abs differentially influenced α-MSH-mediated feeding and anxiety.9,10 α-MSH is a 13 amino-acid (a.a.) peptide11 critically involved in regulation of energy balance by decreasing food intake and increasing energy expenditure via activation of the melanocortin receptor type 4 (MC4R),12 both centrally and peripherally.13,14 α-MSH also regulates mood and emotion, for example, increasing anxiety.15,16 Determining the origin of α-MSH-reactive auto-Abs may, hence, shed new light on the ED etiology.
A molecular mimicry concept has been developed to explain the origin of auto-Abs crossreacting with microbial pathogens and host proteins, and that may cause some infection-triggered autoimmune diseases.17 By applying this concept to the origin of auto-Abs crossreactive with α-MSH, we previously studied by an in silico approach, the sequence homology, of at least five consecutive amino acids, between appetite-regulating peptide hormones and proteins from bacteria, viruses, fungi and archea.18,19 To our surprise, such homology was present in several bacterial species of the gut microbiota, for example, between α-MSH and both commensal and pathogenic Escherichia coli bacteria.19 This indicates that some gut bacteria may be constitutively involved in production of host Ig modulating the biological activity of peptide hormones, and, hence, may be physiologically and/or pathophysiologically involved in regulation of appetite and emotion.20 In support of this link, studies in germ-free mice showed stimulatory effects of gut microbiota on plasma levels of all classes of Ig.21 The presence of amino-acid sequence homology does not, however, signify functional molecular mimicry, that is, the ability of microbial proteins to stimulate production of auto-Abs crossreactive with host peptide hormones, which should be experimentally validated.
Thus, the aim of the present study was to determine, using a proteomic approach, the putative microbial origin of α-MSH auto-Abs by identification of bacterial α-MSH antigen-mimetic proteins and to validate their relevance to feeding and anxiety and ED. For this purpose, we used E. coli K12, which is a gut commensal and environmental bacterial species. The rationale of using these bacteria was the presence of five consecutive amino-acid sequence homology in several E. coli proteins with α-MSH, as well as significant correlations between presence of E. coli in gut microbiota and body mass index.22 Following identification of a putative α-MSH antigen-mimetic protein in E. coli, we validated its ability to stimulate α-MSH auto-Abs production and to affect food intake and anxiety in mice using immunization. Further, we determined the effects of immunization-induced auto-Abs on MC4R signaling in vitro. In the next step, we studied whether delivery of α-MSH antigen-mimetic protein in the gut, as a natural component of E. coli bacteria, may influence host α-MSH auto-Abs production and food intake, as compared with mutant E. coli bacteria lacking the expression of the protein of interest. Finally, we studied plasma samples of patients with AN, BN and BED for the presence of antibodies directed against the identified bacterial protein mimetic of α-MSH and potential correlations with patients EDI-2 scores.
Materials and methods
E. coli K12 culture and protein extraction
The bacterial strain used in this study was E. coli K12, provided by UMR 6270 CNRS Laboratory in Rouen University, France. E. coli K12 was grown in 250 ml Luria Bertani (LB) broth (MP Biomedicals, Illkirch, France) at 37 °C for 24 h. Protein extraction was performed as described by Marti et al.23 In brief, bacteria were harvested by centrifugation at 4000 g for 30 min at 4 °C and the resulting pellet was resuspended in extraction buffer (300 mM NaCl and 20 mM Tris-HCl, pH 8). The suspension was disrupted by sonication (3 × 3 min, pulse ON 1 s, OFF 1 s at 21% of amplitude) and centrifuged at 10 000 g for 10 min at 4 °C. The supernatant was recovered and ultracentrifuged at 4 °C for 45 min at 60 000 g to further separate proteins into cytoplasmic (supernatant) and envelope (pellet) fractions. Protein concentrations were measured using 2-D Quant Kit (GE Healthcare, Piscataway, NJ, USA).
Two-dimensional polyacrylamide gel electrophoresis
For two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE), 400 μg of E. coli K12 protein extract were added to iso-electro focusing buffer (7 M urea, 2 M thiourea and 0.5% ampholytes, pH 4–7, 20 mM dithiothreitol, 2 mM tributyl phosphine, 2% CHAPS and 0.005% bromophenol blue) and solubilized for 60 min at room temperature with slight shaking. The first-dimensional gel separation was carried out using ReadyStrip IPG Strip (18 cm, pH 4–7 NL, Bio-Rad, Marnes-la-Coquette, France). After 24 h of passive rehydration of the strip with iso-electro focusing buffer, the protein sample was added to the strips through a loading cup placed at 1.5 cm from the cathode. Iso-electro focusing was performed with the Ettan IPGphor 3 System (GE Healthcare) in four steps (31 500 Vh): 500 V for 1 h, 1000 V gradient, 10 000 V gradient and 10 000 V for 2 h. After two equilibration steps with 2% dithiothreitol and 2.5% iodoacetamide, respectively, the second dimension, that is, a SDS-PAGE, (10% polyacrylamide gel, 20 cm × 18 cm × 1 mm) was performed on an Ettan Daltsix vertical electrophoresis system (GE Healthcare) with 12 mA per gel. After SDS-PAGE, the 2D gel was fixed for 2 h in 2% (vol:vol) orthophosphoric acid and in 50% (vol:vol) methanol at room temperature. Gels were then rinsed with water, and the protein spots were visualized by CBB G-250 (Bio-Rad Laboratories, Hercules, CA, USA) staining (34% (vol:vol) methanol, 17% (wt:vol) ammonium sulfate, 2% (vol:vol) orthophosphoric acid and 0.66 g CBB G-250 per liter).
Immunoblotting
Following 2D-PAGE, E. coli cytoplasmic proteins were transferred onto Hybond-ECL polyvinylidene difluoride membrane (GE Healthcare) via a dry transfer method (Trans Blot Cell, Bio-Rad) and a constant current of 0.8 mA cm−2 of the membrane size for 2 h. After transfer, membranes were blocked with 5% (wt:vol) milk (Regilait, Macon, France) in phosphate-buffered saline (PBS; 10 mmol l−1 Tris, pH 8, and 150 mM l−1 NaCl) plus 0.05% (vol:vol) Tween 20. After washes, membranes were incubated overnight at 4 °C with polyclonal rabbit anti-α-MSH IgG (1:1000, Peninsula Laboratories, San Carlos, CA, USA), followed by washes and incubation with polyclonal swine anti-rabbit horseradish peroxidase-conjugated Igs (1:3000; Dako, Trappes, France). Immunoblots were revealed by the ECL detection system (GE Healthcare) and were scanned with ImageScanner II (GE Healthcare) and digitalized with Labscan 6.00 software (GE Healthcare). The same procedure was performed after adsorption of rabbit anti-α-MSH IgG with 10−6 M of α-MSH peptide (Bachem AG, Bubendorf, Switzerland) overnight at 4 °C.
