Abstract
Individual changes in dopamine-related genes influence prefrontal activity during cognitive-affective processes; however, the extent to which common genetic variations combine to influence prefrontal activity is unknown. We assessed catechol-O-methyltransferase (COMT) Val108/158Met (rs4680) and dopamine D2 receptor (DRD2) G-T (rs2283265) single nucleotide polymorphisms and functional magnetic resonance imaging during an emotional response inhibition test in 43 healthy adults and 27 people with schizophrenia to determine the extent to which COMT Val108/158Met and DRD2 G-T polymorphisms combine to influence prefrontal response to cognitive-affective challenges. We found an increased number of cognitive-deficit risk alleles in these two dopamine-regulating genes predict reduced prefrontal activation during response inhibition in healthy adults, mimicking schizophrenia-like prefrontal hypoactivity. Our study provides evidence that functionally related genes can combine to produce a disease-like endophenotype.
Similar content being viewed by others
Introduction
In humans, the dopamine system has a crucial role in mediating cognitive and affective processes. Aberrant dopamine neurotransmission is thought to underlie the symptoms of schizophrenia, a disease with a genetic basis. The extent to which common genetic variations controlling cortical dopamine signalling can combine to influence cognitive-affective neural processing is uncertain.
Pharmacological intervention studies with dopamine antagonists/agonists in healthy individuals have revealed dopaminergic modulation of prefrontal cortex activity during executive control and working memory1, 2, 3 and in limbic circuitry during emotion perception and regulation.4,5 Polymorphisms in genes controlling human dopamine neurotransmission influence prefrontal activity.6, 7, 8 Common single nucleotide polymorphisms (SNPs) in the catechol-O-methyltransferase (COMT) gene (rs4680) determines activity of the main enzyme that catabolizes cortical dopamine9 and the dopamine D2 receptor (DRD2) gene (rs2283265) results in an increase in alternatively spliced short (D2S) isoform relative to long isoform (D2L) in the cortex.6,10 Prefrontal function and its dependent cognitive processes are regulated by opposing D1- and D2-mediated action.10,11 Dopamine-dependent prefrontal response arguably relies on the regulation of both dopamine availability and the relative balance of D2S/D2L receptor-mediated action.6,10
Association studies of COMT and DRD2 polymorphisms have produced inconclusive results in relation to schizophrenia risk.12,13 Several studies have reported an association between the COMT Val allele or the DRD2 T allele and reduced performance on prefrontal cognitive tests in conjunction with changes in prefrontal activity in healthy individuals.6,14,15 This is consistent with the idea that genetic variability in dopamine signalling relates more directly to the intermediate endophenotype of relatively compromised prefrontal function as opposed to psychiatric diagnoses. What is not known is whether and how these genetic variations combine to confer a prefrontal ‘risk state’ in healthy people during cognitive-affective challenges. Given evidence of differential effects of dopamine genotypes on prefrontal function in schizophrenia,15,16 it is also unclear whether this putative genetic influence in healthy individuals would be similar to the illness state.
We aimed to determine the extent to which COMT Val108/158Met (rs4680) and DRD2 G-T (rs2283265) polymorphisms combine to influence prefrontal response to cognitive-affective challenges in healthy individuals and in schizophrenia. We predicted that inheritance of a greater number of prefrontal dysfunction ‘risk alleles’ (COMT Val and DRD2 T alleles) would be associated with reduced prefrontal activation in healthy individuals, producing a state similar to prefrontal hypoactivity observed in schizophrenia during cognitive-affective processing.17 We further predicted that because of DRD2 antagonism by antipsychotics, the oligogenic influence on prefrontal activity may be obscured in schizophrenia.
Materials and methods
Participants
Forty-eight healthy adults and 39 people with schizophrenia or schizoaffective disorder participated in the study. All participants were screened for the following exclusion criteria: (1) a history of neurological disorder, (2) head injury with loss of consciousness, (3) cardiovascular or metabolic disease such as uncontrolled hypertension or diabetes, (4) a history of developmental disorder, such as dyslexia, (5) substance dependence or abuse in the past 5 years, and (6) contraindications for magnetic resonance imaging (MRI), including the presence of ferromagnetic implants, pregnancy and claustrophobia. Healthy participants were also excluded if they had a personal history of any psychiatric disorder and/or a first degree relative with a psychotic disorder and people with schizophrenia were also excluded if they had a concurrent Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition axis I diagnosis. See Table 1 for a demographic characterization of the groups.
