Abstract
Studying monoaminergic seasonality is likely to improve our understanding of neurobiological mechanisms underlying season-associated physiological and pathophysiological behavior. Studies of monoaminergic seasonality and the influence of the serotonin-transporter-linked polymorphic region (5-HTTLPR) on serotonin seasonality have yielded conflicting results, possibly due to lack of power and absence of multi-year analyses. We aimed to assess the extent of seasonal monoamine turnover and examined the possible involvement of the 5-HTTLPR. To determine the influence of seasonality on monoamine turnover, 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA) were measured in the cerebrospinal fluid of 479 human subjects collected during a 3-year period. Cosine and non-parametric seasonal modeling were applied to both metabolites. We computed serotonin (5-HT) seasonality values and performed an association analysis with the s/l alleles of the 5-HTTLPR. Depressive symptomatology was assessed using the Beck Depression Inventory-II. Circannual variation in 5-HIAA fitted a spring-peak cosine model that was significantly associated with sampling month (P=0.0074). Season of sampling explained 5.4% (P=1.57 × 10−7) of the variance in 5-HIAA concentrations. The 5-HTTLPR s-allele was associated with increased 5-HIAA seasonality (standardized regression coefficient=0.12, P=0.020, N=393). 5-HIAA seasonality correlated with depressive symptoms (Spearman’s rho=0.13, P=0.018, N=345). In conclusion, we highlight a dose-dependent association of the 5-HTTLPR with 5-HIAA seasonality and a positive correlation between 5-HIAA seasonality and depressive symptomatology. The presented data set the stage for follow-up in clinical populations with a role for seasonality, such as affective disorders.
Similar content being viewed by others
Introduction
Seasonal variation in monoaminergic transmission may be one of the mechanisms explaining circannual fluctuations in behavior, which range from suicide in humans1, 2, 3, 4 to mating in animals.5, 6 The monoamines dopamine (DA) and serotonin (5-HT) have pivotal roles in human behavior. 5-HT regulates a plethora of behavioral, cognitive and physiological functions, such as mood, aggression, reward, sexuality, attention, memory and perception;7, 8 DA mediates a similarly diverse list of neuroendocrine, behavioral and neurophysiological actions.9 Although preliminary evidence indicates that monoaminergic transmission in human cerebrospinal fluid (CSF) varies between seasons, the results of such studies are inconsistent.10, 11, 12, 13 Limited sample sizes and absence of multi-year analyses constitute the foremost explanations behind such discrepancies. Insufficient power of these investigations further precludes reliable estimations of the explained variances in monoamine transmission by seasonal factors. As a consequence, it has not been unequivocally determined whether and to what degree seasonal factors explain variation in monoaminergic transmission.
Studying monoaminergic seasonality is likely to improve our understanding of neurobiological mechanisms underlying season-associated physiological and pathophysiological behavior, such as suicide and seasonal affective disorder (SAD). Although the heritability (h2) of seasonality in monoamine turnover is unknown, twin studies indicate that seasonal fluctuations in human behavior (for example, sleep and mood) are highly heritable, particularly in men (h2=69%).14, 15 The genotype most extensively investigated for its role in monoaminergic seasonality is the s/l (short/long) polymorphism of the 5-HT transporter-linked polymorphic region (5-HTTLPR). By measuring internal jugular venoarterial 5-hydroxyindoleacetic acid (5-HIAA), the minor s-allele of this genotype was found to be associated with a more than twofold greater brain 5-HT turnover rate than the major allele.16 Notably, seasonality in 5-HT transporter (5-HTT) binding measured with positron emission tomography (PET) in the putamen is also associated with the s-allele of the 5-HTTLPR.17 Furthermore, other lines of evidence hint at an impact of this polymorphism on a variety of 5-HT seasonality measures in humans, including 5-HT concentrations in blood, seasonal behavior and 5-HTT binding measured with PET and single-photon emission computed tomography.18, 19, 20, 21, 22 However, these studies investigating the influence of the 5- HTTLPR on measures of 5-HT seasonality are inconsistent with regard to seasonal directionality, that is, whether the s-allele increases or diminishes 5-HT seasonality. Such incongruities may have originated from the small and diverse study populations they were based on.17, 18, 19, 20, 21 A well-powered genetic study targeting 5-HT seasonality in a homogeneous sample of subjects has the potential to resolve these inconsistencies. In addition, unraveling the seasonality of monoamine turnover and clarifying the impact of the 5-HTTLPR on 5-HT seasonality may open avenues for clinical research. For example, 5-HTT aberrations have been detected in SAD23 and comparisons of seasonal patterns in (5-HTTLPR mediated) 5-HT turnover between SAD cases and controls may aid in the elucidation of the unknown pathophysiology of this disorder.