Protein identification
The protein spots of interest were excised from CBB G-250-stained 2D gels using the Ettan Spot Picker (GE Healthcare), and automated in-gel digestion of proteins was performed on the Ettan Digester (GE Healthcare). Protein extracts were then resuspended in 10 μl of 5% (vol:vol) acetonitrile/0.1% (vol:vol) formic acid and then analyzed with a nano-LC1200 system coupled to a 6340 Ion Trap mass spectrometer equipped with a nanospray source and an HPLC-chip cube interface (Agilent Technologies, Courtaboeuf, France). In brief, peptides were enriched and desalted on a 40-nl RP-C18 trap column and separated on a Zorbax (30-nm pore size, 5-μm particle size) C18 column (43 mm long × 75 μm inner diameter; Agilent Technologies). A 9-min linear gradient (3–80% acetonitrile in 0.1% formic acid) at a flow rate of 400 nl min−1 was used, and the eluent was analyzed with an Ion Trap mass spectrometer. For protein identification, MS/MS peak lists were extracted and compared with the protein databases by using the MASCOT Daemon version 2.2.2 (Matrix Science, Boston, MA, USA) search engine. The searches were performed with the following specific parameters: enzyme specificity, trypsin; one missed cleavage permitted; no fixed modifications; variable modifications, methionine oxidation, cysteine carbamidomethylation, serine, tyrosine and threonine phosphorylation; monoisotopic; peptide charge, 2+ and 3+; mass tolerance for precursor ions, 1.5 Da; mass tolerance for fragmentations, 0.6 Da; electrospray ionization-Trap as instrument; taxonomy, E. coli; National Center for Biotechnology Information (NCBI) database (NCBInr 20120531 (18280215 sequences, 6265275233 residues); Bethesda, MD, USA). Protein hits were automatically validated if they satisfied one of the following criteria: identification with at least two top-ranking peptides (bold and red) each with a MASCOT score of >54 (P<0.01), or at least two top-ranking peptides each with a MASCOT score of >47 (P<0.05). To evaluate false-positive rates, all the initial database searches were performed using the ‘decoy’ option of MASCOT. Results were considered relevant if the false-positive rate never exceeded 1%.
Protein separation by OFFGEL
High-resolution E. coli K12 protein separation into 24 fractions was done onto the 3100 OFFGEL fractionator using the OFFGEL pH3–10 kit (Agilent Technologies). Protein samples (400 μg) preparation and assembly of all parts of the OFFGEL systems were done according to the procedures described in the Agilent Quick start Guide. OFFGEL fractionation was performed using the standard program OG24PRO with maximum limited current parameters (8000 V, 50 μA and 200 mW) until 64 KVh was reached after 30 h. At the end of the experiment, all fractions were transferred into a 0.8-ml deep well (Thermo Fisher Scientific, Illkirch, France) and stored at −20 °C. Nine protein-containing fractions recovered from the central part of the OFFGEL were studied by western blot using rabbit anti-α-MSH IgG (Peninsula Laboratories) followed by protein identification as described above.
Immunization and behavior in mice
All experimental protocols were conducted according to US National Institutes of Health guidelines and European Union directives, and animal experiments were approved by the Institutional Ethical Committees. Two-month-old male C57Bl6 mice (Janvier Laboratories, L’Arbresle, France) were acclimated to the animal facility for 1 week with 12 h light–dark cycle, lights on at 0700 hours and were kept in standard mouse-holding cages (n=8) in each. Mice were fed ad libitum with standard pelleted rodent chow (RM1 diet, SDS, Essex, UK) with drinking water always available and were manipulated daily by gentle holding and measuring body weight. During acclimatization, mice were distributed between four cages to obtain the similar mean body weight per mouse per cage. After 1 week of acclimatization, mice from each cage were assigned to one of four study group and received following treatments: (i) Group 1, ClpB immunization (n=8): ClpB protein (Delphi Genetics, Gosselies, Belgium) 50 μg per mouse in 200 μl of 1:1 (vol:vol) of PBS with Complete Freund’s Adjuvant (Sigma, St Louis, MO, USA), intraperitoneally (i.p.); (ii) Group 2, adjuvant injection controls (n=8): 200 μl of Complete Freund’s Adjuvant in PBS (1:1 (vol:vol), i.p.); (iii) Group 3, PBS injection controls (n=8): 200 μl of PBS (i.p.); and (iv) Group 4, intact controls (n=8): received no injections, and then all mice were returned to their holding cages. Fifteen days later, mice were given a boost immunization and the following treatments: (i) Group 1 (n=8), ClpB protein (Delphi Genetics) 50 μg per mouse in 200 μl of 1:1 (vol:vol) of PBS with Incomplete Freund’s Adjuvant (Sigma) i.p.; (ii) Group 2 (n=8): 200 μl of Incomplete Freund’s Adjuvant in PBS (1:1 (vol:vol), i.p.); (iii) Group 3, (n=8): 200 μl of PBS (i.p.); and iv) Group 4, (n=8): received no injections.
Next day after the boost, mice were placed individually into the BioDAQ mouse cages (Research Diets, New Brunswick, NJ, USA) each equipped with an automatic feeding monitor. Food (Serlab, Montataire France) and drinking water were available ad libitum and body weight was measured daily. After 3 days of acclimatization to the BioDAQ cages, mice received the following treatments: Groups 1, 2 and 3 (each n=8), that is, mice that have been immunized with ClpB, injected with adjuvants and with PBS, respectively, all received an acute injection of α-MSH peptide (Bachem AG), 100 μg kg−1 body weight in ~100 μl of PBS (i.p.) at 1000 hours. The control mice (n=8) received PBS only (i.p.). Feeding data was continuously monitored and analyzed using the BioDAQ data viewer 2.3.07 (Research Diets). For the meal pattern analysis, the inter-meal interval was set at 300 s.
After the feeding study, mice were placed in individual mouse-holding cages with food and water available ad libitum, and were analyzed for locomotor activity and anxiety in O-maze (Med Associate, St Albans, VT, USA) tests performed during 2 consecutive days. Two hours after the O-maze test, mice were killed by decapitation in a guillotine and trunk blood was collected into EDTA-containing tubes. Plasma was separated by centrifugation at 3500 r.p.m. (1.4 g) for 10 min at 4 °C and stored at −80 °C before assay.
Locomotor activity and anxiety tests in mice
After feeding study in the BioDAQ cages, mice were analyzed for locomotor activity using a Versamax Animal Activity Monitor (AccuScan Instruments, Columbus, OH, USA). Next day after the locomotor activity test, all mice were tested for their anxiety in an elevated O-maze.24 The elevated-O-maze is a variation of more commonly used elevated plus-maze pharmacologically validated for anxiety testing in rodents.25 The advantage of the O-maze is that it lacks the ambiguous central square of the traditional plus-maze. The O-maze consisted of a circular infrared platform (outer diameter 120 cm) elevated 80 cm above the floor, featuring two open and two closed segments made of gray plastic. The closed segments were enclosed by walls extending 20 cm above the surface of the maze and covered with a black infrared plexiglas lid. Each test started by placing the mouse into one of the two closed segments. The test lasted 5 min and was recorded using a video camera placed above the O-maze and the EthoVision video tracking software (Noldus IT, Wageningen, The Netherlands). Measurements of distance and time spent in the open and closed segments were analyzed. Between each mouse tests, the O-maze was cleaned with 30% ethanol.