Diagnosis in people with schizophrenia or schizoaffective disorder was confirmed by means of a standardized Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.18 Symptom severity was assessed with the Positive and Negative Syndrome Scale.19 Estimates of current full-scale intelligent quotient were obtained from an abbreviated version of the WAIS-III20 that includes Digit Symbol Substitution, Arithmetic, Picture Completion and Similarities subtests, and premorbid intelligent quotient estimates were assessed using the WTAR 21 in people with schizophrenia. All of the people with schizophrenia were receiving antipsychotics: amisulpride (n=3), aripiprazole (n=2), clozapine (n=8), clozapine and aripiprazole (n=1), clozapine and risperidone (n=1), olanzapine (n=4), quetiapine (n=2), quetiapine and ziprasidone (n=1), risperidone (n=3), risperidone and olanzapine (n=1), zuclopenthixol and quetiapine (n=1).
All participants gave written informed consent according to the procedures approved by the South Eastern Sydney and Illawarra Area Health Service and the University of New South Wales Human Research Ethics Committees.
Genotyping and oligogenic score
DNA was isolated from 8 ml samples of whole blood collected in EDTA tubes using a PUREGENE DNA purification kit (QIAGEN, Chadstone Centre, VIC, Australia) following the manufacturer’s protocols. Genomic DNA from each individual was prepared at a dilution of 10 ng/μl. Genotyping was performed using Applied Biosystems (Mulgrave, VIC, Australia) TaqMan SNP assays designed for use with an ABI Prism 7900HT Fast Real Time quantitative PCR system for the DRD2 SNP rs2283265 (G-T) and the COMT Val108/158Met SNP rs4680. A PCR solution consisting of 2.5 μl of 2 × Universal mastermix with ROX, 0.125 μl genotyping probe and 0.375 μl double-distilled H2O was prepared, added into a 384-well plate containing 1 μl of genomic DNA from each sample and pipetted up and down to ensure the genomic DNA and PCR solution were sufficiently mixed. All SNP genotyping results were then analysed with Sequence Detection Software version 2.3 (ABI, Life Technologies, Mulgrave, VIC, Australia). Both SNPs were found to be in the Hardy–Weinberg equilibrium in both the healthy sample and people with schizophrenia.
We tallied the number of risk alleles for each individual to generate an ‘oligogenic score’, which we tested as a predictor of prefrontal cortex activation. Oligogenic score was defined by the number of Val alleles and T alleles, such that individual scores ranged from 0 to 4 (see Table 2). To calculate the oligogenic score, we propose a parsimonious model of combined genetic influence by assuming an equal and additive contribution of both genetic polymorphisms based on the observation of a similar magnitude of change in prefrontal DRD2 mRNA levels6,15 and prefrontal COMT enzymatic activity9 based on these SNPs and similar odds ratios22, 23, 24, 25 for these SNPs.
Magnetic resonance imaging
MRI was performed using a 3 Tesla Phillips Achieva MRI scanner, with an eight-channel bird cage head coil at Neuroscience Research Australia, Randwick, Australia. A T1-weighted high-resolution anatomical scan was obtained for each participant for registration purposes and to screen for anatomical abnormalities (TR: 5.4 ms; TE: 2.4 ms; FOV: 256 mm; matrix: 256 × 256; sagittal plane; slice thickness: 1 mm; 180 slices). Functional T2*-weighted images were obtained using a gradient echo-planar imaging sequence, TR/TE=3000/30; 32 interleaved slices, covering the whole brain, thickness=3 mm, gap=1 mm; voxel size: 3 × 3 × 3 mm3; scan repetitions=212; flip angle=90°; field of view=24 cm.
