Abstract
Although aerobic methane (CH4) release from plants leads to an intense scientific and public controversy in the recent years, the potential functions of endogenous CH4 production in plants are still largely unknown. Here, we reported that polyethylene glycol (PEG)-induced osmotic stress significantly increased CH4 production and soluble sugar contents in maize (Zea mays L.) root tissues. These enhancements were more pronounced in the drought stress-tolerant cultivar Zhengdan 958 (ZD958) than in the drought stress-sensitive cultivar Zhongjiangyu No.1 (ZJY1). Exogenously applied 0.65 mM CH4 not only increased endogenous CH4 production, but also decreased the contents of thiobarbituric acid reactive substances. PEG-induced water deficit symptoms, such as decreased biomass and relative water contents in both root and shoot tissues, were also alleviated. These beneficial responses paralleled the increases in the contents of soluble sugar and the reduced ascorbic acid (AsA), and the ratio of AsA/dehydroascorbate (DHA). Further comparison of transcript profiles of some key enzymes in sugar and AsA metabolism suggested that CH4 might participate in sugar signaling, which in turn increased AsA production and recycling. Together, these results suggested that CH4 might function as a gaseous molecule that enhances osmotic stress tolerance in maize by modulating sugar and AsA metabolism.
Similar content being viewed by others
Introduction
Methane (CH4) is the most abundant reduced organic compound in the atmosphere, and also the second important greenhouse gas after carbon dioxide1,2. It was previously considered as a degradation product of organic substance by microbes under anoxic conditions3. Keppler et al.4 further reported a surprising discovery that terrestrial plants can produce CH4 under aerobic conditions. Although much controversy and debate followed this original work, a number of researchers have attempted to provide alternate explanations of the aerobic CH4 formation from plants using different scales of measurement5.
In fact, the non-microbial CH4 production from plants constitutes a significant fraction of the global CH4 sources2,6. A comprehensive understanding of CH4 in plants is that the living plants and fungi can not only emit CH4 to the atmosphere7, but also produce CH4 in plants in vivo8. Interestingly, this phenomenon was observed in the researches of nitric oxide9, carbon monoxide10, as well as hydrogen gas11. In some plant species, several chemical compounds, such as lignin, cellulose, leaf surface wax, ascorbic acid (AsA), the methyl groups of sulphur-containing amino acid methionine (Met), were suggested to be the potential precursors of CH42,5,12,13. Based on the original work, it was further reported that the aerobic CH4 formation is significantly increased by ultraviolet (UV) radiation14, heat15, water stress16, bacterial pathogen14, and physical injury17. However, the potential function of endogenous CH4 for plant responses to various stresses is unclear.
Drought is the most pervasive limitation to the achievement of potential yield in maize, and triggers various interacting events including increased abscisic acid (ABA) and reactive oxygen species (ROS) levels18. This decreased availability of water is quantified as a decrease in water potential. During water deficit stress, osmotic stress sensing and signaling are pivotal to plant water status and lead to rapid changes in gene expression19. Normally, polyethylene glycol (PEG)-6000 is considered as the best solute for mimicking a water deficient stress that is reflective of the type of stress imposed by a drying soil20. Limited water availability caused by osmotic stress and drought, could provoke a shift in the balance between ROS production and their elimination in plants21. In fact, osmotic stress-induced ROS overproduction and corresponding oxidative reactions could result in the increases in lipid peroxidation and even the loss of biomass. For example, ROS overproduction is harmful to nucleic acids, proteins, lipids, and sugar if accumulated over a certain threshold in plants22,23. In order to avoid the overproduction of ROS caused by osmotic stress, plants have evolved specific antioxidant systems to ensure a control of the cellular redox state24, and these include the non-enzymatic components (ascorbic acid, AsA; glutathione, GSH; etc), and the antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (POD), etc25. Induced sugar metabolism is also observed when plants are subjected to short periods of oxidative or osmotic stress, suggesting that soluble sugars may function as osmoprotectants during stress26. Actually, sugars are being recognized as important regulatory molecules with both signaling and putative ROS scavenging functions in plants27. Since both CH4 and sugars (in particular) are involved in carbon metabolism, the relationship between CH4 and sugar metabolism under osmotic stress still remains to be elucidated.
To explore the potential role of CH4 in PEG-induced osmotic stress and corresponding mechanism(s), two maize cultivars (Zhengdan 958 and Zhongjiangyu No.1) differing in osmotic stress tolerance were compared in this study. Time course analysis revealed that CH4 production was induced by PEG-6000 treatment in maize root tissues, especially in osmotic stress-tolerant cultivar (Zhengdan 958; ZD958). Comparatively, more toxic responses were observed in osmotic stress-sensitive cultivar (Zhongjiangyu No.1; ZJY1). Subsequent work showed that CH4 pretreatment, not only mimicked the responses of PEG-6000 in the induction of endogenous CH4 production, but also alleviated PEG-induced osmotic stress by modulating sugar and AsA metabolism in maize seedlings. Meanwhile, the suppression of ROS production was observed. Our results therefore point to a beneficial role of CH4 in plant tolerance against osmotic stress.
Results
Phenotypic comparison of two maize cultivars under PEG treatment
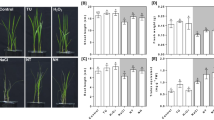
In this study, two highly productive maize cultivars, namely the osmotic-stress tolerant Zhengdan 958 (ZD958) and the sensitive Zhongjiangyu No.1 (ZJY1), were selected for osmotic stress sensitivity analysis. 5-d-old seedlings were treated with different concentrations of PEG-6000 for another 5 d, and phenotypic changes of both root and shoot tissues were monitored. PEG-6000 treatment significantly inhibited maize seedling growth in a dose-dependent manner (Fig. 1a), and the growth inhibition was more pronounced in ZJY1 than that of ZD958. For example, compared to the PEG-free control samples, the fresh weight of root tissues in ZD958 with 15%, 20%, and 25% PEG-6000 treatments decreased to 86.5 ± 7.7%, 70.9 ± 6.5%, and 39.6 ± 10.2%, and with 53.4 ± 5.4%, 39.4 ± 5.6%, and 24.5 ± 4.2% in ZJY1 (Fig. 1b). A similar trend was observed for the shoot tissues of these two maize cultivars under different concentrations of PEG-6000.