ClpB and α-MSH auto-Ab assay
Plasma levels of auto-Abs reacting with ClpB, α-MSH and adrenocorticotropic hormone were measured using enzyme-linked immunosorbent assay according to a published protocol.26 In brief, ClpB protein (Delphi Genetics), α-MSH or adrenocorticotropic hormone peptides (Bachem AG) were coated onto 96-well Maxisorp plates (Nunc, Rochester, NY, USA) using 100 μl and a concentration of 2 μg ml−1 in 100 mM NaHCO3 buffer, pH 9.6, for 72 h at 4 °C. Plates were washed (5 min for three times) in PBS with 0.05% Tween 200, pH 7.4, and then incubated overnight at 4 °C with 100 μl of mouse plasma diluted 1:200 in PBS to determine free auto-Ab levels or diluted 1:200 in dissociative 3 M NaCl and 1.5 M glycine buffer, pH 8.9, to determine total auto-Ab levels. The plates were washed (three times) and incubated with 100 μl of alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (1:2000) or anti-mouse IgM (1:1000), all obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Following washing (three times), 100 μl of p-nitrophenyl phosphate solution (Sigma) was added as AP substrate. After 40 min of incubation at room temperature, the reaction was stopped by adding 3 N NaOH. The optical density was determined at 405 nm using a microplate reader Metertech 960 (Metertech, Taipei, Taiwan). Blank optical density values resulting from the reading of plates without addition of plasma samples were subtracted from the sample optical density values. Each determination was done in duplicate. The variation between duplicate values was <5%. Similar protocol was used to measure anti-ClpB IgG and IgM in human plasma (1:400) using corresponding anti-human IgG or IgM AP-conjugated antibodies (1:2000, Jackson ImmunoResearch Laboratories).
Adsorptions of ClpB antibodies with α-MSH
Plasma samples of mice, diluted 1:200 in PBS, or humans, diluted 1:400 in PBS, were preincubated with 10−6 M α-MSH peptide (Bachem AG) overnight at 4 °C before adding the samples to 96-well Maxisorp plates (Nunc) coated with ClpB protein (Delphi Genetics). IgG and IgM antibodies reactive with ClpB were detected by enzyme-linked immunosorbent assay using corresponding anti-mouse or anti-human AP-conjugated antibodies (Jackson ImmunoResearch Laboratories) as described above. Percentage of ClpB antibodies crossreactive with α-MSH were calculated relative to levels of anti-ClpB antibodies detected without absorption in each individual plasma sample equal 100%.
IgG purification from plasma
IgG purification and affinity assay were performed according to a published protocol.27 Extraction of plasma globulins was done by plasma acidification and separation on C18 SEP column (Phoenix Pharmaceuticals, Burlingame, CA, USA), then 500 μl of mouse plasma was mixed with 500 μl of buffer A (1% trifluoroacetic acid in water). The column was activated in 1 ml of buffer B (60% acetonitrile in 1% trifluoroacetic acid) by 3 min centrifugation with 700 r.p.m. and rinsed three times with 3 ml of buffer A. Diluted plasma (1:1 in buffer A) was added to the column and the effluent (1 ml) was saved (frozen at −20 °C) for further purification of IgG. Total IgG were purified from the effluents of mouse plasma samples using the Melon Gel Kit (Thermo Fisher Scientific, Rockford, IL, USA). Plasma effluents diluted 1:4 in kit’s purification buffer was added on washed melon gel deposited in a column. Column was spun 1 min at 6000 r.p.m., and the IgG containing effluent was saved and frozen at −20 °C before lyophilization. Lyophilized IgG were reconstituted in the 0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant P20 buffer (GE Healthcare).
For the cyclic adenosine monophosphate (cAMP) experiment, IgG purified from eight mice of the ClpB and of the adjuvant control group were combined, respectively, into two pools that were divided in two parts. One part was used directly in cAMP assay and the other was further purified using affinity chromatography for α-MSH (Bachem AG) coated on activated UltraLink beads (Pierce, Rockford, IL, USA). The α-MSH IgG-depleted IgG effluents were saved, lyophilized and diluted in PBS to the final concentration of 0.5 mg ml−1 to be used in cAMP assay.
Affinity kinetics assay
Affinity kinetics of mouse IgG for ClpB and α-MSH was determined by a biospecific interaction analysis based on the surface plasmon resonance phenomenon on a BIAcore 1000 instrument (GE Healthcare). α-MSH (Bachem AG) or ClpB protein (Delphi Genetics) were diluted to 0.5 mg ml−1 in 10 mM sodium acetate buffer, pH 5.0 (GE Healthcare), and were covalently coupled on the sensor chips CM5 (GE Healthcare) by using the amine coupling kit (GE Healthcare). All measures were performed on the same α-MSH or ClpB-coated chips. For the affinity kinetic analysis, a multicycle method was run with five serial dilutions of each IgG sample: 3360, 1680, 840, 420 and 210 (nmol), including a duplicate of 840 nmol and a blank sample (0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v Surfactant P20 buffer only). Each cycle included 2 min of analyte injection and 5 min of dissociation with flow speed 30 μl min−1 at 25 °C. Between injections of each sample, the binding surface was regenerated with 10 mM NaOH, resulting in the same baseline level of the sensorgram. The affinity kinetic data were analyzed using BiaEvaluation 4.1.1 program (GE Healthcare). For fitting kinetic data, the Langmuir’s 1:1 model was used, and the sample values were corrected by subtracting the blank values.
In vitro cAMP assay
Stable cell line of human embryonic kidney-293 cells expressing human MC4R was generated using a lentiviral transduction technology and purchased from Amsbio (Oxon, UK). High expression of MC4R mRNA in transfected cells was validated by reverse transcription PCR in Amsbio and in our laboratory. The presence of the transgene in cells before each experiment was verified by the visualization at a fluorescence microscope of the green fluorescent protein, which gene was inserted in the same with MC4R lentivector but under a different promoter. The α-MSH peptide (Bachem AG) was diluted in the induction buffer: PBS, 500 μM IBMX, 100 μM RO 20–1724 (Sigma), 20 mM MgCl2 to the final concentrations of 2, 1, 750, 500, 250, 100, 75, 50 and 10 nM corresponding to the α-MSH doses of 0.6, 3, 4.5, 6, 15, 30, 45, 60 and 120 pmol, respectively, and also included one blank sample.