Emotional go/no-go task
All participants received a functional MRI (fMRI) scan while completing an emotional go/no-go test in which they respond to visually presented words with neutral meaning while inhibiting responses to words with negative emotional meaning. We selected a verbal emotional response inhibition test as it robustly produces activation of prefrontal cognitive control circuitry in healthy people and it is sensitive to diagnostic differences in which people with schizophrenia show prefrontal hypoactivity.17 The words used in the emotional go/no-go test were selected from the Affective Norms for English Words26 stimulus set, which provides normative valence and arousal ratings. Four conditions were alternated in a block design: (1) responding to negative words while inhibiting responses to neutral words, (2) responding to neutral words while inhibiting responses to negative words, (3) responding to positive words while inhibiting responses to neutral words and (4) responding to neutral words while inhibiting responses to positive words. A simple instruction cue (for example, ‘NEGATIVE’) was presented on screen at the start of each block indicating the valence of the stimuli requiring a response. All stimuli were visually presented in the centre of the screen. Participants were asked to press a response button as quickly as possible when a stimulus of the required valence appeared. Each task block consisted of 10 stimuli and each condition was presented four times for a total of 160 stimuli. For the purpose of this study, we focused on the negative versus neutral conditions, given prior evidence of more pronounced diagnostic group differences on negative go/no-go conditions17 and the association among negative affect, COMT genotype,27, 28, 29 and D2 receptor blockade.30
Statistical analyses
Behavioural analyses
Before scanning, all participants rated the word stimuli used in the fMRI test as positive, negative or neutral using a tick box questionnaire format, which allowed us to take into account individual differences in stimulus ratings when analysing the behavioural performance data acquired during fMRI scanning (for example, if a normative ’neutral’ stimulus was rated as ‘negative’ by a participant, a button press response following that stimulus was scored as correct on a ‘NEGATIVE’ task block and as an error on a ‘NEUTRAL’ block). Repeated measures analysis of variances were performed on the mean percentage correct and on the average reaction times (RTs) for ‘GO’ trials with group (healthy controls vs people with schizophrenia) as a between-subjects variable and task condition (inhibit negative vs inhibit neutral) as a within-subjects variable. The analysis was repeated with ‘risk status’ (people with schizophrenia vs high-risk controls vs low-risk controls) as the between-subjects variable. Finally, a series of correlation analyses was performed to test the relationship of oligogenic score to demographic (including education and intelligence) and performance variables (RT and accuracy) in the healthy control sample.
fMRI processing and analysis
All processing and analyses were performed with SPM8 (Wellcome Trust Centre for Neuroimaging). All data sets were screened for excessive motion (>3 mm in x, y or z direction or >3° rotation) and magnetic resonance artefacts. We excluded five participants because of incidental findings of abnormalities on structural MRI (two healthy controls; three patients), seven because of excessive movement (two healthy controls; five patients), four because of scanning artefacts (one healthy control; three patients) and one patient because of very poor task performance (at chance level), such that the analysed sample consisted of 70 people (27 patients and 43 healthy adults). Movement parameters were also included as regressors in the first-level model. Three dummy scans were obtained before each fMRI data acquisition to allow for the equilibration of the MRI signal. Functional images were realigned to the first image in the time series and coregistered to the anatomical image. All images were normalized to the Montreal Neurological Institute (MNI) anatomical template using a nonlinear 12 parameter affine transformation. Images were smoothed with a 10-mm full width half maximum Gaussian kernel.
At the first level of analysis, the contrast of interest was defined as condition 2 (inhibit responses to negative words) minus condition 1 (inhibit responses to neutral words) to assess the magnitude of the difference in blood oxygenation level-dependent (BOLD) signal for inhibiting responses to negative words. At the second level, we conducted a whole-brain single sample T-test in the healthy control group to reveal areas of significant activation at the group level. To correct for false-positive errors, we used a modified double threshold approach, which was originally proposed by Forman et al.31 To ensure that we were able to identify all the major task-relevant activation clusters, we used a P-value of 0.005 combined with a voxel extent of 58, based on Monte Carlo simulations conducted with a custom script (cluster_threshold_beta.m obtained from www2.bc.edu/~slotnics/scripts.htm), employing the following parameters: acquisition matrix (80 × 80), original voxel dimensions (3 × 3 × 3), number of slices (32), full width half maximum set to 10 resampled voxel resolution (2 × 2 × 2), mask (none), corrected P-value (0.05), voxel-based P-value (0.005) and iterations (1000).
The resulting clusters were selected as functional regions of interest (ROIs) and contrast values were extracted for each ROI using MarsBar,32 representing the mean value across all voxels within that ROI. Before running between groups’ ROI analyses, outlier contrast values were defined as ±2 s.d. from the group mean and data were removed from further analysis if outlier values occurred for the majority of ROIs. This resulted in an additional exclusion of data from one patient and two controls, such that n=41 for the controls and n=26 for the patients in the ROI analysis. Differences in BOLD response as a function of diagnostic group were assessed by means of the general linear model, which included group as a between-subjects factor and age, education level and gender as demographic covariates. The contrast values for each of the ROIs were subsequently entered into separate regression analyses with the oligogenic score as the predictor variable separately in the control and patient groups.