(a) 5-d-old seedlings of ZD958 and ZJY1 were transferred to half-strength Hoagland solutions containing the indicated concentrations of PEG-6000 (PEG) for another 5 d. Photographs were then taken. Bar = 10 cm. Meanwhile, fresh weight (b) and relative water content (RWC; c) were measured in both root and shoot tissues. Data are presented as means ± SE (5 root or shoot parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
To further compare responses of ZD958 and ZJY1 under PEG-6000 treatment, relative water content (RWC) was analyzed (Fig. 1c). Although no significant difference was observed in root and shoot tissues upon 15% PEG-6000 treatment in two cultivars, ZD958 exhibited higher RWC than those of ZJY1 under more severe stress conditions. Combined with the changes in fresh weight, these results confirmed thatZD958 is more osmotic stress tolerant than ZJY1 under our experimental conditions. Therefore, ZD958 was regarded as an osmotic stress tolerant cultivar and ZJY1 as a sensitive cultivar in this study. Additionally, 20% PEG-6000, a moderate concentration, was used in the following experiments.
Both methane and soluble sugar content were increased in response to PEG-induced osmotic stress
Previous results revealed that CH4 emission was increased under climatic stress conditions, and this phenomenon led to the “greenhouse effect”4,16. To investigate whether endogenous CH4 production was involved in PEG-induced osmotic stress, the endogenous CH4 production in root tissues of maize seedlings were monitored by gas chromatography (GC; Fig. 2a). Compared with the control conditions, CH4 production in root tissues of two cultivars continuously increased during a 5-d exposure to PEG-6000. Comparatively, CH4 production was more strongly enhanced in ZD958 (a tolerant cultivar) than in ZJY1 (a sensitive cultivar) under stress conditions. These results suggested that the inducement of CH4 production in vivo may be related to a protective response against osmotic stress. A similar increasing trend was observed in the soluble sugar content in the root tissues of ZD958 (in particular) and ZJY1 upon stress (Fig. 2b). Additionally, we observed that under control conditions, a higher CH4 or sugar content was detected in root tissues of ZD958 than that of ZJY1. Combined with the corresponding phenotypes (Fig. 1), these results clearly indicated a possible interrelationship between CH4 production and soluble sugar content in maize seedlings upon PEG-6000 treatment.
5-d-old maize seedlings of ZD958 and ZJY1 were transferred to half-strength Hoagland solutions containing 20% PEG-6000 for another 5 d. Endogenous methane (a) and total soluble sugar (b) contents in root tissues were determined at the indicated time points. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root parts per experiment performed three times).
PEG-induced osmotic stress was attenuated by CH4
Since sugar metabolism and signaling function exist as part of an integrated redox system, including quenching ROS and contributing to stress tolerance, especially in tissues or organelles with high soluble sugar concentrations27, we asked the question whether exposure to CH4 could reduce oxidative damage in maize plants upon PEG stress, so as to adapt to osmotic stress.
Different concentrations of CH4 (0.13, 0.65, and 1.30 mM CH4) were used to mimic endogenous CH4-related responses in ZD958 under PEG stress. Our experiments showed that pretreatments with CH4 partially alleviated the loss of fresh and dry weight in both root and shoot tissues of ZD958 caused by PEG (Fig. 3a–c). The maximal protective response was observed in 0.65 mM CH4-pretreated plants. When CH4 was applied alone, only dry weight in root tissues of ZD958 was obviously increased, by comparison with the control samples. Soluble sugar content was significantly enhanced by PEG-6000, which was further strengthened after CH4 pretreatment, with maximal responses at 0.65 and 1.30 mM (Fig. 3d). To further assess the protective effects of CH4, PEG-induced oxidative damage to cell membranes in root tissues was investigated. Because of the stressed plant tissues containing anthocyanin and other interfering compounds, the TBARS content, an important indicator of lipid peroxidation and free radical generation, were measured. Treatment with 20% PEG-6000 for 5 d caused a significant increase in TBARS content compared with the control samples (Fig. 3e). By contrast, the amount of the increased TBARS content triggered by PEG-6000 was reduced by CH4 pretreatment, with the maximal reduction observed at 0.65 mM CH4. No significant changes occurred in CH4-pretreated alone samples. Therefore, 0.65 mM CH4 was applied in the following experiments.
(a) 5-d-old seedlings were preincubated in the solution containing the indicated concentrations of CH4 for 1 d, and then transferred to half-strength Hoagland solutions with or without 20% PEG-6000 for another 5 d. Photographs were then taken. Bar = 10 cm. Meanwhile, fresh weight (b) and dry weight (c) were measured in both root and shoot tissues. The contents of soluble sugar (d) and TBARS (e) in root tissues were also determined. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root or shoot parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
To further investigate the regulatory roles of CH4 in PEG-induced osmotic stress, we compared the differences between ZD958 and ZJY1 after the pretreatment with CH4 (Fig. 4). CH4 alone could not influence the RWC in the root and shoot tissue of two cultivars. However, there were higher levels of RWC in both root and shoot tissues of ZD958 than those of ZJY1 upon CH4 followed by PEG-6000 stress (Fig. 4b). As expected, CH4 pretreatment increased endogenous CH4 and soluble sugar concentrations in PEG-treated plants, especially in root tissues of ZD958 than ZJY1 (Fig. 4c,d). Exogenously applied CH4 alone increased endogenous CH4 production to some extent. A similar induction was observed upon PEG-6000 treatment, particularly in ZD958. Therefore, we deduced that CH4 may protect against PEG-induced osmotic stress by improving sugar content.