After unfreezing, the cells were cultured in 250 ml tissue culture flasks (BD-Falcon, Beckton-Dickinson, Bedford, MA, USA) in Dulbecco’s modified Eagle medium 4.5 g l−1 glucose (Eurobio, Courtaboeuf, France) supplemented with (2 mM L-glutamine, 10% fetal calf serum, 0.1 mM nonessential amino acids and 1% penicillin–streptavidin) in humidified cell culture incubator at 37 °C, 5% CO2 for 8–10 days. At the day of experiment, cultured MC4R human embryonic kidney-293 cells were treated with 0.25% trypsin-EDTA (Sigma-Aldrich, Gillingham, UK) and cell pellets were resuspended in PBS to obtain ~5000 cells per well (10 μl) in a nontreated bioluminescence white 96-microwell plate (Nunc, Roskilde, Denmark). The cAMP production was measured using the bioluminescent assay cAMP-Glo Max Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. In brief, the cells were incubated for 15 min at room temperature with different concentrations of α-MSH peptide alone or α-MSH together with mouse IgG pools from ClpB-immunized or adjuvant control groups, and which were added to the cells just before α-MSH. Serial dilutions of cAMP standard (provided by the kit) were assayed on the same microplate. cAMP detection solution was added to each well, then the cells were homogenized by agitation and centrifuged 2 min at 1000 r.p.m. and then incubated for 20 min at 23 °C. Kinase-Glo reagent substrate was added in each well and after 10 min of incubation at 23 °C, the luminescence was read with a bioluminescence instrument (Safas Spectrometer, Monaco). Three tests for each dilution were performed in separate wells and were repeated at two separate days resulting in n=6 for each point of the cAMP activation curve when native IgG were used. After depletion of native IgG from anti-α-MSH IgG fraction, the same experiment was performed with each α-MSH concentration and IgG as described above.
E. coli gavage in mice
One-month-old male C57Bl6 mice (Janvier Laboratories) were acclimated to the animal facility for 1 week and maintained as described above. Mice were distributed into four groups (n=8 in each) as follows: (i) gavaged with 108 E. coli K12 bacteria; (ii) gavaged with 108 E. coli K12 bacteria deficient for ClpB; (iii) gavaged with LB medium only; and (iv) controls that did not receive any treatments. The ClpB mutant strain was generated in the Bernd Bukau’s Laboratory (ZMBH, Heidelberg University, Heidelberg, Germany) and was kindly provided together with the corresponding wild-type (WT) E. coli bacteria by Dr Axel Mogk. Mice were placed individually into the BioDAQ cages (Research Diets) and intragastrically gavaged daily before the onset of dark phase for 21 days with 0.5 ml of LB medium with or without bacteria. During the last day of gavage, mice feces were collected and frozen. After gavage, mice were killed by decapitation and trunk blood was collected into EDTA-containing tubes. Plasma was separated by centrifugation at 3500 r.p.m. (1.4 g) for 10 min at 4 °C and stored at −80 °C before assay. Plasma levels of anti-ClpB and anti-α-MSH IgG and IgM were assayed as described above.
ClpB DNA assay
DNA was extracted from the cultures of the WT and ClpB mutant strains, and was also purified from mice feces using the QIAampR DNA Stool Mini Kit (Qiagen, Courtaboeuf, France). Bacteria were dissolved in water and boiled at 100 °C during 5 min, after 1 min of centrifugation at 11 000 r.p.m., the supernatant containing the DNA was stored at −20 °C. Using the NCBI primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), we designed the following nucleotide primers that amplify 180-base pair DNA region coding for the ClpB protein fragment containing one identified α-MSH-like epitope (Figure 1e), forward: 5′-GCAGCTCGAAGGCAAAACTA-3′ and reverse: 5′-ACCGCTTCGTTCTGACCAAT-3′ (Invitrogen Custom Primers, Cergy Pontoise, France). PCR was performed in a thermocycler with MicroAmp tubes (Eppendorf, Hambourg, Germany). The reaction was carried out in a 50-μl volume containing 25 μl of Go Taq Green Master Mix 2 × (Promega), 1 μl (20 pmol) of each primer, 21 μl of bi-distilled water and 1 μl of bacterial DNA. PCR conditions were as follows: 3 min at 94 °C followed by 35 cycles at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 1.5 min. PCR products were visualized on a 1% agarose gel (Sigma), with the expected size of 180 base pair and the specificity validated using ClpB mutant strain.
Proteomic identification of molecular mimicry between E. coli K12 proteins and α-MSH. (a) 2D GE of E. coli cytoplasmic proteins. (b, c) Immunoblots of E. coli proteins detected with rabbit anti-α-MSH IgG, preadsorbed (c) or not (b) with α-MSH. Circles in red surround the spots specifically recognized by α-MSH IgG which were used for protein identification. Circles in blue indicate nonspecific spots. Proteins identified in the spots 1–4 are isoforms of ClpB. (d) α-MSH and ClpB amino-acid sequence alignments using the Stretcher program. (e) Western blot of the recombinant ClpB, revealed with anti-α-MSH IgG. Lanes 1 and 2, 20 and 10 μg of ClpB, respectively. IP, isoelectric point.
Human subjects
The ED patients and controls were all female with the following body mass index and age (mean±s.d.). Body mass index (kg/m2): controls, 22.8±3.8; AN, 15.01±2.0; BN, 21.7±3.8 and BED, 33.3±8.1. Age (years): controls, 25.8±8.9; AN, 18.62±4.9; BN, 22.7±6.8 and BED, 30.6±11.6. All experimental protocols were conducted according to US National Institutes of Health guidelines and European Union directives, and were approved by the Institutional Ethical Committees. AN, BN and BED were diagnosed by a psychiatrist and a clinical psychologist according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV). Plasma samples from healthy female volunteers served as the control group. All study patients and controls gave their informed written consent for study participation. Plasma samples from both patients and controls were taken on the day of completing the EDI-2 test, instantly frozen and kept at −70 °C then shipped on dry ice and stored again at −80 °C until analyzed.
Statistical analysis
Data were analyzed and the graphs were plotted using the GraphPad Prism 5.02 (GraphPad Software, San Diego, CA, USA). Normality was evaluated by the Kolmogorov–Smirnov test. Group differences were analyzed by the analysis of variance or the nonparametric Kruskal–Wallis test with the Tukey’s or Dunn’s post tests, respectively, according to the normality results. Body weight changes were analyzed with two-way repeated measurements analysis of variance and the Bonferroni post tests. Individual groups were compared using the Student’s t-test or the Mann–Whitney test according to the normality results. Effects of absorptions of ClpB antibodies with α-MSH were analyzed using paired t-test. Pearson’s or Spearman’s correlation coefficients were calculated according to the normality of the variable. The cAMP production was analyzed using a nonlinear regression fit (log(α-MSH) vs normalized cAMP response), which equation was Y=100/(1+10(LogEC50-X) × HillSlope). Data are shown as mean±s.e.m., and for all test, P<0.05 was considered statistically significant.