To further assess whether an increased load on the prefrontal risk alleles was associated with schizophrenia-like hypofrontality, the control group was divided into a low-allelic load (‘low risk’) group (oligogenic score <2; n=15) and a high-allelic load (‘high risk’) group (oligogenic score ⩾2; n=26). We performed univariate analysis of variances on the contrast values from each of the ROIs, with a group factor (high-allelic load controls, low-allelic load controls and people with schizophrenia) while controlling for age, sex and education. Significant main effects were followed-up with post hoc least significant difference tests. Finally, as concurrent DRD2 blockade via antipsychotics may affect the same neural pathways as those presumed to be influenced by dopaminergic polymorphisms,33 we examined the effect of mean daily chlorpromazine equivalent dose34,35 on brain activation in people with schizophrenia. We constructed general linear models for each of the ROIs with mean daily chlorpromazine equivalent dose as a continuous predictor to assess the relationship with BOLD response.
Results
Behavioural results
Significant main effects of group (schizophrenia vs control) indicated that people with schizophrenia were impaired relative to the healthy controls in terms of accuracy, F(1,68)=14.44, P<0.001 and RT, F(1,68)=7.80, P<.01. The pattern of responding across conditions was similar between groups, as indicated by a main effect of condition on accuracy, F(1,68)=7.1, P<0.01 and RT, F(1,68)=54.4, P<0.001, and no significant interaction (F<1 for accuracy and RT). Responses were more accurate and faster in the ‘inhibit neutral’ as compared with the ‘inhibit negative’ condition (see Supplementary Data 1). An additional analysis of variance was performed to examine performance differences among the healthy controls categorized by their oligogenic score (high-risk healthy controls versus low-risk healthy controls) and people with schizophrenia. The main effect of task condition on accuracy, F(1,67)=6.10, P=0.016 and on RT, F(1,67)=54.10, P<0.001 showed slower and less accurate responses during inhibition to negative as compared with neutral words. There were significant main effects of group on accuracy, F(2,67)=7.19, P=0.001 and on RT, F(2,67)=4.07, P=0.021, but no significant interaction effects for accuracy or RT (F<1). Least significance difference post hoc tests revealed main effects of group with increased accuracy for both the high- and low-risk control groups relative to the people with schizophrenia during the inhibit negative condition, whereas no significant differences were observed between the high-risk and low-risk control subgroups in terms of accuracy or RT (see Supplementary Data 1 for detailed results).
Within groups, correlation analysis revealed no significant relationships between oligogenic score and task performance (accuracy and RT) or measures of general cognitive ability in either the people with schizophrenia or the healthy controls (see Supplementary Data 2).
fMRI results
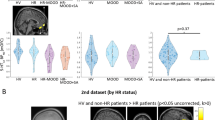
Whole-brain fMRI analysis in healthy individuals revealed five large clusters of increased activation during response inhibition to negative emotional words: right insula (MNI peak coordinates: 32 32 -2), left middle frontal gyrus (Brodmann area (BA) 10) (MNI peak coordinates: -28 48 10), right middle frontal gyrus (BA 10) (MNI peak coordinates: 28 52 10), right supplementary motor area (MNI peak coordinates: 10 24 50) and right middle frontal gyrus (BA 9) (MNI peak coordinates: 46 34 30). These areas were defined as ROIs for further analysis (see Figure 1a; for detailed results see Supplementary Data 3). There were no activation clusters surviving the statistical threshold in the sample of people with schizophrenia.
Regions showing significant activation in the healthy adults during performance of the emotional go/no-go test. (a) The contrast shown reflects inhibition of responses to negative stimuli versus neutral stimuli in the middle frontal gyrus (Brodmann area (BA) 10), the right dorsolateral prefrontal cortex (BA 9), the right supplementary motor area and the right insula. A detailed overview of the activation clusters is presented in Supplementary Data 3. (b) The bar graphs illustrate the differential effect of oligogenic score on the brain activity in healthy controls and in people with schizophrenia relative to comparison group in one of the ROIs (right BA 10). Bar graphs for the additional ROIs showing a linear relationship between oligogenic score and brain activation are provided in Supplementary Data 4.