Based on a recent study by our group,12 we reasoned that season of sampling and season of birth influence 5-HIAA but not homovanillic acid (HVA) concentrations in human CSF. To unambiguously assess the association of such seasonal factors with monoamine turnover, CSF measurements were carried out in 479 individuals over three consecutive years. Given the influence of 5-HTTLPR s-allele carrier status on seasonality in 5-HTT binding,17 we hypothesized that a relatively large sample size would allow us to detect dose-dependent effects of the 5-HTTLPR on seasonality of 5-HT turnover (here defined as CSF 5-HIAA concentrations). We accordingly aimed to clarify whether this polymorphism predicts seasonal variation in 5-HIAA levels in human CSF. To that end, we first computed per-subject 5-HIAA seasonality values and subsequently analyzed the association of these values with the 5-HTTLPR s/l polymorphism. Finally, to extend the findings to the behavioral level, we correlated 5-HIAA seasonality with depressive symptomatology.
Materials and methods
Subjects
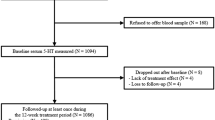
Subject collection procedures have been described elsewhere.12 In brief, 479 volunteers were recruited at outpatient preoperative screening services in four hospitals in and around Utrecht, The Netherlands, from 28 August 2008 until 31 August 2011. The ethics committee at the University Medical Center Utrecht (UMCU) and all local ethics committees approved this study. Written informed consent was obtained from the participants. Seasonal variation in monoaminergic transmission was also analyzed in a subset of the study population in a previous study (N=223, that is, 47% of the study population).12 We included patients (i) undergoing spinal anesthesia for minor elective surgical procedures; (ii) ranging between 18–60 years of age; and (iii) with four grandparents born in The Netherlands or other North-Western European countries (Belgium, Germany, the United Kingdom, France and Denmark). Each candidate participant received a personal telephone interview to exclude subjects with a self-reported history of psychotic or major neurological disorders (stroke, brain tumors and neurodegenerative diseases) and to record any use of psychotropic medication. A self-reported history of non-psychotic unipolar affective, attention-deficit-hyperactivity and anxiety disorders was allowed and recorded if applicable.
Collection of CSF, DNA and 5-HIAA measurements
These methods have been described elsewhere.12 In brief, a single sample of 6 ml of CSF was suctioned from each (sitting) participant and transported the same day to the laboratory, where fractions of 0.5 and 1 ml were immediately stored at −80 °C. Concentrations of 5-HIAA and HVA in CSF were measured using high-performance liquid chromatography, as described previously.12 A whole-blood sample was used to extract genomic DNA using standard techniques in a subset of the study population (N=414).
Genotyping procedures
As part of an on-going project, genotype data of 414 subjects were collected using the Illumina HumanOmniExpress Beadchip at the UCLA Neurosciences Genomics Core (UNGC). Quality control was conducted as described in the Supplementary Methods, leaving 398 individuals. We used a recently developed machine-learning method of vertex discriminant analysis validated for Northern European populations to predict the 5-HTTLPR polymorphism.24 In brief, this genotype prediction method prognosticates the 5-HTTLPR genotype based on eight single-nucleotide polymorphisms (SNPs). Three of the eight SNPs had been imputed, as described in the Supplementary Methods. Imputation r2 values for these three SNPs were 1.00 (rs1487971; rs7217677) and 0.94 (rs887469). All eight SNPs passed the quality control thresholds outlined in the Supplementary Methods (<2% genotyping missingness, Hardy–Weinberg equilibrium P>1 × 10−6, and minor allele frequency >0.05).