Results
Proteomic identification of bacterial α-MSH mimetics
To identify bacterial proteins with molecular mimicry to α-MSH, we developed a research strategy based on proteomic technology. Total protein was extracted from E. coli K12 cultures, the cytoplasmic fraction was resolved by 2D gel electrophoresis (Figure 1a) and transferred to a polyvinylidene difluoride membrane. To increase the probability of detection of multiple α-MSH-like epitopes in bacterial proteins, the membrane was revealed with polyclonal anti-α-MSH IgG. We found 13 immunopositive protein spots (Figure 1b), among which the spots 1–8 disappeared after preadsorption of antibodies with 10−6 M α-MSH (Figure 1c), confirming specific α-MSH-mimetic epitopes. Using mass spectrometry, protein spots 1,2,3 and 4, displaying the strongest α-MSH-like staining, were identified as isoforms of the heat-shock protein named ClpB, a 857-a.a. protein, (molecular weight: 95526 Da, accession number: NP_417083.1). Less intensely stained α-MSH-like spots 5–8 (with the highest MASCOT scores of 880, 877, 874 and 800, respectively) were isoforms of the 548-a.a. protein chaperonin GroEL, (molecular weight: 57293 Da; accession number: YP_001732912.1). We also used an alternative strategy of E. coli protein separation, using an OFFGEL fractionator followed by one-dimensional gel electrophoresis and western blot with anti-α-MSH IgG preadsorbed or not with α-MSH (Supplementary Figure 1). One band was specifically recognized by anti-α-MSH IgG and was found to contain the ClpB protein (with the highest MASCOT score of 1065). Based on these results, ClpB was selected as a target protein for further validation of its molecular mimicry with α-MSH.
To analyze the amino-acid sequence homology between α-MSH and bacterial ClpB, both sequences were aligned in the Emboss Stretcher program that use the Needleman-Wunsch algorithm (http://www.ebi.ac.uk/Tools/emboss/). The alignments revealed a site of the ClpB protein displaying discontinuous 5 a.a. sequence homology with α-MSH (Figure 1d). This putative α-MSH-like epitope was located in an inter-helical loop of the ClpB protein structure,28 indicating that it is exposed on the protein surface, that is, accessible to auto-Abs binding.
Western blot of the recombinant ClpB protein revealed with anti-α-MSH IgG showed a 96-kDa band (Figure 1e), confirming that the ClpB protein contains α-MSH-like epitope(s). These results show that the presence of at least five consecutive amino-acid sequence homology, according to the molecular mimicry concept,29 is not an obligatory condition for bacterial proteins to be recognized by IgG crossreacting with a neuropeptide. Furthermore, the E. coli K12 proteins that displayed such consecutive amino-acid homology with α-MSH by in silico analysis (Supplementary Figure 2) were not identified here as in vitro α-MSH mimetics. This mismatch can be related to hidden α-MSH-like epitopes that escaped recognition by anti-α-MSH IgG.
Immunization of mice with ClpB
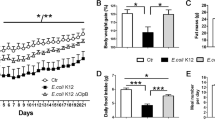
To investigate whether E. coli ClpB may induce auto-Abs crossreactive with α-MSH, influencing feeding and anxiety, we immunized mice with the recombinant bacterial ClpB protein. Mice that received ClpB together with adjuvant or adjuvant alone displayed lower body weight for a few days after injections (Figure 2a). However, 4 weeks later, ClpB-immunized mice had higher body weight (+5%) vs controls (Figure 2a). The mean daily food intake, as measured during the last 10 days of the experiment, was also higher (+13%) in ClpB-immunized mice as compared with other groups (Figure 2b). The increase in food intake was owing to increased meal size (Figure 2c), as meal number did not change (Figure 2d), indicating that the ClpB immunization interfered with satiation rather than with hunger mechanisms. This is in agreement with the known role of α-MSH to induce satiation.30,31 To further validate the relevance of ClpB immunization to α-MSH anorexigenic effect, mice received i.p. injection of α-MSH. The following 24 h food intake and body weight were not affected in ClpB-immunized mice (Figure 2e), indicating that they were not sensitive to the anorexigenic effect of administered α-MSH that was present in nonimmunized mice.
ClpB immunization in mice. ClpB-immunized mice (ClpB+Adj) were compared with mice receiving adjuvant (Adj), PBS or controls (Ctr). (a) Body weight changes during 32 days of the study. Food intake and feeding pattern were studied during the last 2 weeks in the BioDAQ cages. Mean daily food intake (b), meal size (c) and meal number (d) during last 10 days of the study. (e) Food intake during 24 h after injection of α-MSH (100 μg kg−1 body weight, i.p.) or PBS. (f) Plasma levels of ClpB-reactive IgG before and after adsorption with 10−6 M α-MSH. (g) Affinity of anti-ClpB IgG shown as the dissociation equilibrium constants (KD values). (h) Plasma levels of α-MSH-reactive total IgG. (i) Affinity (KD) of anti-α-MSH IgG. (j) cAMP assay in human embryonic kidney-293 cells overexpressing MC4R after stimulation by α-MSH alone or together with IgG (0.5 mg ml−1) pooled from ClpB-immunized or from Adj-injected mice. (k) The cAMP assay was performed with IgG depleted from anti-α-MSH IgG. (a) Two-way repeated measurement analysis of variance (ANOVA) before α-MSH injection (100 μg kg−1 body weight, i.p.), P<0.0001, Bonferroni post tests *a at least, P<0.05 ClpB group vs Ctr; *b at least, P<0.05 Adj group vs Ctr.; *c, P<0.05, Student’s t-test ClpB group vs PBS; and *d, P<0.05, Student’s t-test ClpB group vs Ctr. (b) ANOVA P=0.0002, Tukey’s post tests ***P<0.001, **P<0.01, #P<0.05, Student’s t-test. (c) ANOVA P=0.007, Tukey’s post tests **P<0.01. (e) Student’s t-test, *P<0.05. (f, g) ANOVA P<0.0001, Tukey’s post tests ***P<0.001 ClpB +Adj vs other groups, paired t-test ##P<0.01, ###P<0.001. (h) ANOVA P=0.0002, Tukey’s post tests ***P<0.001, *P<0.05; (i) Kruskal–Wallis test P=0.003, Dunn’s post test **P<0.01, (mean±s.e.m., n=8). (j) ANOVA P=0.005, Tukey’s post test *P<0.05; ANOVA P=0.04, Student’s t-test #P<0.05, aClpB vs α-MSH, bClpB vs Adj. (mean±s.e.m.; j, n=6, k, n=3).
After the feeding experiments, locomotor activity and anxiety-related behavior in mice were studied in the open field and O-maze tests. The total locomotor activity and the time spent in the open vs border areas did not significantly differ between the study groups (Supplementary Figures 3a and b). However, in the closed arms of the O-maze, the ClpB-immunized mice moved a shorter distance as compared with controls (Supplementary Figure 3d) and spent less time as compared with all other groups (Supplementary Figure 3f), indicating decreased anxiety.
To confirm the efficiency of immunization, we assayed plasma levels of anti-ClpB IgG and measured their affinity. In ClpB-immunized mice, a strong increase in anti-ClpB IgG levels (Figure 2f and Supplementary Figure 4a) with lower affinities (Figure 2g, and Supplementary Figures 4b and c) were found, in agreement with recent IgG induction. Increased plasma levels of α-MSH-reactive IgG were also found in ClpB-immunized mice (Figure 2h and Supplementary Figure 4d); these IgG were similarly characterized by lower affinities for α-MSH, as compared with controls (Figure 2i, and Supplementary Figures 4e and f). Adsorption of mouse plasma with α-MSH, significantly reduced plasma levels of anti-ClpB IgG, confirming that a fraction, but not all of the anti-ClpB IgG, were crossreactive with α-MSH (Figure 2f). Plasma levels of α-MSH IgM auto-Abs did not significantly differ between the groups (Supplementary Figure 4g). We also analyzed whether ClpB immunization may induce auto-Abs crossreacting with the adrenocorticotropic hormone, a 39-a.a. peptide containing the α-MSH sequence. No significant differences in plasma adrenocorticotropic hormone-reactive IgG were found (Supplementary Figures 4h and i), showing the selectivity of the conformational mimicry between ClpB and α-MSH.