Analysis of diagnostic group differences in BOLD response in the ROIs revealed a relative decrease in activation in people with schizophrenia during inhibition of responses to negative words in the left BA 10, F(1,62)=5.59, P=0.021, right BA 10, F(1,62)=8.06, P=0.006 and right BA 9, F(1,62)=6.03, P=0.017. No significant differences were observed in the insula and supplementary motor area ROIs. This confirms earlier findings of reduced prefrontal activation in people with schizophrenia during cognitive-affective inhibition.17
Further analysis, breaking down the healthy control group into high-risk and low-risk groups, revealed significant group differences in BOLD response in the left BA 10, F(2,61)=5.75, P=0.005, the right BA 10, F(2,61)=7.68, P=0.001 and the right BA 9, F(2,61)=6.23, P=0.003. Post hoc tests revealed that within the healthy control group, the subgroup with low genetic risk showed significantly higher levels of activation of the bilateral middle frontal gyrus (BA 10) and right middle frontal gyrus (BA 9) compared with the subgroup with high genetic risk, and the schizophrenia group (see Figure 2). The high prefrontal risk allele load subgroup did not differ significantly from the schizophrenia group in relation to activation in any of the ROIs.
Results from the univariate analysis of variances (ANOVAs) on the contrast values from each of the ROIs, with a group factor (high-allelic load controls, low-allelic load controls and people with schizophrenia) while controlling for age, sex and education. After obtaining a significant ANOVA, we performed post hoc Least significance difference (LSD) tests to compare the groups directly. The high-allelic load group did not differ from people with schizophrenia on any of the ROIs. *P<0.05; **P<0.01, ***P<0.001.
As predicted, we detected a significant linear association between increasing risk allele load and reduced activation of the left rostral prefrontal cortex BA 10, beta=−0.47, t(39)=3.28, P=0.002, right rostral prefrontal cortex BA 10, beta=−0.43, t(39)=2.98, P=0.005, right supplementary motor area, beta=−0.31, t(39)=2.05, P=0.047 and right dorsolateral prefrontal cortex BA 9, beta=−0.37, t(39)=2.52, P=0.016, in healthy participants (see Figure 1b and Supplementary Data 4). We then determined whether this allele-dose response was present or absent in schizophrenia. We found no relationship between risk allele load and brain activation in the same ROIs in schizophrenia (see Figure 1b and Supplementary Data 4, all regions P’s>0.3). Supplementary Data 4 also provides results of a power analysis to determine the power of detecting an effect in our patient sample that would have been equivalent to the effect obtained in the healthy controls. Across all ROIs, the power was estimated to be medium to large.
Finally, we also determined whether the brain activity was related to antipsychotic dosage in schizophrenia. We observed a negative association between daily chlorpromazine dose and activation of the right dorsolateral prefrontal cortex (BA 9) during the task in schizophrenia, R2=0.18, beta=−0.42, t(25)=2.27, P=0.033 (see Figure 3).
Scatter plot demonstrating the negative association between daily dose of antipsychotics expressed in daily chlorpromazine equivalents (CPZ) and dorsolateral prefrontal cortex activation in schizophrenia. Medication dose significantly predicted reduced activation of the dorsolateral prefrontal cortex (right Brodmann area (BA) 9) during the emotional response inhibition test.
Discussion
These results provide evidence that genetic variation controlling DRD2 characteristics and synaptic dopaminergic availability combine to shape prefrontal cortical response during cognitive-affective challenges and that common genetic variation may relate to schizophrenia endophenotypes through small but additive effects. In this case, inheritance of only two risk alleles on different chromosomes, both associated with prefrontal functional changes, combined to produce blunted prefrontal brain activation similar to that found in people with schizophrenia. Prefrontal cortical dopamine acting through the dopamine D1 receptor has been shown to be critical for sustaining neuronal activity during ‘prefrontal’ tasks.36 Our results extend the role of DRD2 by suggesting that prefrontal dopamine acting through DRD2 can also make critical contributions to neuronal activity during inhibitory control.