Seasonal modeling
Collinearity between sampling and birth dates was not detected (Spearman’s rho=0.03, P=0.5). To test the association of season of sampling and season of birth with 5-HIAA concentrations, nonlinear quantile regression (nlqr)—modeling seasonal variation in the median 5-HIAA values per month assuming a nonlinear cosine-shaped relationship—was chosen before inspecting circannual metabolite concentration variability.12 To validate the cosine function and model concentrations per day non-parametrically, LOESS (locally weighted scatterplot smoothing, span=0.75) was employed.12 Given the highly significant previous seasonal findings, we reasoned that a twice as large study population would provide sufficient power to detect seasonal fluctuations.12 The same covariates as in a previous study on monoamine metabolite seasonality were incorporated, that is, age and sex for 5-HIAA and weight and lumbar interspace level (L3-L4 vs L4-L5 vs L5-S1) in addition to these two for HVA (also see this reference for all candidate covariates tested, for example, time of day of sampling and psychotropic medication).12 Nlqr models were fitted for a one- and two-peak scenario as outlined in the Supplementary Methods. The best fitting model of these two scenarios (defined as the one showing less deviance) was chosen per metabolite. The nlqr significance level was Bonferroni corrected (α=0.05/4=0.0125 as both season of sampling and season of birth effects were tested for 5-HIAA and HVA). Whenever 3 months with highest median metabolite concentrations were sequential, the Kruskal–Wallis (K–W) test was used to compare metabolite levels between that season and the other seasons taken together (α=0.05). Although duration of bright sunlight has been associated with the rate of 5-HT production,13 our data (Results section) were not compatible with a correlation between sunlight exposure and 5-HIAA as these concentrations were highest in mid spring, whereas rapidly dropping in summer. We therefore did not investigate sunlight exposure effects on 5-HIAA concentrations in CSF. We then computed the explained variance in metabolite concentration by season using analysis of covariance, setting metabolite concentrations as dependent variables; sampling or birth season (dichotomized: three consecutive peak months vs all other months of the year) as independent variables; and incorporating age and sex as covariates. The sum of squares for seasonal effect was then divided by the total sum of squares (α=0.05). These analyses were performed with the statistical software packages SPSS (IBM SPSS Statistics for Mac, Version 20.0, Armonk, NY, USA) and RStudio (www.rstudio.com).
Computation of 5-HIAA seasonality values and association testing of the 5-HTTLPR
To assess the genetic association with 5-HIAA seasonality, we first calculated 5-HIAA seasonality values for the entire study population. We define 5-HIAA seasonality as the degree to which CSF 5-HIAA levels vary by season of sampling, that is, the difference between the observed 5-HIAA value and the 5-HIAA value predicted by the best fitting nlqr cosine model. Measured 5-HIAA values above the cosine during the 6 months surrounding the peak of the cosine (here: January–June) and values below the cosine during the rest of the year were defined as positively seasonal, whereas all other values were deemed negatively seasonal. In other words, positive 5-HIAA seasonality values correspond to larger predicted amplitudes than negative values (see Figure 1a for a graph of the measured values and their direction of seasonality). In the Supplementary Methods, we give examples of how observed 5-HIAA values translate into seasonality values and of the 5-HIAA values and numbers of subjects per sampling month.
(a). Graph displaying measured 5-HIAA data points per sampling month and 5-HIAA seasonality values (N=479). Measured 5-HIAA concentrations (nmol l−1) per subject are plotted against sampling month. Positively seasonal values are green and negatively seasonal values red. The covariates age and sex are included in the model, explaining why some values in January–June are green when under and red when above the cosine curve (and vice versa for July–December). Red line represents the cosine; black line the LOESS; dashed line the 95% confidence intervals (CIs) of the cosine; and whiskers 95% CIs of median CSF 5-HIAA concentrations per sample month. (b) Median CSF 5-HAA concentrations (in nmol l−1) are plotted against month of CSF sampling (N=479). Bold line represents cosine, thin line represents LOESS and dashed lines represent 95% CIs of the cosine. Whiskers indicate 95% CIs of median CSF 5-HIAA concentrations.