Mouse IgG effects on MC4R signaling
To determine the impact of ClpB immunization-induced α-MSH crossreactive IgG on MC4R signaling, we studied their effects on α-MSH-induced cAMP production in MC4R-expressing cells. We found that cAMP concentrations were lower when α-MSH was preincubated with IgG from ClpB-immunized mice, as compared with α-MSH alone or α-MSH preincubated with IgG from adjuvant-injected mice, with a reduction of 8–10% at the two highest α-MSH concentrations (Figure 2j). After depletion of α-MSH-reactive IgG from the pooled IgG, the remaining IgG from the ClpB-immunized mice did not show any effect on α-MSH-induced cAMP release (Figure 2k), indicating that anti-α-MSH crossreactive IgG in ClpB-immunized mice were responsible for lowering cAMP production in response to α-MSH. The reduction in MC4R activation and signaling may, hence, account for the increased food intake and decreased anxiety observed in ClpB-immunized mice. We cannot, however, exclude that ClpB-induced anti-α-MSH IgG may interfere with α-MSH binding to other than MC4R melanocortin receptors, for example, MC3R.32 The putative direct effect of ClpB on MC4R-mediated cAMP production was not possible to evaluate owing to the ClpB ATPase activity33 interfering with ATP-dependent cAMP production.
Intragastric delivery of E. coli in mice
To test whether E. coli may induce immunogenic response against the ClpB protein, resulting in production of anti-ClpB auto-Abs crossreactive with α-MSH, WT and ΔClpB strains of E. coli K12 were given daily to mice by intragastric gavage during 3 weeks. Another group of mice was gavaged with the bacterial culture medium only, and the control group did not receive any treatment. The first days of gavage were accompanied by a decrease in body weight and food intake in mice receiving WT E. coli, which then gradually returned to control levels (Figures 3a and b). Again, during the last week of gavage, feeding pattern was affected in mice receiving E. coli WT showing a decrease in meal size but increase in meal number (Figures 3c and d). Remarkably, mice receiving ΔClpB E. coli did not significantly differ from controls in either body weight gain, food intake or feeding pattern at any time point. These data support specific involvement of bacterial ClpB in the host acute decrease of food intake as well as in the chronic regulation of feeding pattern following E. coli infection. Expectedly, ClpB DNA was more abundant in feces of mice receiving E. coli WT, although its low level was detected in some control mice (Figure 3e).
E. coli supplementation in mice. Effects of intragastric daily gavage (days 1–21) in mice with either E. coli K12 wild-type (WT), ClpB-deficient (ΔClpB) E. coli K12 or LB medium on body weight (a), food intake (b), meal size (c) and meal number (d). (e) PCR detection of a 180-base pair fragment of the bacterial ClpB DNA, first lane, molecular weight marker, second lane DNA from in vitro cultures of E. coli K12 WT, third lane DNA from in vitro cultures of E. coli K12 ΔClpB, and the remaining lanes DNA from mice feces collected at day 21. Plasma levels in optical density in enzyme-linked immunosorbent assay of anti-ClpB IgM (f) and IgG (g) before and after adsorption with 10−6 M α-MSH. Plasma levels of anti-α-MSH IgM (h) and IgG (i). (j) Affinity (equilibrium constant) of anti-α-MSH IgG. (a) Two-way repeated measurements analysis of variance (ANOVA), P=0.3, Bonferroni post test day 2, **P<0.01 control (Ctr) vs E. coli WT. (b) ANOVA days 1–2, P=0.0006, Tukey’s post tests ***P<0.001, *P<0.05, E. coli WT vs aCtr and bLB. (c) Kruskal–Wallis (K–W) test third week P=0.0001, Dunn’s post tests, ***P<0.001, **P<0.01, E. coli WT vs aCtr, bLB and CΔClpB. (d) ANOVA days 1–2, P=0.006, Tukey’s post tests **P<0.01, *P<0.05, K–W test third week P<0.0001, Dunn’s post tests, ***P<0.001, **P<0.01, E. coli WT vs aCtr, bLB and CΔClpB. (f) K–W test, before adsorption P=0.02, Dunn’s post tests *P<0.05, ANOVA after adsorption, P<0.0001, Tukey’s post tests **P<0.01, E. coli WT vs other groups. (g) ANOVA before adsorption, P=0.01, Tukey’s post tests *P<0.05, E. coli WT vs other groups, paired t-test ##P<0.01. (h) Student’s t-test, E. coli WT vs other groups *P<0.05. (j) K–W test P=0.02, Dunn’s post test *P<0.05, Mann–Whitney test, #P<0.05. (mean±s.e.m., n=8).
After 3 weeks of gavage, plasma levels of both anti-ClpB IgM and IgG were elevated in mice that received E. coli WT as compared with controls and ΔClpB E. coli groups (Figures 3f and g). Adsorption of plasma with α-MSH reduced anti-ClpB IgG levels in E. coli WT-gavaged mice (Figure 3g), indicating the presence of anti-ClpB IgG crossreactive with α-MSH. Interestingly, plasma levels of the IgM class of anti-ClpB auto-Abs were increased after adsorption with α-MSH, suggesting that α-MSH caused dissociation of α-MSH IgM immune complexes crossreactive with ClpB that were increased in E. coli WT-gavaged mice (Figure 3f). Plasma levels of anti-α-MSH IgM were also increased by E. coli WT delivery as compared with all other groups (Figure 3h), while anti-α-MSH-reactive IgG were only slightly increased without reaching significance (Figure 3i). Nevertheless, affinity kinetic analysis of α-MSH IgG revealed lower values of the dissociation equilibrium constants in E. coli-gavaged mice (Figure 3j), without significant changes of the association or dissociation rates (not shown). These changes, including increased levels of the IgM class of α-MSH-reactive auto-Abs, might reflect an immune response towards ClpB as to a novel antigen. In fact, low or undetectable levels of ClpB DNA in feces of mice that did not receive E. coli WT indicates that ClpB-expressing microorganisms were not major gut commensals in the studied mice. Thus, in contrast to ClpB-immunized mice, which showed increased levels of low-affinity anti-α-MSH IgG associated with increased meal size and body weight gain, E. coli-gavaged mice showed increased production of both anti-α-MSH-reactive IgM and IgG with increased affinities associated with decreased meal size and body weight. These results indicate that production of α-MSH-reactive auto-Abs with different properties can be linked either positively or negatively with appetite and body weight gain and corroborate previous data showing opposite correlations between α-MSH-reactive IgG and IgM and behavioral traits in AN and BN patients.8
Anti-ClpB antibodies in ED patients
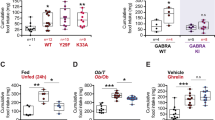
As we validated the ability of the E. coli ClpB protein to stimulate production of α-MSH crossreactive auto-Abs, we next determined the relevance of bacterial ClpB to ED by studying anti-ClpB antibodies in patients with AN, BN or BED. We found that both anti-ClpB IgG and IgM were readily detectable in plasma of ED patients as well as healthy subjects with no significant differences of their mean levels (Figures 4a and b). However, there was high variability in all study groups, indicating a different individual history in encountering ClpB-like antigens. To verify whether human anti-ClpB antibodies similarly were crossreactive with α-MSH, plasma samples were adsorbed with 10−6 M α-MSH, leading to significant reduction of anti-ClpB IgG and IgM detectable levels in all study groups (Figures 4c and d). Further, the relative levels of α-MSH crossreactive anti-ClpB IgG were increased in all three groups of ED patients, in particular BN and BED vs healthy controls (Figure 4e). Elevated levels of α-MSH crossreactive anti-ClpB IgM were found in AN as compared with BN (Figure 4f).