Our second main finding was that this allele-dose effect on prefrontal activation was not present in people with schizophrenia who were currently receiving antipsychotics. If prefrontal response is determined in part by dopamine acting through cortical DRD26,10 then exogenous application of a DRD2 antagonist, as occurs with antipsychotic treatment, would be expected to obscure the additive effects of common genetic polymorphisms that normally translate into functional variability in the healthy prefrontal cortex. Indeed, a higher relative dose of DRD2 blockade correlated with decreased activity of the dorsolateral prefrontal cortex, which fits with previous findings that drugs with higher affinity to the DRD2 cause a decrease in cortical BOLD signal.37 Thus, antipsychotic treatment could be considered an overriding environmental factor that blunts underlying dopaminergic genetic effects in people with schizophrenia who have been administered antipsychotics. This suggests that hypofrontality, commonly observed in schizophrenia in the context of antipsychotic treatment,17,38 has at least two potential sources: first, inheritance of risk alleles biasing the prefrontal cortex to be underactive during cognitive-affective challenges and, second, as a consequence of DRD2 blockade.
The current study has some limitations. First, the sample sizes were relatively small. This may limit generalizability and the findings thus require replication in a larger sample. However, the detection of a significant relationship between oligogenic score and brain activation in the control group does suggest that the combination of dopaminergic gene variants examined here could have a robust impact on prefrontal function and that the study was not statistically underpowered to detect this effect. In addition, the novel finding of a schizophrenia-like prefrontal activation pattern in high-risk controls is certainly noteworthy, but requires replication. Second, the absence of a linear relationship between brain function and oligogenic score in schizophrenia does not preclude that a non-linear relationship exists in schizophrenia, or that it is simply obscured by greater variability in prefrontal response and cognitive function. We performed a power analysis and the results showed that our power to detect an effect in the ROIs examined for the patient sample was medium to large, which suggests that the results are not because of lack of power in the smaller patient sample. Third, there were behavioural differences between high-risk controls and people with schizophrenia. This result may appear to be incongruent with the finding of hypofrontality in high-risk controls. This may suggest that while genetic variability in dopamine signalling in healthy individuals may have an impact on prefrontal brain activation, this is not necessarily reflected in a simple and linear way to alterations in behavioural output. However, the emotional inhibition test used in our study was not designed to be difficult and it did not have varying degrees of difficulty as other more typical executive tests such as the n-back working memory task. Thus, hypofrontality during this cognitive-affective challenge may not be reflected very well in behaviour owing to the low task demands. Regarding performance decline obtained in people with schizophrenia, it is probable that additional illness-related factors negatively affect both neural responses and associated behavioural outcomes in the emotional go/no-go task. Finally, the patient sample consisted of chronically ill patients who were medicated at the time of testing. Although our findings suggest that medication effects may have a role in reducing brain activity, many other factors contribute to increased variability in schizophrenia samples, including generalized cognitive deficits. On the basis of our findings, acutely ill, medication-free, first-episode patients would be predicted to show activation patterns that were consistent with the high-risk control group, but further research is required to clarify the relative impact of genetic variability on dopaminergic function in the context of varying illness severity or stage of illness.
In summary, we found that common polymorphisms in dopaminergic regulating genes can additively combine to produce a hypofrontality endophenotype that is characteristic of schizophrenia. However, DRD2 blockade may also contribute to prefrontal hypoactivity, which could explain the relative treatment resistance of cognitive dysfunction, suggesting that some restoration of DRD2-mediated prefrontal dopaminergic signalling may be of therapeutic benefit in schizophrenia.
References
Kimberg DY, Aguirre GK, Lease J, D'Esposito M . Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp 2001; 12: 246–257.
Gibbs SE, D'Esposito M . A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience 2006; 139: 359–371.
Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E et al. The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology 2009; 207: 35–45.
Diaconescu AO, Menon M, Jensen J, Kapur S, McIntosh AR . Dopamine-induced changes in neural network patterns supporting aversive conditioning. Brain Res 2010; 1313: 143–161.
Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR . Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 2002; 27: 1036–1040.
Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA 2007; 104: 20552–20557.
Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci 2005; 25: 5038–5045.
Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry 2006; 60: 1250–1258.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75: 807–821.
Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000; 408: 199–203.
Seamans JK, Yang CR . The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 2004; 74: 1–58.
Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry 2005; 57: 139–144.
Bertolino A, Blasi G . The genetics of schizophrenia. Neuroscience 2009; 164: 288–299.
Mier D, Kirsch P, Meyer-Lindenberg A . Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry 2010; 15: 918–927.
Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain 2009; 132 ((Pt 2)): 417–425.
Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E et al. Epistasis between the DAT 3' UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci USA 2009; 106: 13600–13605.
Vercammen A, Morris R, Green MJ, Lenroot R, Kulkarni J, Carr VJ et al. Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. J Psychiatry Neurosci 2012; 37: 379–388.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P). Biometric Research Department, New York State Psyciatric Institute: New York, NY, USA, 2007.