(a) 5-d-old maize seedlings were preincubated in the solution containing 0.65 mM CH4 for 1 d, and then transferred to half-strength Hoagland solutions with or without 20% PEG-6000 for 5 d. Photographs were then taken. Bar = 10 cm. (b) Relative water content (RWC) was measured in both root and shoot tissues. Meanwhile, the contents of endogenous methane (c) and total soluble sugar (d) in root tissues were determined. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root or shoot parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
Expression of genes responsible for sugar metabolism
To further establish the relationship between CH4 and sugar metabolism under PEG treatment, we analyzed the transcriptional profiles of some key genes in sugar metabolism and signaling pathways (Supplementary Fig. S1). Similar to the previous reports27,28, PEG treatment significantly increased the transcript levels of sucrose synthase 1 (SH1) and UDP-glucose dehydrogenase (UDPGDH), while decreased the gene expression of soluble acid invertase 2 (IVR2) and hexokinase 3 (HXK3) in root tissues of two maize cultivars (Fig. 5). Compared with PEG alone, the transcript levels of sucrose synthase 2 (SUS1), soluble acid invertase 1 (IVR1), and hexokinase 1/9 (HXK1/9), were dramatically enhanced by CH4 pretreatment in ZD958, a tolerant cultivar. However, no such striking differences were observed in root tissues of ZJY1 (a sensitive cultivar), showing no significant changes or weak induction in these three transcripts. These results indicated that CH4 conferred plant tolerance against osmotic stress by improving sugar metabolism.
5-d-old maize seedlings of ZD958 and ZJY1 were preincubated in the solution containing 0.65 mM CH4 for 1 d, and then transferred to half-strength Hoagland solutions with or without 20% PEG-6000 for 2 d. Relative gene expression of sucrose synthase 1 (SH1; X02400), sucrose synthase 2 (SUS1; L22296), soluble acid invertase 1 (IVR1; AF171874), soluble acid invertase 2 (IVR2; U31451), hexokinase 1/3/9 (HXK1/3/9; NM_001158821/XM_008676343/XM_008658658), UDP-glucose dehydrogenase (UDPGDH; EU961705) in root tissues were analyzed. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
CH4 regulated redox status in response to PEG treatment
The experiments described above indicated that CH4 pretreatment was able to reduce TBARS content in PEG-stressed root tissues (Fig. 3e). Since PEG-induced oxidative stress in root tissues was associated with ROS production29, the antioxidant enzymes responsible for ROS scavenging were investigated. The total activities of SOD, POD, and CAT in root tissues of ZD958 were higher than those of ZJY1 under the control conditions (Supplementary Fig. S2). Although the SOD activities were approximately similar in the two maize cultivars under different treatments, the total activities of POD and CAT were increased by PEG treatment in both cultivars. The only significant effect of CH4 pretreatment was a decrease in POD activity in root tissues of ZD958.
PEG stress-induced ROS has been demonstrated to cause oxidative damage to plants, and H2O2 and O2− are believed to be the most important components. The effect of CH4 on the PEG-induced ROS overproduction was further investigated. Our results showed that PEG induced significant increases in the levels of H2O2 and O2− in root tissues of both cultivars (Fig. 6a; Supplementary Fig. S3). CH4 pretreatment significantly reduced PEG-induced ROS production, with more effects observed in root tissues of ZD958 than in ZJY1.
5-d-old maize seedlings of ZD958 and ZJY1 were preincubated in the solution containing 0.65 mM CH4 for 1 d, and then transferred to half-strength Hoagland solutions with or without 20% PEG-6000 for 2 d. Afterwards, the contents of endogenous H2O2 (a) in root tissues was analyzed. Meanwhile, the contents of reduced ascorbic acid (AsA; b), dehydroascorbate (DHA; c), and AsA/DHA ratio (d) in root tissues were determined according to the method described by Law et al.51. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
Since the glutathione-ascorbate cycle is a metabolic pathway that detoxifies H2O2, AsA and GSH homeostasis were analyzed. CH4 alone enhanced the reduced AsA content and AsA/dehydroascorbate (DHA) ratio in both ZD958 and ZJY1 when compared to the control conditions (Fig. 6b–d). By contrast, PEG decreased AsA content and significantly increased DHA content, therefore resulting in a lower AsA/DHA ratio. However, CH4 pretreatment obviously blocked the decrease of AsA content and the increase of DHA level induced by PEG, thus resulting in the higher level of AsA/DHA ratio, in comparison with PEG alone. Moreover, higher reduced and total contents of ascorbate (AsA+DHA) were observed in ZD958 than ZJY1 under all of the tested conditions (Table 1).
As expected, treatment with PEG triggered an obvious increase in content of GSH and an decrease of GSSG in roots (Table 2). By contrast, CH4 pretreatment significantly eliminated the effects of PEG alone on GSH and GSSG contents. Meanwhile, a higher ratio of GSH/GSSG, an important parameter for the intracellular redox status, was observed in the CH4 pretreatment cultivars (ZD958 in particular) followed by PEG exposure, with respect to PEG alone samples. These results implied that CH4 may be involved in the reestablishment of redox status in PEG-treated maize seedlings via the modulation of AsA and GSH homeostasis.