Genotype summary statistics were generated in Plink V1.07.25 To test for the association between the 5-HTTLPR s/l polymorphism and the 5-HIAA seasonality, a linear additive model was run in Plink V1.07.25 The same linear model was applied to Beck Depression Inventory II (BDI-II) scores to rule out direct effects of this genotype on depressive symptomatology. As age and sex were included in the model used for computation of 5-HIAA seasonality values, we provide the following association statistics for linear models with and without these covariates (α=0.05): standardized regression coefficients (β) that correspond to a change in 5-HIAA seasonality by an increase in the number of s-alleles, that is, from 0 to 1 to 2; and P-values. As a test of robustness, we repeated the analysis on a subset of patients resulting from excluding subjects currently on psychotropic medication or with a self-reported psychiatric history. To rule out direct associations of the 5-HTTLPR with CSF 5-HIAA, the association of the 5-HTTLPR s/l polymorphism with absolute concentrations of CSF 5-HIAA was tested.
Assessment of depressive symptoms and correlation analysis with 5-HIAA seasonality
Affective disorders (SAD and bipolar II disorder, in particular) are most commonly associated with seasonal behavioral fluctuations.26 We therefore assessed the association of seasonal variation in 5-HT transmission with depressive symptomatology as a quantitative trait. To that end, all participants were asked to fill out the Dutch version of the BDI-II27 online within 2 weeks after their surgical elective procedure. We chose web-based symptom questionnaires as these have been validated as reliable assessment tools in a range of epidemiological studies28, 29 and may decrease socially desirable responses compared to face-to-face interviews or questionnaires that are filled out in clinical settings.30, 31 To test whether depressive symptoms show seasonal variation, we applied the best fitting of models 1a and b (Supplementary Methods) to BDI-II scores. We then used the non-parametric Spearman’s correlation test to estimate the possible correlation between 5-HIAA seasonality values and BDI-II total scores (α=0.05), as total BDI-II scores were not normally distributed (K–S two-tailed P<0.001, Supplementary Methods). To rule out direct associations between absolute 5-HIAA values and BDI-II scores, correlations between the CSF 5-HIAA and BDI-II scores were also estimated using Spearman’s correlation.
Results
Descriptive statistics
The study population consisted of 479 subjects, of whom 71% were male (Table 1). The mean (s.d.) concentrations of 5-HIAA and HVA were 169 (68.6) and 217 (75.1) nmol l−1, respectively (Supplementary Figure 1). Twenty-three subjects (4.8%) had a self-reported history of psychiatric illness and 13 (2.7%) were on psychotropic medication during the lumbar puncture.
Monoamine metabolite seasonality
(Figures 1 and 2) For 5-HIAA, the one-peak nlqr-model sampling model showed less deviance than the two-peak model (Supplementary Methods) and was significant (P of the amplitude of the cosine=0.0074, Figure 1b). The LOESS followed a similar pattern to the cosine, validating the chosen model (Figure 1b). Figure 1a provides the raw 5-HIAA measurements per subject and sampling month. Peak and trough concentrations were in late March (tmax=3.8) and September (tmin=9.8), respectively. Concentrations were 44% increased in spring compared with the other months. In the Supplementary Methods, summary statistics per nlqr-model are given. Three consecutive spring months (March, April and May) showed highest mean and median 5-HIAA levels and differed from the other months considered together: the mean (s.d.) 5-HIAA concentration in these three months was 195 (73.1), whereas in the other months the mean was 158 (64.1) nmol l−1 (K–W test P=6.4 × 10−7, Figure 2). The explained variance in 5-HIAA concentrations by sampling in these three months was 5.4% (P=1.57 × 10−7). The 5-HIAA season of birth results were nonsignificant.
For HVA, no significant associations with sampling month or birth month were found (Supplementary Methods).
Association of the 5-HTTLPR genotype with 5-HIAA seasonality
(Figure 3) After genotype quality control (Supplementary Methods), 398 subjects remained, for 393 from whom 5-HIAA levels were available. The genotypes were in Hardy–Weinberg equilibrium (P>0.5) and the numbers of subjects per genotype were: 73 (19%) S/S, 192 (49%) S/L and 128 (33%) L/L. The mean (s.e.) 5-HIAA seasonality values per genotype were: 11.39 (7.61), 0.13 (4.96) and −10.22 (5.41), respectively (Figure 3). A dose-dependent positive association of the s-allele with 5-HIAA seasonality was detected in the models with and without covariates age and sex (β=0.12, P=0.020; and β=0.11, P=0.023, respectively). When excluding subjects on psychotropic mediation or with a self-reported psychiatric history, the results did not change (β=0.12, P=0.019). Neither absolute concentrations of CSF 5-HIAA nor BDI-II scores were associated with the 5-HTTLPR (P=0.2 and 0.8, respectively).