Kay SR, Fiszbein A, Opler LA . The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–276.
Wechsler D . Wechsler Adult Intelligence Scale. 3rd edn,The Psychological Corporation: San Antonio, TX, USA, 1997.
Wechsler D . Wechsler Test of Adult Reading (WTAR). Psychological Corporation: San Antonio, TX, USA, 2001.
Zheng C, Shen Y, Xu Q . Rs1076560, a functional variant of the dopamine D2 receptor gene, confers risk of schizophrenia in Han Chinese. Neurosci Lett 2012; 518: 41–44.
Glatt SJ, Faraone SV, Tsuang MT . Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry 2003; 160: 469–476.
Glatt SJ, Faraone SV, Lasky-Su JA, Kanazawa T, Hwu HG, Tsuang MT . Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol Psychiatry 2009; 14: 885–893.
Munafo MR, Bowes L, Clark TG, Flint J . Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry 2005; 10: 765–770.
Bradley MM, Lang PJ . Norms for English Words (ANEW): Instruction Manual and Affective Ratings. University of Florida: FL, USA, 1999.
Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci 2005; 25: 836–842.
Williams LM, Gatt JM, Grieve SM, Dobson-Stone C, Paul RH, Gordon E et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. NeuroImage 2010; 53: 918–925.
Herrmann MJ, Wurflein H, Schreppel T, Koehler S, Muhlberger A, Reif A et al. Catechol-O-methyltransferase Val158Met genotype affects neural correlates of aversive stimuli processing. Cogn Affect Behav Neurosci 2009; 9: 168–172.
Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB et al. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry 2007; 164: 630–637.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC . Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33: 636–647.
Brett M, Anton J-L, Valabregue R, Poline J-B . Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan; available on CD-ROM in NeuroImage, Vol 16, No. 2.
Weickert TW, Goldberg TE, Mishara A, Apud JA, Kolachana BS, Egan MF et al. Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol Psychiatry 2004; 56: 677–682.
Bollini P, Pampallona S, Nieddu S, Bianco M, Tibaldi G, Munizza C . Indicators of conformance with guidelines of schizophrenia treatment in mental health services. Psychiatr Serv 2008; 59: 782–791.
Woods SW . Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64: 663–667.
Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22: 3708–3719.
Roder CH, Hoogendam JM, van der Veen FM . FMRI, antipsychotics and schizophrenia. Influence of different antipsychotics on BOLD-signal. Curr Pharm Des 2010; 16: 2012–2025.
Berman KF, Torrey EF, Daniel DG, Weinberger DR . Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Arch Gen Psychiatry 1992; 49: 927–934.
Acknowledgements
We thank Alice Rothwell, Deborah Rothmond, Katherine Allen and Heng Giap Woon for their assistance with the collection and processing of the blood samples and genetic analyses. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia, grant number 568807; the University of New South Wales School of Psychiatry; Neuroscience Research Australia; the Schizophrenia Research Institute utilizing funding from the NSW Ministry of Health and the Macquarie Group Foundation; and the Australian Schizophrenia Research Bank, supported by the NHMRC of Australia, the Pratt Foundation, Ramsay Health Care, and the Viertal Charitable Foundation. CSW is a recipient of a National Health and Medical Research Council (Australia) Senior Research Fellowship (#1021970). AJS is a recipient of the Ian Scott Scholarship awarded by Australian Rotary Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Vercammen, A., Weickert, C., Skilleter, A. et al. Common polymorphisms in dopamine-related genes combine to produce a ‘schizophrenia-like’ prefrontal hypoactivity. Transl Psychiatry 4, e356 (2014). https://doi.org/10.1038/tp.2013.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2013.125
Keywords
This article is cited by
-
Association of dopamine-based genetic risk score with dynamic low-frequency fluctuations in first-episode drug-naïve schizophrenia
Brain Imaging and Behavior (2023)
-
Increased levels of a pro-inflammatory IgG receptor in the midbrain of people with schizophrenia
Journal of Neuroinflammation (2022)
-
Elevated endogenous GDNF induces altered dopamine signalling in mice and correlates with clinical severity in schizophrenia
Molecular Psychiatry (2022)
-
A splicing-regulatory polymorphism in DRD2 disrupts ZRANB2 binding, impairs cognitive functioning and increases risk for schizophrenia in six Han Chinese samples
Molecular Psychiatry (2016)