Expression of genes responsible for AsA metabolism
In order to investigate the relationship between CH4 and AsA metabolism, the transcriptional profiles of some key genes in AsA production and recycling (Supplementary Fig. S1) were analyzed. As shown in Fig. 7, CH4 pretreatment alone brought about much higher transcript levels of GDP-L-galactose phosphorylase (GGP), L-galactono-1,4-lactone dehydrogenase (GalLDH), ascorbate peroxidase 1/3/6 (APX1/3/6), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) in ZD958; no such significant increases were observed in ZJY1 except for the gene expression of MDHAR. Compared with the control conditions, PEG treatment significantly decreased the transcript levels of GGP and APX3 in both ZD958 and ZJY1, but increased the expression of APX6. Except for APX1 and APX6 in ZJY1, higher transcript levels of all the tested genes were observed in CH4 pretreatment alone than those in only PEG-6000 stress. After CH4 pretreatment followed by PEG stress, there were significant increases in the levels of GalLDH, APX1/3, and MDHAR mRNA in the root tissues of ZD958 (in particular) and ZJY1 compared to PEG alone, all of which were consistent with the decreased H2O2 concentration and high level of AsA/DHA ratio (Fig. 6a,d).
5-d-old maize seedlings of ZD958 and ZJY1 were preincubated in the solution containing 0.65 mM CH4 for 1 d, and then transferred to half-strength Hoagland solutions with or without 20% PEG-6000 for 2 d. Relative gene expression of GDP-L-galactose phosphorylase (GGP; DT943063), L-galactono-1,4-lactone dehydrogenase (GalLDH; DT943591), ascorbate peroxidase 1 (APX1; NM_001177011), ascorbate peroxidase 3 (APX3; NM_001159274), ascorbate peroxidase 6 (APX6; NM_001139033), dehydroascorbate reductase (DHAR; DR807318), and monodehydroascorbate reductase (MDHAR; CO461725) in root tissues were analyzed55. Control seedlings were incubated in Hoagland solution alone. Data are presented as means ± SE (5 root parts per experiment performed three times). Bars with different letters denote significant differences according to multiple comparisons (P < 0.05).
Possible precursor(s) of CH4 production
Some compounds, such as amino acid L-methionine, have been suggested as likely sources of CH4 emission partly due to the degree of methylation1,4,7,12. To investigate whether these compounds could also induce endogenous CH4 production in maize, L-methionine contents was analyzed. In comparison with the control conditions, CH4 alone had no significant effect on L-methionine production. L-Methionine production in the two cultivars was significantly increased by PEG-6000 stress, especially in ZD958 (Supplementary Fig. S4). Pretreatment with L-methionine induced endogenous CH4 production, especially in ZD958. Together, although the other source(s) for endogenous CH4 production can’t be ruled out, our results clearly confirm that L-methionine is a possible precursor of endogenous CH4 production.
Discussion
A comprehensive understanding of CH4 in plants is that the living plants and fungi can not only emit CH4 to the atmosphere7, but also firstly produce CH4 in vivo8. Similar phenomenon occurred in nitric oxide9 and carbon monoxide10, etc. Although CH4 emission and/or release have previously considered as a non-enzymatic process rather than an enzyme-mediated process4, whether or how plants produce endogenous CH4 production is still elusive. Here, we demonstrated that PEG treatment triggered endogenous CH4 production in maize cultivars with an approximately time-dependent manner (Fig. 2). Thus, our study suggested that CH4 production in plants can be induced by environmental stimuli, including osmotic stress and salinity23.
Among the potential chemical compounds related to CH4 emission and/or release, it was reported that methyl compounds can act as precursors for CH4 formation under aerobic conditions14. For example, Keppler et al.4 suggested the role of methyl groups on pectin as a source for CH4 formation from plant tissues. After the digestion of pectin with pectin methyl esterase (PME), Bruhn et al.15 further observed a decrease of CH4 efflux in solutions of Citrus limon fruit pectin after UV-B treatment, in comparison with stress alone. Partly consistent with above results, our tests showed that seedling roots of ZD958, an osmotic stress tolerant cultivar, contain a relatively higher level of pectin upon PEG stress, compared to that in the sensitive cultivar ZJY1 (Supplementary Fig. S5). Endogenous CH4 production was progressively triggered by PEG stress, particularly in ZD958 (Fig. 2). However, the causal link between pectin and CH4 production is not fully elucidated, and endogenous CH4 production might be mediated by multiple pathways5,12,14.
Subsequent results revealed that the endogenous L-methionine production was induced by PEG stress (Supplementary Fig. S4a). The exogenous addition of L-methionine increased endogenous CH4 levels both in ZD958 (in particular) and ZJY1 (Supplementary Fig. S4b). A similar result was found in the papers from Frank Keppler’s lab7,30. Based on the addition of 13CH3-Met and stable isotope measurements, the δ13C(CH4) values in the headspace of L. angustifolia and P. sapidus supplemented with 13C-methionine showed a continuous increase during the entire incubation period. Thus, L-methionine might be one of the precursors of CH4. Combined with our results in Supplementary Fig. S4, a stress-induced CH4 production from L-methionine might exist in plants8,30. Certainly, genetic and biochemical methods should be further provided to confirm this possibility.
Significant CH4 generation was previously demonstrated in animals31,32. It was further confirmed that CH4 is a critical molecule implicated in the anti-inflammatory diseases in mammals, and in protecting against ischemia reperfusion induced oxidative and nitrosative stresses33. This report triggers our interests to investigate the potential roles of CH4 in osmotic stressed plants.