Correlation between 5-HIAA seasonality and depressive symptoms
Four hundred and six (85%) of the 479 subjects filled out the BDI-II; the mean total score (s.d.) was 4.48 (5.32). For 345 subjects, both 5-HIAA seasonality values and BDI-II scores were available. No seasonal patterns in mood symptoms were detected (Supplementary Methods). 5-HIAA seasonality correlated positively with total BDI-II scores (Spearman’s rho=0.13, P=0.018), whereas CSF 5-HIAA did not correlate with BDI-II scores (Spearman’s rho=0.04, P=0.45).
Discussion
In this 3-year study of CSF monoamine turnover (N=479), we confirm that 5-HIAA concentrations fit a cosine model that displays a peak in spring and a trough in fall. We additionally show that a significant proportion of the variance (5.4%) in 5-HIAA concentrations is explained by seasonality. Furthermore, we demonstrate a dose-dependent association of the 5-HTTLPR s-allele with seasonality of 5-HT turnover, whereas this polymorphism was not associated with absolute 5-HIAA levels. Finally, 5-HIAA seasonality correlated positively with depressive symptoms.
The current data are based on the largest number of years and participants in seasonal research of CSF monoaminergic transmission in humans or non-human primates to date. Multi-year sampling offers the advantage of curtailing the type-I error likelihood (in this context referring to stochastic seasonal patterns either due to chance or unique factors influencing CSF 5-HIAA in a particular season or year). Our design has thus enabled us to clarify some of the issues related to the much-debated and inconsistent findings in seasonal research of CSF monoaminergic transmission.5, 6, 10, 11, 12, 32, 33 The CSF 5-HIAA peak in spring that we detected is in agreement with a previous study (for which part of the current study population was used).12 The 5-HIAA increases from trough to peak and the summary statistics of the non-quantile regression were similar to those previous findings. Although season of birth and 5-HIAA fitted a cosine model in that study,12 the exceptionally high September concentrations constituted a limitation. The current sample size, which is more than twice the previous one and collected over almost a twofold longer period, allows for more reliable conclusions and thus makes it unlikely that 5-HIAA in human CSF is influenced by season of birth. The lack of HVA seasonality findings is in agreement with the previous study.12
We furthermore demonstrate that seasonality explains over 5% of the variation in CSF 5-HIAA, which is substantial for a biological trait. Previously discussed explanations behind explained variance in 5-HIAA by season may be divided into conception-related and melatonin metabolism-dependent.12 In brief, from an evolutionary perspective and given the association of CSF 5-HIAA with reproductive behavior,5, 6 increased 5-HT availability in spring may have driven up spring birth rates and thereby advanced survival until recently in human history. Alternatively, because 5-HT is the precursor of melatonin, metabolism pathways of these two molecules are likely to be interdependent. Melatonin is a seasonal zeitgeber for reproduction and higher basal melatonin concentrations have been demonstrated in fall than in spring across a range of species.34, 35 A CSF 5-HIAA spring peak could therefore be consequential to seasonal shifts in melatonin metabolism. A final explanation behind increased 5-HIAA in spring not previously discussed relates to 5-HTT availability. As demonstrated by single-photon emission computed tomography imaging techniques,21 5-HTT availability rises in winter. Possibly, upregulated 5-HTT facilitates reuptake of 5-HT and thereby prevents its breakdown in winter, whereas 5-HT breakdown into 5-HIAA may increase when more 5-HTT is available in spring.