Anti-ClpB antibodies in ED patients. Plasma levels of anti-ClpB IgG (a) and IgM (b) in healthy women (control, Ctr) and in patients with AN, BN and BED. Plasma levels of ClpB IgG (c) and IgM (d) before and after adsorption with 10−6 M α-MSH. Percentage of α-MSH crossreactive anti-ClpB IgG (e) and IgM (f). (b) Student’s t-test *P<0.05. (c, d) Paired t-tests, ***P<0.001, **P<0.01. (e) Kruskal–Wallis test P<0.0001, Dunn’s post test, **P<0.01, Mann–Whitney test #P<0.05. (f) Analysis of variance P=0.02, Tukey’s post test *P<0.05. (mean±s.e.m., Ctr, n=65, AN, n=27 BN, n=32 and BED, n=14).
To further determine the relevance of anti-ClpB IgG and IgM to ED, we studied whether their plasma levels may correlate with behavioral traits in ED patients and controls measured by the EDI-2. We found that in controls, ClpB IgG correlated inversely with the normal range of a few psychological traits, but in AN patients, ClpB IgG levels correlated positively with the core psychopathological traits such as body dissatisfaction and drive for thinness (Table 1). Moreover, in AN and BED patients, EDI-2 subscale scores correlated with ClpB IgM in the opposite way, being negative in AN but positive in BED (Table 1). However, in BED patients, ClpB IgM correlated negatively with age, suggesting that the highest anti-ClpB IgM levels were associated with the acute form of the disease. Remarkably, the correlations found in AN patients between ClpB IgG or IgM and drive for thinness or interpersonal distrust, respectively, resembled closely the correlations between the same psychological traits and α-MSH-reactive IgG or IgM found in a different group of AN patients in a previous study.8
Discussion
Our data, for the first time reveal a molecular link between ClpB-expressing gut bacteria and host regulation of feeding and emotion via production of anti-ClpB antibodies crossreactive with α-MSH. It is in support of a mechanism of molecular mimicry as an underlying cause of ED. The molecular mimicry concept was initially used to explain the development of autoimmune diseases caused mainly by viral proteins.29 Our work extends this concept by implicating it in the (patho)physiological interactions between gut bacteria and host peptidergic signaling involved in the regulation of motivated behavior and emotion. It suggests that specific alterations of gut microbiota may lead to behavioral and emotional abnormalities as observed in ED patients. Such alterations may result from dietary changes,34 which are gender-specific.35 A combination of host microbiota changes with other ED-related risk factors36 is in line with the ‘hygiene hypothesis’ of AN37 and with data supporting an infectious origin of neuropsychiatric disorders.38 Although our results indicate that immunity to bacterial ClpB is a physiological phenomenon that may influence host phenotype, our findings of increased levels of anti-ClpB IgG crossreactive with α-MSH in ED patients and correlations of anti-ClpB antibodies with patient’s psychopathological traits support the involvement of ClpB-expressing microorganisms in increased ClpB antibody production and establishment of abnormal feeding behavior and emotion.
Here we identified and validated bacterial ClpB as an α-MSH antigen-mimetic protein. Indeed, the absence of an anti-α-MSH response in mice gavaged with ΔClpB E. coli suggests that ClpB is the principal α-MSH-mimetic protein in E. coli. ClpB has been well characterized for its functional role in bacteria as a heat-shock-induced protein disaggregation chaperone,39 and it is peculiar to find molecular mimicry between ClpB, which is known to protect bacteria from thermal and oxidative stress,40,41 and α-MSH, which is a stress-induced hormone42 that is also anti-pyretic.43 Although such functional similarity might be coincidental, it may also suggest a phylogenetic link in development of coordinated responses to stress in bacteria and the mammalian host. Furthermore, such a link would implicate bacterial stress-activated proteins in the pathophysiology of stress-related diseases in humans. Because the ClpB protein with the same α-MSH-like epitope is expressed by several Gram-negative bacteria, including intestinal pathogens Salmonella enterica, Shigella dysenteriae and Yersinia enerocolitica, we predict that these pathogens should be all able to stimulate production of α-MSH crossreactive auto-Abs. The ability of humans to rise antibody response to ClpB has been reported in patients with Campylobacter jejuni-induced enteritis.44 Besides the altered α-MSH-mediated satiety and anxiety, other hormonal pathways involved in regulation of appetite and emotion are implicated in the pathophysiology of ED,45 suggesting that the identification of bacterial antigen-mimetic proteins of other peptide hormones could be a new promising strategy for understanding the origin of altered peptidergic signaling in ED. For instance, a recent study revealed that increased affinity of plasma IgG for ghrelin, a hunger hormone, is responsible for its enhanced orexigenic effect in obese humans,46 however, the antigenic origin of such IgG is currently unknown. The proteomic approach used in our study can be applied to identify bacterial antigen mimetics of other peptide hormones or can be improved by replacing gel-based identification mass spectrometry by more sensitive and efficient multidimensional liquid chromatography techniques coupled to tandem mass spectrometry after affinity purification of bacterial proteins.47
In conclusion, the present work identifies ClpB as a protein responsible for the origin of auto-Abs crossreactive with α-MSH, associated with psychopathological traits in ED patients and, hence, suggest that ClpB-expressing microorganisms should be further validated as a novel potential specific target for diagnostics and treatment of ED.
References
Hudson JI, Hiripi E, Pope J, Harrison G,, Kessler RC . The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007; 61: 348–358.
van Elburg A, Treasure J . Advances in the neurobiology of eating disorders. Curr Opin Psychiatry 2013; 26: 556–561.
Kaye WH, Fudge JL, Paulus M . New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 2009; 10: 573–584.
Friederich H-C, Wu M, Simon JJ, Herzog W . Neurocircuit function in eating disorders. Int J Eating Disord 2013; 46: 425–432.
Trace SE, Baker JH, Penas-Lledo E, Bulik CM . The genetics of eating disorders. Annu Rev Clin Pychol 2013; 9: 589–620.
Kaye W, Treasure J, Kasper S, Aigner M, Bailer U,, Brambilla F et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Pharmacological Treatment of Eating Disorders. World J Biol Psychiatry 2011; 12: 400–443.