Subsequent work showed that the increased endogenous CH4 production may benefit maize under osmotic stress. The following results support this conclusion: (i) the osmotic stress-tolerant maize cultivar ZD958 exhibited more CH4 production than the sensitive cultivar ZJY1 (Figs 1 and 2a); (ii) pretreatments with 0.65 mM CH4, which enhanced CH4 production in our experimental conditions (Fig. 4c), could significantly alleviate PEG-6000-induced symptoms of osmotic stress in both root and shoot tissues, including the improvement of seedling growth stunt (Fig. 3a–c); (iii) CH4 significantly blocked the increase in ROS production and TBARS content caused by PEG-6000 stress (Figs 3e and 6a, Supplementary Fig. S3); meanwhile, both AsA and GSH homeostasis were reestablished by CH4 (Tables 1 and 2, Fig. 6b–d). These finding parallels the situation encountered in animals, in which CH4 was shown to inhibit intestinal superoxide anion generation parallel to decreased activity of myeloperoxidase (MPO), a ROS producing enzyme32. Since the maintenance of ROS homeostasis has been linked to the increased tolerance of plants to a wide range of environmental stresses, our results preliminarily suggested that CH4 may enhance the adaptive plant responses against osmotic stress by alleviating PEG-induced oxidative damage.
A beneficial role of CH4 against salinity stress has been recently reported in alfalfa plants, which was mainly attributed to the induction of antioxidant defence23. To investigate the regulatory mechanism of CH4 on redox status, the total activities of SOD, POD, and CAT were determined in PEG-stressed plants (Supplementary Fig. S2). Unlike no obvious changes of SOD and CAT activities, the reduction of POD activity caused by CH4 only appeared in ZD958 upon PEG stress. In the presence of CH4 and PEG, the levels of H2O2 in two maize cultivars were lower than PEG alone (Fig. 6a), suggesting that CH4 might inhibit ROS production through a putative mechanism. Normally, plant PODs exist in a large number isozymatic forms and exhibit diverse functions depending on their substrates. Besides being responsible for scavenging H2O2, POD can generate H2O2 in the leaf apoplast of cowpea34. Therefore, we deduced that CH4-mediated reduction of POD might be partially responsible for the decreased H2O2 and TBARS contents (Figs 3e and 6a). Another possible explanation is that some potent non-enzymatic antioxidants, such as AsA, GSH, carotenoids, and tocopherols which directly interact with and detoxify ROS25, may be involved in the alleviation of PEG-induced oxidative damage triggered by CH4.
Sucrose is a pivotal integrating regulatory molecule that controls gene expression related to plant metabolism, stress resistance, growth and development27. The only known enzymatic pathways of sucrose cleavage in plants are catalyzed by invertase and sucrose synthases35. Similar to the responses of osmotic stress28, CH4 pretreatment followed by PEG stress differentially increased the transcript levels of SH1, SUS1, and IVR1 in ZD958, the osmotic stress tolerant cultivar (Fig. 5), and may induce the production of glucose and UDP-glucose (Supplementary Fig. S1). Meanwhile, no significant changes or weak induction in these transcripts were observed in the osmotic stress sensitive cultivar. HXKs catalyze the conversion of glucose and fructose into hexose monophosphates, thereby permitting entry of carbon skeletons such as glucose-6-P into catabolism. Although it was reported that there are nine HXKs in maize, only HXK1/3/9 contain stress-responsive cis elements in the promoter region36. In our experimental conditions, compared with PEG alone, the transcript levels of HXK1/9 were dramatically enhanced by CH4 pretreatment followed by PEG in ZD958 (Fig. 5). Therefore, sugar metabolism or signaling may be involved in CH4-induced osmotic stress tolerance.
On the other hand, UDPGDH oxidizes UDP-D-glucose to UDP-D-glucuronate, which is a glycosyl donor for pectin biosynthesis37. Pectin content and its degradation affect cell wall integrity, which is closely linked with abiotic stress sensitivity38. It was reported that polysaccharide remodeling is an important process that controls the mechanical properties of the cell wall and the water status of plant cells under water deficit39. Here, we showed that CH4 pretreatment can slightly increase the pectin contents in ZD958 under PEG stress (Supplementary Fig. S5). As overexpression of genes related to the synthesis of matrix polysaccharides benefits the resistance of plants to water stress40, we therefore speculated that CH4-induced pectin production may be one of the mechanisms to increase osmotic stress tolerance in maize.
Interestingly, D-galacturonic acid derived from pectin breakdown could also be a source of AsA41. AsA is one of the key players in a redox hub that integrates metabolic information and environmental stimuli to different responses within the cellular signaling network25. A high level of endogenous AsA is essential to effectively maintain the antioxidant system that protects plants from oxidative damage42. In our experimental conditions, CH4 pretreatment could increase the reduced AsA contents and AsA/DHA ratio (Fig. 6b–d, Table 1), thus decreasing PEG-induced H2O2 production (Fig. 6a). The comparison of transcript profiles of some key enzymes in sugar and AsA metabolism (Figs 5 and 7), confirmed that CH4 may participate in sugar signaling, which in turn increases AsA production and recycling, resulting in the reduced oxidative damage caused by PEG. Finally, ROS homeostasis was reestablished, and osmotic stress tolerance was successfully enhanced.
Together, this is the first report of the physiological significance of endogenous CH4 production in the protection of higher plants from osmotic stress. This conclusion was confirmed by the improved biomass and RWC. Subsequent studies showed that at least in our experimental conditions, CH4 improves sugar and AsA metabolism, thus suppressing ROS production and resulting in the alleviation of PEG-induced osmotic stress in maize. Further genetic and molecular investigations are required for better understanding of the detailed molecular mechanisms of CH4-induced stress tolerance.