The genetic findings imply that in our study population 5-HTTLPR s-allele homozygous subjects are positively seasonal (that is, tend to have relatively high CSF 5-HIAA in spring or low 5-HIAA in fall), whereas heterozygotes follow the expected cosine circannual pattern (that is, their mean seasonality value is ∼0) and L-homozygotes are negatively seasonal (that is, show a flattened seasonal curve). By demonstrating a dose-dependent association of the 5-HTTLPR s-allele with seasonal variation in 5-HIAA, we extend the results of a PET study comparing s-allele carriers to l-homozygous subjects.17 Our finding is furthermore consistent with a meta-analysis that detected less-frequent s-allele genotype frequencies in subjects with low seasonal variation in affective symptoms.22 Finally, the previously signaled seasonal variation in 5-HTT binding measured by PET in s-allele carriers17 appears in phase with the CSF 5-HIAA spring peak presented here that in turn is heightened in s-allele homozygous subjects. That this 5-HIAA peak comes one season after the increase in 5-HTT binding is in keeping with the abovementioned hypothesis about the impact of 5-HTT availability on CSF 5-HIAA. To our knowledge, no genetic study on CSF 5-HIAA seasonality in healthy subjects had been reported. In addition, the approaches adopted by all but one group17 investigating seasonality of 5-HT measures entailed dividing the year into four seasons, as opposed to per-month modeling of 5-HT seasonality. Moreover, in contrast to the previous studies on 5-HT seasonality,17, 18, 19, 21 the current sample size (N=414) yields adequate genotype distributions to analyze 5-HTTLPR dose-dependent associations with 5-HIAA seasonality.
Our observation of the positive correlation between 5-HIAA seasonality and depressive symptomatology suggests that not absolute 5-HIAA concentrations but seasonal patterns in 5-HT turnover variability could influence susceptibility to depressive symptomatology. Such a contention would be in line with epidemiological evidence pointing to a latitude-dependent seasonal variation in suicide rates, that is, spring peaks in high-latitude regions and absence of seasonal variation around the equator.36, 37, 38 Speculatively, seasonal variation in CSF 5-HIAA may be more pronounced at higher latitudes, which in turn would confer risk for season-associated depression, and thereby suicide. Furthermore, meta-analytical evidence indicates that monoamine depletion does not diminish mood in healthy subjects,39 pleading against direct causal effects of 5-HIAA concentrations in depressive symptomatology.
A limitation of this study is that psychiatric illness was not systematically assessed. However, running the same nlqr models after excluding the subjects on psychotropic medication during the lumbar puncture (N=13) and those with a self-reported history of psychiatric illness (N=23) did not change the CSF 5-HIAA seasonality findings (Supplementary Figures 2A and 2B, summary statistics not shown). A second potential caveat is that the 5-HTTLPR prediction model that we applied24 explains 85% of the variation in the 5-HTTLPR, although this figure exceeds the R2 of a different SNP-based 5-HTTLPR prediction model.40 Notably, there is no ‘gold standard’ for 5-HTTLPR genotyping and direct genotyping is notoriously cumbersome.24 Moreover, the genotype distributions reported here are identical to previously reported distributions in Dutch healthy individuals.24 Regarding the correlation between 5-HIAA seasonality and BDI-II symptoms, the sample size (N=345) and Spearman’s rank correlation coefficient (r=0.13) were relatively small. We also recognize that the absences of an independent replication cohort and repeated measurements—both of which are due to intricacies inherent in human CSF collection—constitute constraints. Finally, although subjects had fasted at least 6 h before the lumbar puncture, we cannot correct for dietary factors as such data were unavailable for the current study.
Our findings contribute to unmasking the molecular basis of seasonality of 5-HT turnover and the potential role of the 5-HTTLPR. Larger sample sizes or within-subject designs are needed to replicate our findings and further investigate the genetic basis of 5-HT seasonality. Targeted studies, such as PET, could be performed to examine the role of MAO-A,41, 42, 43 a major enzyme in the 5-HT pathway. MAO-A PET has demonstrated relevance for studying psychiatric phenotypes.41, 42, 43 Future studies should involve sampling around the equator as well as at different latitudes in the Northern and Southern hemispheres to gain a better understanding of how 5-HT turnover in CSF varies between seasons and around the globe. Moreover, such a study would provide the means to investigate the latitude-dependent role of the 5-HTTLPR and other genetic factors in 5-HIAA seasonality and possible relationships with neurobehavioral traits (such as SAD, bipolar disorder and suicide). When complemented with assessments of additional 5-HT-associated phenotypes (for example, 5-HTT binding measured with PET, platelet 5-HT uptake, platelet 5-HT content, and plasma tryptophan and melatonin levels44), such projects may comprehensively dissect the genetic determinants of 5-HT physiology. This in turn may open avenues for applications in clinical populations with an established role for seasonality, such as affective disorders.