Fetissov SO, Hallman J, Oreland L, af Klinteberg B, Grenbäck E, Hulting AL et al. Autoantibodies against α-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci USA 2002; 99: 17155–17160.
Fetissov SO, Harro J, Jaanisk M, Järv A, Podar I, Allik J et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci USA 2005; 102: 14865–14870.
Hamze Sinno M, Do Rego JC, Coëffier M, Bole-Feysot C, Ducrotte P, Gilbert D et al. Regulation of feeding and anxiety by α-MSH reactive autoantibodies. Psychoneuroendocrinology 2009; 34: 140–149.
Coquerel Q, Hamze Sinno M, Boukhettala N, Coëffier M, Terashi M,, Bole-Feysot C et al. Intestinal inflammation influences α-MSH reactive autoantibodies: Relevance to food intake and body weight. Psychoneuroendocrinology 2012; 37: 94–106.
Harris JI, Lerner AB . Amino-acid sequence of the alpha-melanocyte-stimulating hormone. Nature 1957; 179: 1346–1347.
Fan W, Boston BA, Kesterson RA, Hruby VJ,, Cone RD . Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997; 385: 165–168.
Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK . Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab 2009; 20: 203–215.
Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 2013; 62: 490–497.
Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK . Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 2010; 58: 1009–1018.
Chaki S, Hirota S, Funakoshi T, Suzuki Y, Suetake S,, Okubo T et al. Anxiolytic-like and antidepressant-like activities of MCL0129 (1-[(S)-2-(4-fluorophenyl)-2-(4-isopropylpiperadin-1-yl)ethyl]-4-[4-(2-met hoxynaphthalen-1-yl)butyl]piperazine), a novel and potent nonpeptide antagonist of the melanocortin-4 receptor. J Pharmacol Exp Ther 2003; 304: 818–826.
Oldstone MB . Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol 2005; 296: 1–17.
Fetissov SO In: Neuropsychiatric Disorders and Infection Fatemi SH (ed). Taylor & Francis Books Ltd, 2004, pp 253–262.
Fetissov SO, Hamze Sinno M, Coëffier M, Bole-Feysot C, Ducrotté P, Hökfelt T et al. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition 2008; 24: 348–359.
Fetissov SO, Déchelotte P . The new link between gut-brain axis and neuropsychiatric disorders. Curr Opin Clin Nutr Metab Care 2011; 14: 477–482.
Hansson J, Bosco N, Favre L, Raymond F, Oliveira M,, Metairon S et al. Influence of gut microbiota on mouse B2 B cell ontogeny and function. Mol Immunol 2011; 48: 1091–1101.
Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes 2013; 37: 1460–1466.
Marti S, Nait Chabane Y, Alexandre S, Coquet L, Vila J,, Jouenne T et al. Growth of Acinetobacter baumannii in pellicle enhanced the expression of potential virulence factors. PLoS ONE 2011; 6: e26030.
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT . Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology (Berl) 1994; 116: 56–64.
Crawley JN,, Bailey KR In: Methods of Behavior Analysis in Neuroscience Buccafusco Jerry J. (ed). CRC Press, 2009, pp 77–101.
Fetissov SO . Neuropeptide autoantibodies assay. Methods Mol Biol 2011; 789: 295–302.
Legrand R, Takagi K, Fetissov SO . Immunoglobulin G preparation from plasma samples and analysis of its affinity kinetic binding to peptide hormones. Protoc Exch 2014 doi:10.1038/protex.2014.004.
Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W,, Yoshida M et al. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell 2003; 115: 229–240.
Oldstone MB . Molecular mimicry and immune-mediated diseases. FASEB J 1998; 12: 1255–1265.
Azzara AV, Sokolnicki JP, Schwartz GJ . Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav 2002; 77: 411–416.
Richard CD, Tolle V, Low MJ . Meal pattern analysis in neural-specific proopiomelanocortin-deficient mice. Eur J Pharmacol 2011; 660: 131–138.
Begriche K, Levasseur PR, Zhang J, Rossi J, Skorupa D,, Solt LA et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J Biol Chem 2011; 286: 40771–40781.
Woo KM, Kim KI, Goldberg AL, Ha DB, Chung CH . The heat-shock protein ClpB in Escherichia coli is a protein-activated ATPase. J Biol Chem 1992; 267: 20429–20434.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563.
Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun 2014; 5.
Hilbert A, Pike KM, Goldschmidt AB, Wilfley DE, Fairburn CG, Dohm FA et al. Risk factors across the eating disorders. Psychiatry Res 2014 doi:10.1016/j.psychres.2014.05.054.
Acres MJ, Heath JJ, Morris JA . Anorexia nervosa, autoimmunity and the hygiene hypothesis. Med Hypo 2012; 78: 772–775.
Kneeland RE, Fatemi SH . Viral infection, inflammation and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 2013; 42: 35–48.
Doyle SM, Wickner S . Hsp104 and ClpB: protein disaggregating machines. Trends Biochem Sci 2009; 34: 40–48.
Motohashi K, Watanabe Y, Yohda M,, Yoshida M . Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc Natl Acad Sci USA 1999; 96: 7184–7189.
Lourdault K, Cerqueira GM, Wunder EA, Picardeau M . Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect Immun 2011; 79: 3711–3717.
Charmandari E, Achermann JC, Carel J-C, Soder O, Chrousos GP . Stress response and child health. Sci. Signal. 2012; 5: mr1.
Murphy MT, Richards DB, Lipton JM . Antipyretic potency of centrally administered alpha-melanocyte stimulating hormone. Science 1983; 221: 192–193.
Thies FL, Karch H, Hartung H-P,, Giegerich G . The ClpB protein from Campylobacter jejuni: molecular characterization of the encoding gene and antigenicity of the recombinant protein. Gene 1999; 230: 61–67.
Inui A . Eating behavior in anorexia nervosa—an excess of both orexigenic and anorexigenic signalling? Mol Psychiatry 2001; 6: 620–624.
Takagi K, Legrand R, Asakawa A, Amitani H, François M,, Tennoune N et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat Commun 2013; 4: 2685.
Kussmann M, Raymond F, Affolter M . OMICS-driven biomarker discovery in nutrition and health. J Biotechnol 2006; 124: 758–787.
Acknowledgements
This work was supported by funds from the European Union INTERREG IVA 2 Seas Program (7-003-FR_TC2N), LARC Neuroscience Network of Haute Normandie Region, France, Fonds Français Alimentation et Santé and the Estonian Ministry of Education and Science (IUT20-40).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Tennoune, N., Chan, P., Breton, J. et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl Psychiatry 4, e458 (2014). https://doi.org/10.1038/tp.2014.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.98
This article is cited by
-
Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota
Journal of Animal Science and Biotechnology (2023)
-
Anorexia nervosa and microbiota: systematic review and critical appraisal
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2023)
-
The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice
Nature Microbiology (2023)
-
A critical analysis of eating disorders and the gut microbiome
Journal of Eating Disorders (2022)
-
Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms
Scientific Reports (2021)