Methods
Preparation of culture solution containing methane and determination of methane content
The CH4 gas (99.9%, v/v) from a compressed gas cylinder (Nanjing Special Gas Co., China) was bubbled into 500 ml half-strength Hoagland solutions at a rate of 200 ml min−1 for at least 30 min. The corresponding methane-rich solution was then immediately diluted with half-strength Hoagland solutions to the concentrations required (0.13, 0.65, and 1.30 mM CH4), and maintained at a constant level for at least 12 h. Therefore, methane-rich solution was used twice a day. Similarly in animal research, CH4 was dissolved in 20 ml of physiological saline for 20 min at a speed of 200 ml min−1 to reach a supersaturated level43,44.
For analyzing endogenous CH4 production, seedling roots were homogenized with 5 ml sterile water and transferred to a vial. Sulphuric acid was added to digest plant material, which was adopted in the determination of carbon monoxide and hydrogen gas production45,46. The GC was calibrated using a secondary standard CH4 mixture (2.0 ppm CH4 in N2; Nanjing Special Gas Co., China). For the measurement of samples, 2 ml of the head space air in the vial was injected directly into carrier gas by syringe.
Plant materials and growth conditions
Two maize (Zea mays L.) cultivars Zhengdan 958 (ZD958) and Zhongjiangyu No.1 (ZJY1) were used in this study. After soaking overnight, maize seeds were germinated on filter paper imbibed in distilled water at 25 °C in the darkness for 2 d. Uniform seedlings were then chosen and transferred to an incubator with a 14-h photoperiod at 25 ± 1 °C and 200 μmol m−2 s−1 irradiation. After growing for another 2 d, seedlings were incubated in half-strength Hoagland solutions with or without polyethylene glycol (PEG-6000) as described in the corresponding figure legends. After various treatments for 5 d or the indicated time points, plants were photographed, and shoot or root parts were sampled for used immediately or flash-frozen in liquid nitrogen, and stored at −80 °C for further analysis.
Measurement of relative water content (RWC)
Relative water content (RWC) was measured following the method of Fukao et al.47. Fresh weight (FW) of root or shoot tissues were determined immediately after harvest, and then different tissues were floated on deionized water for 6 h. The rehydrated tissues were re-weighted to determine turgid weight (TW). Finally, different tissues were oven dried at 65 °C for 3 d, and dry weight (DW) of each sample was measured. RWC was calculated using the following equation: RWC (%) = ((FW - DW)/(TW - DW)) × 100%.
Soluble sugar analysis
Total soluble sugar content was analyzed according to the spectroscopic method described by Yemm & Willis48. The anthrone reagent was prepared by dissolving 1.0 g anthrone in 50 ml ethyl acetate, and heated in a boiling water bath. Maize root tissues were crushed in 5 ml distilled water with a mortar and pestle. The suspensions were centrifuged at 10,000 g for 20 min, and the supernatants were collected. Total soluble sugars were analyzed by 100 μl of sample extract reacting with 0.5 ml anthrone reagent and 5 ml H2SO4. The mixture was incubated in a boiling water bath for 10 min. After cooling, the absorbance at 630 nm was determined. Glucose was used to prepare standard solutions for the calibration curve.
Determination of thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was estimated by measuring the amount of TBARS as previously described by Hodges et al.49. Maize root tissues were homogenized with inert sand in 80:20 (v:v) ethanol:water, followed by centrifugation at 10,000 g for 10 min. Samples were crushed with either 20% trichloroacetic acid (TCA) and 0.01% butylated hydroxytoluene or 0.65% 2-thiobarbituric acid (TBA) solution. After heating in a boiling water bath for 30 min, the mixture was quickly cooled, and then centrifuged at 10,000 g for 10 min. The absorbance at 440, 532, and 600 nm was determined. The concentration of lipid peroxides was quantified in terms of TBARS amount using an extinction coefficient of 157 mM−1 cm−1 and expressed as nmol g−1 fresh weight (FW).
Determination of H2O2, dehydroascorbate (DHA) and AsA contents
For the determination of endogenous H2O2 content50, maize root tissues (about 0.2 g) were ground with liquid nitrogen and homogenized with 0.2 M HClO4 at 4 °C. The suspensions were centrifuged at 10,000 g for 20 min, and the supernatants were collected. An aliquot of supernatant (500 μl) was added to 500 μl of assay reagent (0.5 mM ammonium ferrous sulfate, 50 mM H2SO4, 0.2 mM xylenol orange, and 200 mM sorbitol) and the absorbance at 560 nm was determined after 15 min of incubation at 30 °C. Standard curves were obtained by adding variable amounts of H2O2.
Reduced ascorbic acid (AsA) and dehydroascorbate (DHA) contents were measured according to the previous method51. After treatment, fresh root tissues were crushed and homogenized in cold 6% TCA immediately. AsA and DHA content was analyzed by 200 μl of sample extract reacting reagents. Afterwards, samples were vortex-mixed and incubated at room temperature for 1 min, and 0.1 ml of 10 mM dithiothreitol was added. Then 0.5 ml of 10% TCA, 0.4 of 44% ortho-phosphoric acid, 0.4 ml of 4% 2,2′-dipyridyl in 70% ethanol, and 0.2 ml of 3% (w/v) FeCl3, were added. The mixture was incubated at 37 °C for 30 min, and the absorbance at 525 nm was determined. Standard curves were obtained by adding variable amounts of AsA.
Alternatively, another method for the determination of ascrobate was carried out according to the method reported by Ueda et al.52. For the measurement of reduced AsA, the reaction mixture consisted of 10 μL of 0.01 U μL−1 AO, 10 μL of sample, and 80 μL of 0.1 M potassium phosphate buffer (pH 7.0). For the measurement of oxidized AsA, the reaction mixture consisted of 4 mM DTT, 80 μL of 0.1 M potassium phosphate buffer (pH 7.8), and 10 μL of extract. In blank wells, the AO solution or DTT was substituted by potassium phosphate buffer (pH 7.8). After shaking the plate for 5 s, continuous absorbance reading at 265 nm was started by using UV spectrophotometry (UV1102II, Tianmei, China). Finally, total AsA was calculated.