In summary, we highlight a dose-dependent association of the 5-HTTLPR s/l polymorphism with 5-HIAA seasonality and demonstrate that increased 5-HIAA seasonality is associated with depressive symptomatology. Notably, 5-HIAA seasonal variability and not absolute 5-HIAA concentrations show associations with genetic background and mood symptoms, underscoring the biological relevance of 5-HT seasonality.
References
Bjorksten KS, Bjerregaard P, Kripke DF . Suicides in the midnight sun--a study of seasonality in suicides in West Greenland. Psychiatry Res 2005; 133: 205–213.
Partonen T, Haukka J, Viilo K, Hakko H, Pirkola S, Isometsa E et al. Cyclic time patterns of death from suicide in northern Finland. J Affect Disord 2004; 78: 11–19.
Chew KS, McCleary R . The spring peak in suicides: a cross-national analysis. Soc Sci Med 1995; 40: 223–230.
Benedito-Silva AA, Pires ML, Calil HM . Seasonal variation of suicide in Brazil. Chronobiol Int 2007; 24: 727–737.
Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M . CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res 1997; 72: 89–102.
Zajicek KB, Price CS, Shoaf SE, Mehlman PT, Suomi SJ, Linnoila M et al. Seasonal variation in CSF 5-HIAA concentrations in male rhesus macaques. Neuropsychopharmacology 2000; 22: 240–250.
Berger M, Gray JA, Roth BL . The expanded biology of serotonin. Annu Rev Med 2009; 60: 355–366.
Canli T, Lesch KP . Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 2007; 10: 1103–1109.
Bjorklund A, Dunnett SB . Dopamine neuron systems in the brain: an update. Trends Neurosci 2007; 30: 194–202.
Brewerton TD, Berrettini WH, Nurnberger JI Jr., Linnoila M . Analysis of seasonal fluctuations of CSF monoamine metabolites and neuropeptides in normal controls: findings with 5HIAA and HVA. Psychiatry Res 1988; 23: 257–265.
Chotai J, Murphy DL, Constantino JN . Cerebrospinal fluid monoamine metabolite levels in human newborn infants born in winter differ from those born in summer. Psychiatry Res 2006; 145: 189–197.
Luykx JJ, Bakker SC, Lentjes E, Boks MP, van Geloven N, Eijkemans MJ et al. Season of sampling and season of birth influence serotonin metabolite levels in human cerebrospinal fluid. PLoS ONE 2012; 7: e30497.
Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD . Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002; 360: 1840–1842.
Jang KL, Lam RW, Livesley WJ, Vernon PA . Gender differences in the heritability of seasonal mood change. Psychiatry Res 1997; 70: 145–154.
Madden PA, Heath AC, Rosenthal NE, Martin NG . Seasonal changes in mood and behavior. The role of genetic factors. Arch Gen Psychiatry 1996; 53: 47–55.
Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C et al. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry 2008; 65: 38–46.
Kalbitzer J, Erritzoe D, Holst KK, Nielsen FA, Marner L, Lehel S et al. Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biol Psychiatry 2010; 67: 1033–1039.
Hanna GL, Himle JA, Curtis GC, Koram DQ, Veenstra-VanderWeele J, Leventhal BL et al. Serotonin transporter and seasonal variation in blood serotonin in families with obsessive-compulsive disorder. Neuropsychopharmacology 1998; 18: 102–111.
Neumeister A, Pirker W, Willeit M, Praschak-Rieder N, Asenbaum S, Brucke T et al. Seasonal variation of availability of serotonin transporter binding sites in healthy female subjects as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 2000; 47: 158–160.
Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH . Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry 2008; 65: 1072–1078.
Ruhe HG, Booij J, Reitsma JB, Schene AH . Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging 2009; 36: 841–849.
Johansson C, Willeit M, Levitan R, Partonen T, Smedh C, Del Favero J et al. The serotonin transporter promoter repeat length polymorphism, seasonal affective disorder and seasonality. Psychol Med 2003; 33: 785–792.
Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P et al. Enhanced serotonin transporter function during depression in seasonal affective disorder. Neuropsychopharmacology 2008; 33: 1503–1513.
Lu AT, Bakker S, Janson E, Cichon S, Cantor RM, Ophoff RA . Prediction of serotonin transporter promoter polymorphism genotypes from single nucleotide polymorphism arrays using machine learning methods. Psychiatr Genet 2012; 22: 182–188.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Kim DR, Czarkowski KA, Epperson CN . The relationship between bipolar disorder, seasonality, and premenstrual symptoms. Curr Psychiatry Rep 2011; 13: 500–503.
Beck AT SRaBG . Manual for the Beck Depression Inventory-II. Psychological Corporation: San Antonio, TX, USA, 1996.
Gosling SD, Vazire S, Srivastava S, John OP . Should we trust web-based studies? A comparative analysis of six preconceptions about internet questionnaires. Am Psychol 2004; 59: 93–104.
Ekman A, Dickman PW, Klint A, Weiderpass E, Litton JE . Feasibility of using web-based questionnaires in large population-based epidemiological studies. Eur J Epidemiol 2006; 21: 103–111.
Joinson A . Social desirability, anonymity, and internet-based questionnaires. Behav Res Methods Instrum Comput 1999; 31: 433–438.
Buchanan T, Smith JL . Using the Internet for psychological research: personality testing on the World Wide Web. Br J Psychol 1999; 90, Pt 1 125–144.
Csernansky JG, Faull KF, Pfefferbaum A . Seasonal changes of CSF monoamine metabolites in psychiatric patients: what is the source? Psychiatry Res 1988; 25: 361–363.
Losonczy MF, Mohs RC, Davis KL . Seasonal variations of human lumbar CSF neurotransmitter metabolite concentrations. Psychiatry Res 1984; 12: 79–87.
Garidou ML, Vivien-Roels B, Pevet P, Miguez J, Simonneaux V . Mechanisms regulating the marked seasonal variation in melatonin synthesis in the European hamster pineal gland. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1043–R1052.
Macchi MM, Bruce JN . Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 2004; 25: 177–195.
Heerlein A, Valeria C, Medina B . Seasonal variation in suicidal deaths in Chile: its relationship to latitude. Psychopathology 2006; 39: 75–79.
Parker G, Gao F, Machin D . Seasonality of suicide in Singapore: data from the equator. Psychol Med 2001; 31: 549–553.
Partonen T, Haukka J, Nevanlinna H, Lonnqvist J . Analysis of the seasonal pattern in suicide. J Affect Disord 2004; 81: 133–139.
Ruhe HG, Mason NS, Schene AH . Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry 2007; 12: 331–359.
Vinkhuyzen AA, Dumenil T, Ryan L, Gordon SD, Henders AK, Madden PA et al. Identification of tag haplotypes for 5HTTLPR for different genome-wide SNP platforms. Mol Psychiatry 2011; 16: 1073–1075.
Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA et al. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch Gen Psychiatry 2011; 68: 817–826.
Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry 2009; 66: 1304–1312.
Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry 2010; 67: 468–474.
Wirz-Justice A, Richter R . Seasonality in biochemical determinations: a source of variance and a clue to the temporal incidence of affective illness. Psychiatry Res 1979; 1: 53–60.
Acknowledgements
We thank the participants for contributing biofluids and behavioral measures. We also acknowledge Drs JTA Knape, T Kappen, P Keijzers and P Vaessen for their assistance in developing our CSF sampling protocol and for the CSF samplings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Luykx, J., Bakker, S., van Geloven, N. et al. Seasonal variation of serotonin turnover in human cerebrospinal fluid, depressive symptoms and the role of the 5-HTTLPR. Transl Psychiatry 3, e311 (2013). https://doi.org/10.1038/tp.2013.84
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2013.84
Keywords
This article is cited by
-
The effect of meteorological variables on suicide
International Journal of Biometeorology (2020)
-
The effect of seasonal changes and climatic factors on suicide attempts of young people
BMC Psychiatry (2017)
-
Peripheral blood gene expression profiles linked to monoamine metabolite levels in cerebrospinal fluid
Translational Psychiatry (2016)