Determination of GSH and GSSG contents
GSH and GSSG contents were assayed according to the procedure of Gronwald et al.53. GSH content in root of maize was analyzed by the 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB)-glutathione reductase (GR) recycling assay. GSSG was determined by the same method in the presence of 2-vinylpyridine. Absorbance at 412 nm was determined, and the GSH/GSSG ratio was calculated.
RNA isolation and real-time RT-PCR analysis
Total RNA was isolated from maize root tissues with Trizol reagent (Invitrogen, Carlsbad, CA USA) according to the manufacturer’s instructions. Real-time RT-PCR experiments were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Germany) with SYBR pre-mixture kit (BioTeke, China). Using specific primers (Supplementary Table S1), the expression levels of corresponding genes were normalized against two internal control genes β-tubulin (TUB) and actin1 (ACT1) under identical conditions28. The efficiency and specificity of all the primers were checked by both melting curve analysis and agarose gel in our experimental conditions. The data were based on three independent biological replicates and each sample was prepared in triplicate.
Antioxidant enzyme activity assay
As previously described by Huang et al.54, superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed by measuring its capacity of inhibiting the photochemical reduction of NBT. One unit of SOD (U) was defined as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50%. Guaiacol peroxidase (POD, EC 1.11.1.7) activity was determined by measuring the oxidation of guaiacol (extinction coefficient 26.6 mM−1 cm−1) at 470 nm. Catalase (CAT, EC 1.11.1.6) activity was measured by monitoring the consumption of H2O2 (extinction coefficient 39.4 mM−1 cm−1) at 240 nm. Protein was determined by the method of Bradford52, using bovine serum albumin (BSA) as the standard.
Statistical analysis
All data presented were the mean values of three independent experiments. Each value was expressed as means ± SE. Statistical analysis was performed using SPSS 16.0 software. Differences among treatments were analyzed by one-way ANOVA taking P < 0.05 as significant according to multiple comparisons.
Additional Information
How to cite this article: Han, B. et al. Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci. Rep. 7, 46185; doi: 10.1038/srep46185 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Keppler F. et al. Methane formation in aerobic environments. Environ Chem 6, 459–465 (2009).
Wang, Z. P., Chang, S. X., Chen, H. & Han, X. G. Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Sci Rev 127, 193–202 (2013).
Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60, 609–640 (1996).
Keppler, F., Hamilton, J. T. G., Braß, M. & Röckmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 439, 187–191 (2006).
Bruhn, D., Møller, I. M., Mikkelsen, T. N. & Ambus, P. Terrestrial plant methane production and emission. Physiol Plantarum 144, 201–209 (2012).
Kirschbaum, M. U. F., Niinemets, Ü., Bruhn, D. & Winters, A. J. How important is aerobic methane release by plants? Funct Plant Sci Tech 1, 138–145 (2007).
Lenhart, K. et al. Evidence for methane production by saprotrophic fungi. Nat Commun 3, 1046 (2012).
Jia, Z., Cai, Z., Xu, H. & Li, X. Effect of rice plants on CH4 production, transport, oxidation and emission in rice paddy soil. Plant Soil 230, 211–221 (2001).
Planchet, E., Jagadis, G. K., Sonoda, M. & Kaiser, W. M. Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41, 732–743 (2005).
Guenther, A. et al. Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos Environ 34, 2205–2230 (2000).
Das, D. & Veziroğlu, T. N. Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energ 26, 13–28 (2001).
Althoff, F. et al. Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat Commun 5, 4205 (2014).
Bruhn, D., Mikkelsen, T. N., Rolsted, M. M., Egsgaard, H. & Ambus, P. Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol 16, 512–516 (2014).
McLeod, A. R. et al. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol 180, 124–132 (2008).
Bruhn, D., Mikkelsen, T. N., Øbro, J., Willats, W. G. T. & Ambus, P. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol 11, 43–48 (2009).
Qaderi, M. M. & Reid, D. M. Methane emissions from six crop species exposed to three components of global climate change: temperature, ultraviolet-B radiation and water stress. Physiol Plantarum 137, 139–147 (2009).
Wang, Z. P. et al. Physical injury stimulates aerobic methane emissions from terrestrial plants. Biogeosci Discuss 6, 615–621 (2009).
Jiang, M. & Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53, 2401–2410 (2002).
Osakabe, Y., Yamaguchi-Shinozaki, K., Shinozaki, K. & Tran, L. S. P. Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J Exp Bot 64, 445–458 (2013).
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J. & Zhu, J. K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45, 523–539 (2006).
Noctor, G., Mhamdi, A. & Foyer, C. H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol 164, 1636–1648 (2014).
Møller, I. M., Jensen, P. E. & Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481 (2007).
Zhu, K. et al. Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ Exp Bot 129, 37–47 (2016).
Uzilday, B., Turkan, I., Ozgur, R. & Sekmen, A. H. Strategies of ROS regulation and antioxidant defense during transition from C3 to C4 photosynthesis in the genus Flaveria under PEG-induced osmotic stress. J Plant physiol 171, 65–75 (2014).
Foyer, C. H. & Noctor, G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155, 2–18 (2011).
Zanella, M. et al. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J Exp Bot 67, 1819–1826 (2016).
Bolouri-Moghaddam, M. R., Le, R. K., Xiang, L., Rolland, F. & Van, E. W. Sugar signalling and antioxidant network connections in plant cells. The FEBS J 277, 2022–2037 (2010).
Kakumanu, A. et al. Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-Seq. Plant Physiol 160, 846–867 (2012).
Liu, Y., Xu, S., Ling, T., Xu, L. & Shen, W. Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J Plant Physiol 167, 1371–1379 (2010).
Lenhart, K., Althoff, F., Greule, M. & Keppler, F. Techincal note: methionine, a precursor of methane in living plants. Biogeosciences 12, 1907–7914 (2015).
Ghyczy, M. et al. Hypoxia-induced generation of methane in mitochondrial and eukaryotic cells-an alternative approach to methanogenesis. Cell Physiol Biochem 21, 251–258 (2008a).
Ghyczy, M. et al. Oral phosphatidylcholine pretreatment decreases ischemia reperfusion induced methane generation and the inflammatory response in the small intestine. Shock 30, 596–602 (2008b).
Boros, M. et al. The anti-inflammatory effects of methane. Crit Care Med 40, 1269–1278 (2012).
Fecht-Christoffers, M. M., Führs, H., Braun, H. P. & Horst, W. J. The role of hydrogen peroxide-producing and hydrogen peroxide-consuming peroxidases in the leaf apoplast of cowpea in manganese tolerance. Plant Physiol 140, 1451–1463 (2006).
Koch, K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7, 235–246 (2004).
Zhou, M. L. et al. Trehalose metabolism-related genes in maize. Plant Growth Regul 33, 256–271 (2014).
Kärkönen, A. et al. UDP-glucose dehydrogenases of maize: a role in cell wall pentose biosynthesis. Biochem J 391, 409–415 (2005).
Liu, H., Ma, Y., Chen, N., Guo, S., Liu, H. & Xu, Y. Overexpression of stress-inducible OsBURP16, the β subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant, Cell & Environ 37, 1144–1158 (2014).
Gribaa, A. et al. Effect of water deficit on the cell wall of the data palm (Phoenix dactylifera ‘Deglet nour’, Arecales) fruit during development. Plant, Cell & Environ 36, 1056–1070 (2013).
Piro, G., Leucci, M. R., Waldron. K. & Dalessandro, G. Exposure to water stress causes changes in the biosynthesis of cell wall polysaccharides in roots of wheat cultivars varying in drought tolerance. Plant Sci 165, 559–569 (2003).
Ishikawa, T., Dowdle, J. & Smirnoff, N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plantarum 126, 343–355 (2006).
Caverzan, A. et al. Plant responses to stress: Role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35, 1011–1019 (2012).
Song, K. et al. Methane-rich saline attenuates ischemia/reperfusion injury of abdominal skin flaps in rats via regulating apoptosis level. BMC surgery 15, 92 (2015).
Wu, J. et al. Protective effects of methane-rich saline on diabetic retinopathy via anti-inflammation in a streptozotocin-induced diabetic rat model. Biochem Bioph Res Co 466, 155–161 (2015).
Renwick, G. M., Giumarro, C. & Siegel, S. M. Hydrogen metabolism in higher plants. Plant Physiol 39, 303–306 (1964).
Anderson, C. R. & Wu, W. H. Analysis of carbon monoxide in commercially treated tuna (Thunnus spp.) and mahi-mahi (Coryphaena hippurus) by gas chromatography/mass spectrometry. J Agr Food Chem 53, 7019–7023 (2005).
Fukao, T., Yeung, E. & Bailey-Serres, J. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23, 412–427 (2011).
Yemm, E. W. & Willis, A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57, 508–514 (1954).
Hodges, D. M., DeLong, J. M., Forney, C. F. & Prange, R. K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611 (1999).
Ma, F. et al. Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol Biol 85, 49–61 (2014).
Law, M. Y., Charles, S. A. & Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem J 210, 899–903 (1983).
Ueda, Y., Wu, L. & Frei, M. A critical comparison of two high-throughput ascorbate analyses methods for plant samples. Plant Physiol Biochem 70, 418–423 (2013).
Gronwald, J. W. et al. Effect of herbicide antidotes on glutathione content and glutathione S-transferase activity of sorghum shoots. Pestic Biochem Physiol, 29, 66–76 (1987).
Huang, J., Han, B., Xu, S., Zhou, M. & Shen, W. Heme oxygenase-1 is involved in the cytokinin-induced alleviation of senescence in detached wheat leaves during dark incubation. J Plant Physiol 168, 768–775 (2011).
Sanahuja, G. et al. Ascorbic acid synthesis and metabolism in maize are subject to complex and genotype-dependent feedback regulation during endosperm development. Biotech J 8, 1221–1230 (2013).
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (KYTZ201402), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the National Natural Science Foundation of China (J1210056 and J1310015).
Author information
Authors and Affiliations
Contributions
Bin Han and Xingliang Duan performed the measurements. Wenbiao Shen came up with the idea, provided overall supervision and guidance on the experimental aspects. Bin Han analyzed the data and wrote the manuscript. Xingliang Duan revised the manuscript, and other authors Yu Wang, Kaikai Zhu, Jing Zhang, Ren Wang, Huali Hu, Fang Qi, Jincheng Pan, Yuanxin Yan also discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Han, B., Duan, X., Wang, Y. et al. Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci Rep 7, 46185 (2017). https://doi.org/10.1038/srep46185
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46185
This article is cited by
-
Crops’ response to the emergent air pollutants
Planta (2022)
-
Plant gasotransmitters: light molecules interplaying with heavy metals
Reviews in Environmental Science and Bio/Technology (2021)
-
The role of methane in plant physiology: a review
Plant Cell Reports (2020)
-
Hydrogen peroxide is involved in methane-induced tomato lateral root formation
Plant Cell Reports (2019)
-
Methyl-coenzyme M reductase-dependent endogenous methane enhances plant tolerance against abiotic stress and alters ABA sensitivity in Arabidopsis thaliana
Plant Molecular Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.